Abstract
MarR is a key regulator of the marRAB operon involved in antibiotic resistance and solvent stress tolerance in Escherichia coli. We show that two metabolic intermediates, 2,3-dihydroxybenzoate and anthranilate, involved in enterobactin and tryptophan biosynthesis, respectively, can activate marRAB transcription. We also found that a third intermediate involved in ubiquinone biosynthesis, 4-hydroxybenzoate, activates marRAB transcription in the absence of TolC. Of the three, however, only 2,3-dihydroxybenzoate directly binds MarR and affects its activity.
A central regulator of intrinsic antibiotic resistance and organic acid tolerance in Escherichia coli and related enteric bacteria is MarR, a negative autoregulator of the marRAB operon (2, 4, 7-8, 10, 17-19). This protein is a canonical member of a family of transcriptional repressors commonly associated with regulating the expression of multidrug efflux systems, stress response systems, metabolic pathways, and virulence factors (reference 24 and references therein). A common theme among MarR family regulators is the ability to bind structurally disparate anionic lipophilic compounds. In the case of MarR, numerous chemical compounds, such as benzoate, salicylate, 2,4-dinitrophenol, menadione, and plumbagin, have been shown to modulate its activity both in vivo and in vitro (1, 3, 9, 19, 22). Additionally, MarR activity is affected through protein-protein interactions with enzymes such as transketolase A and DNA gyrase subunit A (11-12).
Recently, Rosner and Martin found that in an E. coli tolC mutant, the marRAB promoter was upregulated approximately 2-fold (21). They concluded from these results that upon the loss of TolC-dependent excretory capacity, cells accumulate metabolic intermediates capable of inducing the mar system. Intrigued by this hypothesis, we examined a number of metabolic pathways and hypothesized that those involved in the superpathway of chorismate may be potential effectors of MarR given their chemical similarity to its known ligands. In this work, we show that three such intermediates in aromatic amino acid biosynthesis activate marRAB transcription in vivo when supplied endogenously and that one, 2,3-dihydroxybenzoate, directly binds to MarR in vitro.
Aromatic metabolic intermediates activate the marRAB operon in vivo.
Of the several known inducers of marRAB expression, the best characterized is salicylate, a weak aromatic acid (1, 3, 9, 19, 22). Specifically, salicylate has been shown to directly bind MarR (3, 19). If metabolic intermediates are potential ligands for MarR, we surmised that that they may have chemical structures similar to that of the canonical inducer salicylate. Among the vast number of metabolic intermediates in E. coli metabolism, those involved in the superpathway of chorismate appeared to be the most promising (14).
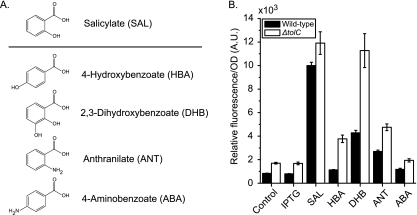
Examination of this superpathway in E. coli K-12 yielded four compounds that were structurally similar to salicylate (Fig. 1 A). Our basis for assessing similarity was the presence of a carboxylate group with adjacent hydroxyl or amine groups on the benzyl ring. Four putative compounds were identified: 4-hydroxybenzoate (HBA), an intermediate in ubiquinone-8 biosynthesis; 2,3-dihydroxybenzoate (DHB), an intermediate in enterobactin biosynthesis; anthranilate (ANT), an intermediate in tryptophan biosynthesis; and 4-aminobenzoate (PABA), an intermediate in tetrahydrofolate biosynthesis.
FIG. 1.
