Abstract
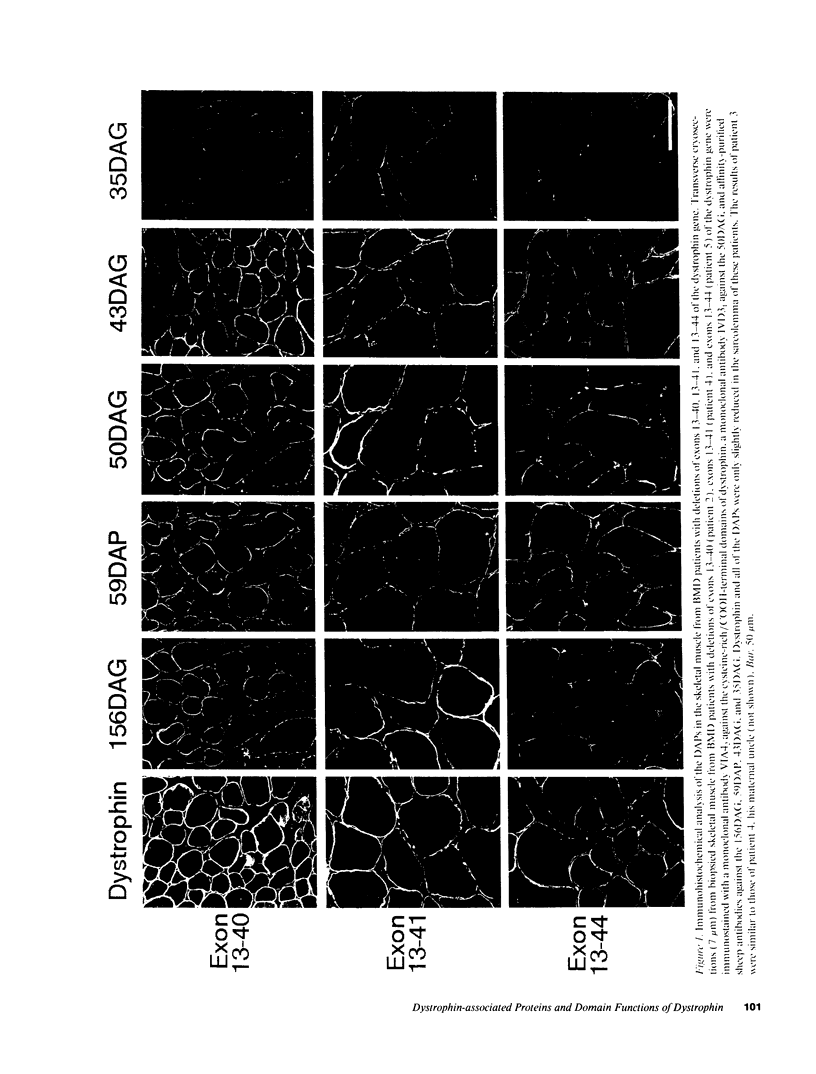
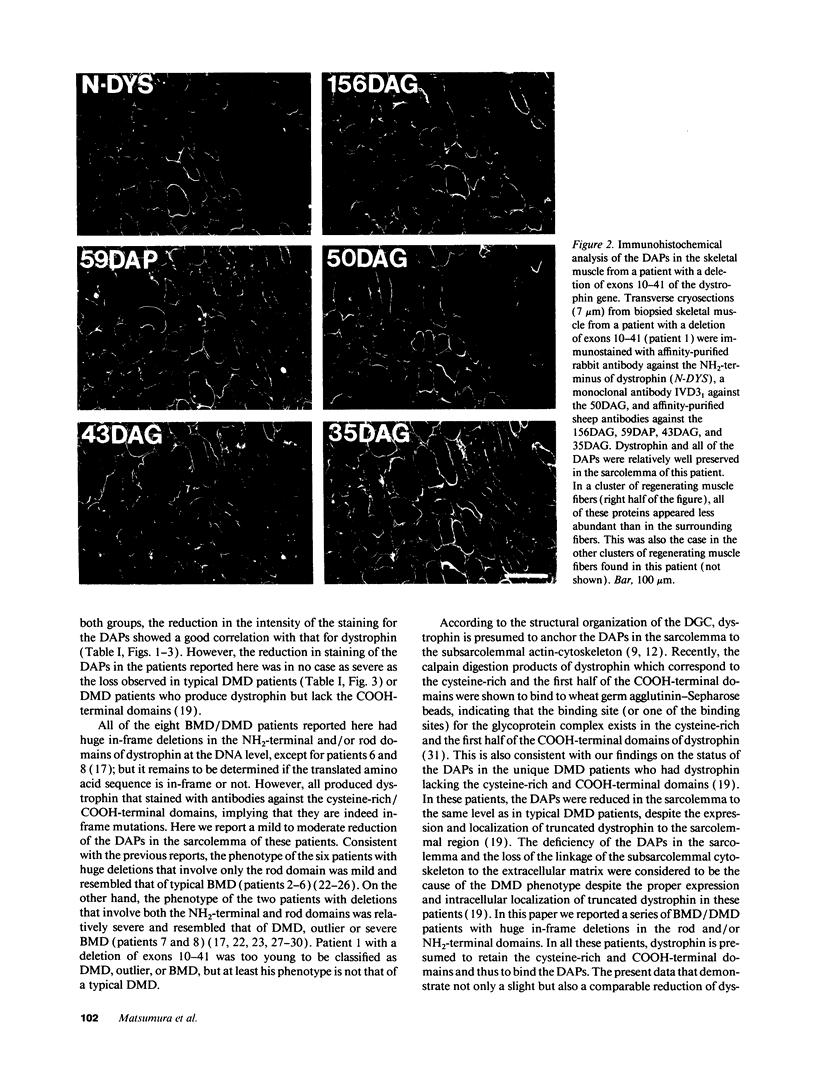
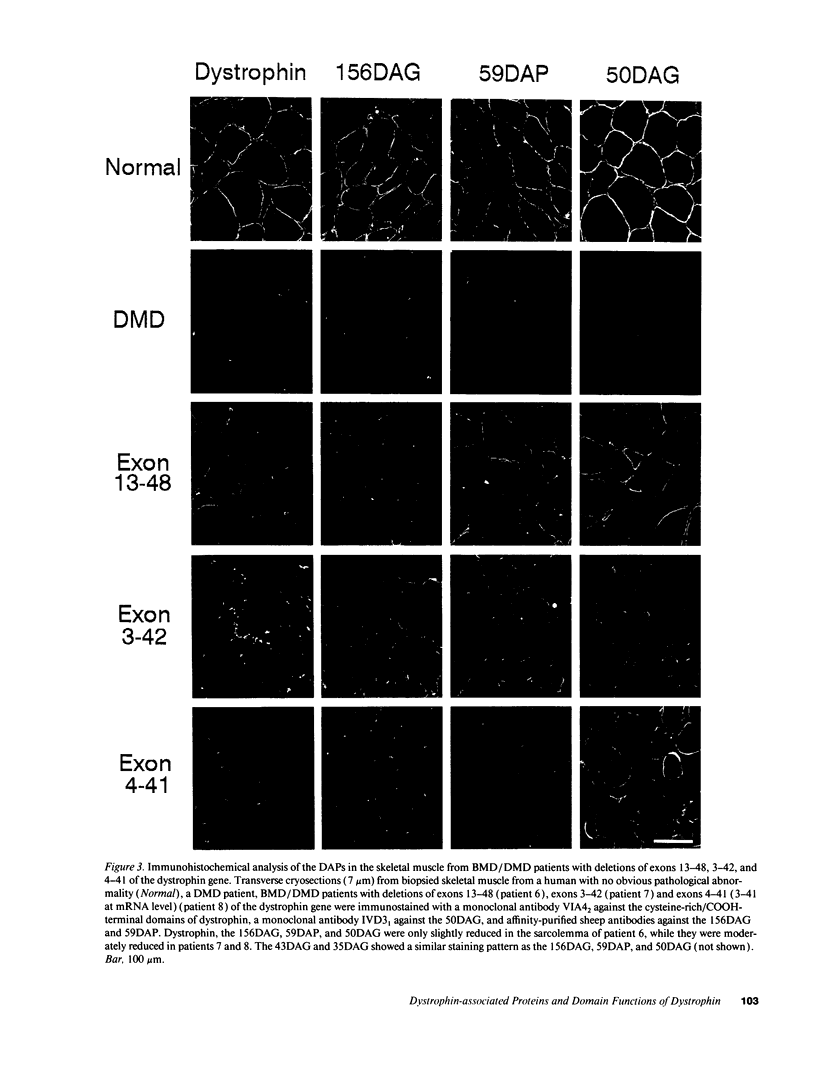
The absence of dystrophin causes the drastic reduction of the dystrophin-associated proteins (DAPs) in the sarcolemma and the loss of the linkage between the subsarcolemmal cytoskeleton and the extracellular matrix in Duchenne muscular dystrophy (DMD) skeletal muscle. Here, we report a mild reduction of the DAPs in the unique Becker muscular dystrophy patients with huge deletions in the rod domain of dystrophin and a moderate reduction of the DAPs in patients with huge deletions that involve both the NH2-terminal and rod domains of dystrophin. The phenotype of the latter patients was more severe than that of the former. In both cases, however, the reduction in the DAPs was milder than in typical DMD patients or DMD patients lacking the COOH-terminal domains of dystrophin. Our results suggest that (a) the NH2-terminal and rod domains of dystrophin may not be essential for the interaction with the sarcolemmal glycoprotein complex; and (b) defects in the actin binding activity of dystrophin may cause disruption of the anchorage of the dystrophin-glycoprotein complex to the subsarcolemmal cytoskeleton, which may render muscle fibers susceptible to degeneration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs A. H., Hoffman E. P., Snyder J. R., Arahata K., Specht L., Shapiro F., Angelini C., Sugita H., Kunkel L. M. Exploring the molecular basis for variability among patients with Becker muscular dystrophy: dystrophin gene and protein studies. Am J Hum Genet. 1991 Jul;49(1):54–67. [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989 Mar 16;338(6212):259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., Gibbs R. A., Ranier J. E., Nguyen P. N., Caskey C. T. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988 Dec 9;16(23):11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S. B., Nicholson L. V., Johnson M. A., Forrest S. M., Love D. R., Zubrzycka-Gaarn E. E., Bulman D. E., Harris J. B., Davies K. E. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990 Jan 11;343(6254):180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Kahl S. D., Campbell K. P. Purification of dystrophin from skeletal muscle. J Biol Chem. 1991 May 15;266(14):9161–9165. [PubMed] [Google Scholar]
- Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990 May 24;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay S. B., Sherratt T. G., Heckmatt J. Z., Dubowitz V., Miller G., Shokeir M., Ray P. N., Strong P. N., Worton R. G. Dystrophin in frameshift deletion patients with Becker muscular dystrophy. Am J Hum Genet. 1992 Sep;51(3):562–570. [PMC free article] [PubMed] [Google Scholar]
- Hemmings L., Kuhlman P. A., Critchley D. R. Analysis of the actin-binding domain of alpha-actinin by mutagenesis and demonstration that dystrophin contains a functionally homologous domain. J Cell Biol. 1992 Mar;116(6):1369–1380. doi: 10.1083/jcb.116.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Ervasti J. M., Leveille C. J., Slaughter C. A., Sernett S. W., Campbell K. P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992 Feb 20;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Ikeya K., Saito K., Hayashi K., Tanaka H., Hagiwara Y., Yoshida M., Yamauchi A., Fukuyama Y., Ishiguro T., Eguchi C. Molecular genetic and immunological analysis of dystrophin of a young patient with X-linked muscular dystrophy. Am J Med Genet. 1992 Jun 1;43(3):580–587. doi: 10.1002/ajmg.1320430315. [DOI] [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Levine B. A., Moir A. J., Patchell V. B., Perry S. V. Binding sites involved in the interaction of actin with the N-terminal region of dystrophin. FEBS Lett. 1992 Feb 17;298(1):44–48. doi: 10.1016/0014-5793(92)80019-d. [DOI] [PubMed] [Google Scholar]
- Love D. R., Flint T. J., Genet S. A., Middleton-Price H. R., Davies K. E. Becker muscular dystrophy patient with a large intragenic dystrophin deletion: implications for functional minigenes and gene therapy. J Med Genet. 1991 Dec;28(12):860–864. doi: 10.1136/jmg.28.12.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S. B., Hart K. A., Klamut H. J., Thomas N. S., Bodrug S. E., Burghes A. H., Bobrow M., Harper P. S., Thompson M. W., Ray P. N. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science. 1988 Nov 4;242(4879):755–759. doi: 10.1126/science.3055295. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Campbell K. P. Deficiency of dystrophin-associated proteins: a common mechanism leading to muscle cell necrosis in severe childhood muscular dystrophies. Neuromuscul Disord. 1993 Mar;3(2):109–118. doi: 10.1016/0960-8966(93)90002-2. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Ervasti J. M., Ohlendieck K., Kahl S. D., Campbell K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992 Dec 10;360(6404):588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Imoto N. [Two long-living brothers of dystrophin-related muscular dystrophy with an in-frame deletion of exon 3 of the dystrophin gene--clinical features and diagnosis]. Rinsho Shinkeigaku. 1991 Mar;31(3):286–290. [PubMed] [Google Scholar]
- Matsumura K., Tomé F. M., Collin H., Azibi K., Chaouch M., Kaplan J. C., Fardeau M., Campbell K. P. Deficiency of the 50K dystrophin-associated glycoprotein in severe childhood autosomal recessive muscular dystrophy. Nature. 1992 Sep 24;359(6393):320–322. doi: 10.1038/359320a0. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Tomé F. M., Ionasescu V., Ervasti J. M., Anderson R. D., Romero N. B., Simon D., Récan D., Kaplan J. C., Fardeau M. Deficiency of dystrophin-associated proteins in Duchenne muscular dystrophy patients lacking COOH-terminal domains of dystrophin. J Clin Invest. 1993 Aug;92(2):866–871. doi: 10.1172/JCI116661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi L., Mora M., Bernasconi P., Mantegazza R., Gebbia M., Balestrini M. R., Cornelio F. Very small dystrophin molecule in a family with a mild form of Becker dystrophy. Neuromuscul Disord. 1993 Jan;3(1):65–70. doi: 10.1016/0960-8966(93)90043-j. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Campbell K. P. Dystrophin constitutes 5% of membrane cytoskeleton in skeletal muscle. FEBS Lett. 1991 Jun 3;283(2):230–234. doi: 10.1016/0014-5793(91)80595-t. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Campbell K. P. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J Cell Biol. 1991 Dec;115(6):1685–1694. doi: 10.1083/jcb.115.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Matsumura K., Kahl S. D., Leveille C. J., Campbell K. P. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 1991 Sep;7(3):499–508. doi: 10.1016/0896-6273(91)90301-f. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Snook J. B., Campbell K. P. Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J Cell Biol. 1991 Jan;112(1):135–148. doi: 10.1083/jcb.112.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Matsumura K., Ionasescu V. V., Towbin J. A., Bosch E. P., Weinstein S. L., Sernett S. W., Campbell K. P. Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology. 1993 Apr;43(4):795–800. doi: 10.1212/wnl.43.4.795. [DOI] [PubMed] [Google Scholar]
- Ragot T., Vincent N., Chafey P., Vigne E., Gilgenkrantz H., Couton D., Cartaud J., Briand P., Kaplan J. C., Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993 Feb 18;361(6413):647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Yoshida M., Yamamoto H., Ozawa E. Glycoprotein-binding site of dystrophin is confined to the cysteine-rich domain and the first half of the carboxy-terminal domain. FEBS Lett. 1992 Aug 17;308(2):154–160. doi: 10.1016/0014-5793(92)81265-n. [DOI] [PubMed] [Google Scholar]
- Vainzof M., Takata R. I., Passos-Bueno M. R., Pavanello R. C., Zatz M. Is the maintainance of the C-terminus domain of dystrophin enough to ensure a milder Becker muscular dystrophy phenotype? Hum Mol Genet. 1993 Jan;2(1):39–42. doi: 10.1093/hmg/2.1.39. [DOI] [PubMed] [Google Scholar]
- Way M., Pope B., Cross R. A., Kendrick-Jones J., Weeds A. G. Expression of the N-terminal domain of dystrophin in E. coli and demonstration of binding to F-actin. FEBS Lett. 1992 Apr 27;301(3):243–245. doi: 10.1016/0014-5793(92)80249-g. [DOI] [PubMed] [Google Scholar]
- Winnard A. V., Klein C. J., Coovert D. D., Prior T., Papp A., Snyder P., Bulman D. E., Ray P. N., McAndrew P., King W. Characterization of translational frame exception patients in Duchenne/Becker muscular dystrophy. Hum Mol Genet. 1993 Jun;2(6):737–744. doi: 10.1093/hmg/2.6.737. [DOI] [PubMed] [Google Scholar]