Activation of the marRAB operon by aromatic acid metabolites in the superpathway of chorismate. (A) Chemical structure of metabolites selected based on their similarity to salicylate. (B) Observed transcriptional activation of the marR′-yfp promoter fusion in the presence of 5 mM concentrations of indicated inducers in wild-type (CR700) and ΔtolC mutant (CR703) backgrounds. Salicylate and IPTG served as positive and negative controls for activation, respectively. Cells were grown overnight in MOPS (morpholinepropanesulfonic acid) minimal medium (20 mM glucose, 0.2% Casamino Acids, pH 7.2) and subcultured 1:200 in fresh medium. Following dilution, 450 μl of culture was transferred to deep 96-well plates and grown at 37°C with aeration at 1,000 rpm to an OD of 0.5. At this time, 100 μl of medium containing dissolved inducer was added. Growth was continued for an additional 2 h prior to fluorescence and optical density measurements made with a Tecan Safire2 microplate reader. An expanded description of the experimental procedures is provided in the supplementary material. A.U., arbitrary units.
To test whether these compounds were inducers of the marRAB operon, we used a chromosomal, single-copy transcriptional fusion of the marRAB promoter to the fast-folding yellow fluorescent protein (YFP) variant Venus to monitor gene expression in the presence of the four potential inducers (20). For comparison, we also explored the abilities of these chemicals to activate expression in a tolC null mutant. Of the four compounds, we found that only DHB and ANT activate the marRAB promoters in wild-type cells (Fig. 1B). We also found that HBA could activate the marRAB promoter in a tolC null mutant. Interestingly, PABA failed to activate the marRAB promoter despite its chemical similarity to salicylate.
MarR binds 2,3-dihydroxybenzoate.
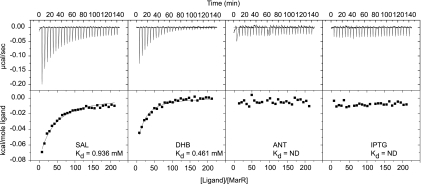
We next tested whether MarR binds directly to DHB, ANT, and HBA. To measure binding, we employed isothermal titration calorimetry (ITC) using purified MarR (Fig. 2). Consistent with previous measurements, we found that the affinity of MarR for salicylate was at a Kd (dissociation constant) of 0.9 mM, well within the ranges reported using other methods (1, 19). Binding was not observed with isopropyl-β-d-thiogalactopyranoside (IPTG), which served as our negative control. We also found that MarR bound DHB with a Kd of 0.5 mM. However, we found that ANT did not bind to MarR even though it was capable of activating the marRAB promoter. Similarly, we found that HBA, which activates the marRAB promoter only in the absence of tolC, also did not bind to MarR (data not shown). This suggests that these two compounds may indirectly regulate marRAB promoter activity.
FIG. 2.
MarR binding of aromatic acid inducers as determined by isothermal titration calorimetry (ITC). Experiments were conducted using a VP-ITC calorimeter (MicroCal) with 1.4 ml MarR at 10 μM and ligands at a concentration of 10 mM, both in Tris-buffered saline (50 mM Tris, 150 mM NaCl, pH 7.4). In the cases of ANT and HBA, higher concentrations of ligand were also tested though again no binding was observed (data not shown). Titration reactions were performed with 28 injections, all 10 μl in volume, with constant stirring at 300 rpm at 25°C. Data acquisition and binding coefficients were determined with the Origin-based MicroCal analysis software.
We note that despite the fact that MarR had similar affinities for salicylate and DHB, salicylate has a greater effect than DHB on marRAB activation in vivo. To explain these results, we imagine that DHB is likely being metabolized by the cell. Consistent with this hypothesis, we observed increased levels of DHB-dependent marRAB promoter activation in a tolC mutant background (Fig. 1B), suggesting that loss of the excretory function of TolC may lead to a buildup of DHB, most likely by preventing the efflux of the downstream metabolite, enterobactin (5).
MarR activity is modulated in vitro by 2,3-dihydroxybenzoate.
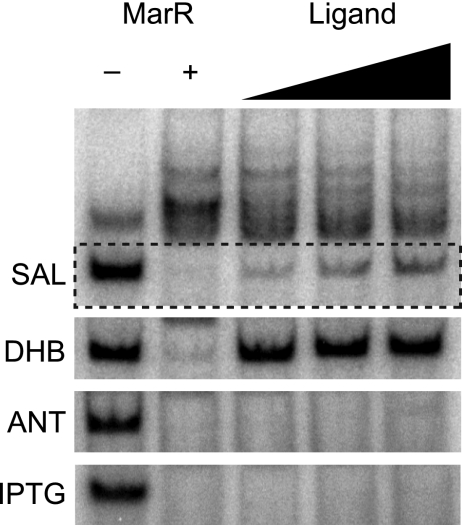
As MarR binds to DHB, we next tested whether DHB affects MarR binding to the marRAB promoter region. To determine the loss of MarR binding activity in the presence of DHB, we employed electrophoretic mobility shift assays using purified MarR and a 150-bp region of the marRAB promoter (see the supplemental material for details). Using salicylate as our positive control and IPTG as our negative control, we found that only DHB directly affected MarR activity (Fig. 3). Interestingly, we observed significantly more unbound DNA in the presence of DHB than salicylate, even though MarR has similar binding affinities for the two. We also tested whether ANT affected MarR activity and found that it did not, consistent with our ITC experiments.
FIG. 3.
MarR DNA binding activity in the presence of aromatic acid metabolites as determined by electromobility shift assays. Binding reaction mixtures consisted of 20 ng of purified MarR and 5 ng of a radiolabeled, 150-bp fragment of the marRAB promoter containing two MarR operator sites. The ligands salicylate (SAL), 2,3-dihydroxybenzoate (DHB), anthranilate (ANT), and IPTG were supplied at increasing concentrations of 2.5 mM, 5 mM, and 10 mM to the binding reaction mixtures. Reactions were displayed on 5% acrylamide, 0.5× Tris-borate-EDTA (TBE)-buffered gels. Loss of MarR DNA binding activity was monitored by the emergence of free DNA in the presence of these ligands. Salicylate and IPTG served as positive and negative controls, respectively. A detailed description of the methods is provided in the supplemental material.
Disruptions in enterobactin and tryptophan biosynthesis affect marRAB promoter activity in tolC mutants.
Our previous results have demonstrated that when added exogenously, DHB and ANT activate the marRAB promoter in vivo. To better correlate the metabolite effector hypothesis of Rosner and Martin to these observations, we disrupted the enzymatic steps in enterobactin and tryptophan metabolism that block either the synthesis or utilization of DHB or ANT. Specifically, mutants lacking the enterobactin synthesis pathway (ΔentCEBAH) or deficient in 2,3-dihydro-2,3-dihydroxybenzoate (DDHB) dehydrogenase (entA), enterobactin synthase (entF), anthranilate synthase (trpE), and phoshoribosyl transferase (the C-terminal region of trpD) activities were constructed in otherwise wild-type and tolC mutant strains and tested for alterations in marRAB promoter activity.
In TolC+ backgrounds, we observed no significant changes in marRAB promoter activity in any of the mutants, presumably due to the ability of these cells to readily excrete accumulated intermediates (data not shown). However, when we repeated these experiments in the absence of TolC, we found that the marRAB promoter was less active in mutants unable to synthesize enterobactin from chorismate (1,559 ± 170 relative fluorescence units per optical density unit [RFU/OD] [ΔtolC mutant] versus 1,387 ± 195 RFU/OD [ΔtolC ΔentCEBAH mutant], P = 0.001). However, when we attempted to accumulate the DHB in the cell by blocking conversion of DHB to enterobactin, we observed a decrease in marRAB promoter activity as opposed to an expected increase (1,559 ± 170 RFU/OD [ΔtolC mutant] versus 1,491 ± 78 RFU/OD [ΔtolC ΔentF mutant], P = 0.04). While statistically significant, the effect is minor and likely not physiologically significant. We suspect that this mutant (ΔtolC ΔentF) does not accumulate significant amounts of DHB. Interestingly, we observed a significant increase in marRAB promoter activity when we blocked the conversion of DDHB to DHB (1,559 ± 170 RFU/OD [ΔtolC mutant] versus 1,737 ± 133 RFU/OD [ΔtolC ΔentA mutant], P = 0.0001). Given the similar chemical structures of DDHB and DHB, differing only by a hydrated 2,3 carbon-carbon bond on the benzyl ring, we suspect that DDHB may accumulate in this mutant and serve as an alternate activator of MarR. Collectively, these results suggest that enterobactin intermediates are physiological activators of the marRAB operon.
We also investigated the effects of tryptophan biosynthesis on marRAB expression in the absence of TolC. We found that mutants unable to convert ANT to tryptophan did not exhibit any significant changes in marRAB promoter activity (data not shown). Interestingly, in the absence of anthranilate synthase, and therefore the ability to synthesize ANT, we observed a statistically significant increase in the marRAB promoter activity (1,516 ± 68 RFU/OD [ΔtolC mutant] versus 1,702 ± 173 RFU/OD [ΔtolC ΔtrpE mutant], P < 0.00002). We suspect that blocking the initial step of tryptophan biosynthesis may redirect metabolic fluxes to other pathways where the metabolite intermediates induce marRAB expression. Taken together, these results indicate that disruption of tryptophan biosynthesis affects marRAB expression, although they suggest that tryptophan intermediates do not contribute directly to the tolC phenotype. Moreover, the effect is not direct nor is the mechanism clear. We were not entirely surprised by this result, as we found that ANT does not directly bind MarR and affect its activity. Rather, the effect appears to be indirect.
Conclusions.
A number of studies have shown that some metabolic intermediates are inducers and substrates for various efflux pumps (6, 13, 15-16, 23). Likely, these mechanisms prevent the buildup of toxic metabolic intermediates. In support of this model, our results demonstrate that MarR directly binds one such intermediate, 2,3-dihydroxybenzoate, that is involved in the biosynthesis of enterobactin, itself a substrate for TolC (5). Whether DHB is a MarR effector under physiological conditions, however, is still unknown.
While these results suggest that enterobactin biosynthesis contributes to the increase in marRAB expression in the absence of TolC, they do not explain the phenotype completely. Due to the magnitude of the changes in marRAB expression caused by mutations in the enterobactin pathway, we suspect that the true source of increased activation is likely a combination of many intracellular metabolites, as proposed by Rosner and Martin (21).
Supplementary Material
Acknowledgments
We thank G. D. Glekas, H. E. Rao, and G. W. Ordal for providing technical assistance in protein purification and M. M. Wood for helpful insights in performing EMSA experiments. Also, we thank S. Saini and J. E. Cott-Chubiz for helpful discussions.
This work was supported in part by grants from the National Science Foundation and the National Institutes of Health.
Footnotes
Published ahead of print on 16 July 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1999. Alteration of the repressor activity of MarR, the negative regulator of the Escherichia coli marRAB locus, by multiple chemicals in vitro. J. Bacteriol. 181:4669-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekshun, M. N., and S. B. Levy. 1997. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob. Agents Chemother. 41:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat. Struct. Biol. 8:710-714. [DOI] [PubMed] [Google Scholar]
- 4.Ariza, R. R., S. P. Cohen, N. Bachhawat, S. B. Levy, and B. Demple. 1994. Repressor mutations in the marRAB operon that activate oxidative stress genes and multiple antibiotic resistance in Escherichia coli. J. Bacteriol. 176:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleuel, C., C. Grosse, N. Taudte, J. Scherer, D. Wesenberg, G. J. Krauss, D. H. Nies, and G. Grass. 2005. TolC is involved in enterobactin efflux across the outer membrane of Escherichia coli. J. Bacteriol. 187:6701-6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carole, S., S. Pichoff, and J. P. Bouch. 1999. Escherichia coli gene ydeA encodes a major facilitator pump which exports l-arabinose and isopropyl-beta-d-thiogalactopyranoside. J. Bacteriol. 181:5123-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chollet, R., C. Bollet, J. Chevalier, M. Mallea, J. M. Pages, and A. Davin-Regli. 2002. mar operon involved in multidrug resistance of Enterobacter aerogenes. Antimicrob. Agents Chemother. 46:1093-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, S. P., H. Hachler, and S. B. Levy. 1993. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J. Bacteriol. 175:1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, S. P., S. B. Levy, J. Foulds, and J. L. Rosner. 1993. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J. Bacteriol. 175:7856-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, S. P., W. Yan, and S. B. Levy. 1993. A multidrug resistance regulatory chromosomal locus is widespread among enteric bacteria. J. Infect. Dis. 168:484-488. [DOI] [PubMed] [Google Scholar]
- 11.Domain, F., X. R. Bina, and S. B. Levy. 2007. Transketolase A, an enzyme in central metabolism, derepresses the marRAB multiple antibiotic resistance operon of Escherichia coli by interaction with MarR. Mol. Microbiol. 66:383-394. [DOI] [PubMed] [Google Scholar]
- 12.Domain, F., and S. B. Levy. 2010. GyrA interacts with MarR to reduce repression of the marRAB operon in Escherichia coli. J. Bacteriol. 192:942-948. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Helling, R. B., B. K. Janes, H. Kimball, T. Tran, M. Bundesmann, P. Check, D. Phelan, and C. Miller. 2002. Toxic waste disposal in Escherichia coli. J. Bacteriol. 184:3699-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keseler, I. M., C. Bonavides-Martinez, J. Collado-Vides, S. Gama-Castro, R. P. Gunsalus, D. A. Johnson, M. Krummenacker, L. M. Nolan, S. Paley, I. T. Paulsen, M. Peralta-Gil, A. Santos-Zavaleta, A. G. Shearer, and P. D. Karp. 2009. EcoCyc: a comprehensive view of Escherichia coli biology. Nucleic Acids Res. 37:D464-D70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, J. Y., P. F. Miller, M. Gosink, and E. R. Olson. 1999. The identification of a new family of sugar efflux pumps in Escherichia coli. Mol. Microbiol. 31:1845-1851. [DOI] [PubMed] [Google Scholar]
- 16.Liu, J. Y., P. F. Miller, J. Willard, and E. R. Olson. 1999. Functional and biochemical characterization of Escherichia coli sugar efflux transporters. J. Biol. Chem. 274:22977-22984. [DOI] [PubMed] [Google Scholar]
- 17.Maneewannakul, K., and S. B. Levy. 1996. Identification of mar mutants among quinolone-resistant clinical isolates of Escherichia coli. Antimicrob. Agents Chemother. 40:1695-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin, R. G., P. S. Nyantakyi, and J. L. Rosner. 1995. Regulation of the multiple antibiotic resistance (mar) regulon by marORA sequences in Escherichia coli. J. Bacteriol. 177:4176-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin, R. G., and J. L. Rosner. 1995. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc. Natl. Acad. Sci. U. S. A. 92:5456-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai, T., K. Ibata, E. S. Park, M. Kubota, K. Mikoshiba, and A. Miyawaki. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20:87-90. [DOI] [PubMed] [Google Scholar]
- 21.Rosner, J. L., and R. G. Martin. 2009. An excretory function for the Escherichia coli outer membrane pore TolC: upregulation of marA and soxS transcription and Rob activity due to metabolites accumulated in tolC mutants. J. Bacteriol. 191:5283-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seoane, A. S., and S. B. Levy. 1995. Characterization of MarR, the repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli. J. Bacteriol. 177:3414-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dyk, T. K., L. J. Templeton, K. A. Cantera, P. L. Sharpe, and F. S. Sariaslani. 2004. Characterization of the Escherichia coli AaeAB efflux pump: a metabolic relief valve? J. Bacteriol. 186:7196-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson, S. P., and A. Grove. 2006. Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Curr. Issues Mol. Biol. 8:51-62. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.