Abstract
The bacterial nucleoid-associated protein H-NS, which preferentially targets and silences A+T-rich genes, binds the ubiquitous reporter gene gfp and dramatically reduces local transcription. We have redesigned gfp to reduce H-NS-mediated transcription silencing and simultaneously improve translation in vivo without altering the amino acid sequence of the GFP protein.
The green fluorescent protein (GFP) from the jellyfish Aequorea victoria is widely used as a reporter of transcriptional activity and protein localization in both eukaryotes and prokaryotes (reviewed in reference 16). The DNA encoding gfp and many of its variants has a high A+T content (∼60%) and strong predicted DNA curvature, both of which are key features of DNA that is targeted by the histone-like nucleoid structuring protein (H-NS) (10, 12, 14, 15, 22). H-NS is a global gene silencer that represses transcription by condensing chromosome and plasmid DNA in many Gram-negative bacteria. For example, H-NS binds and represses ∼15% of the Escherichia coli and Salmonella enterica genomes (13), including A+T-rich genes that have been acquired horizontally (5). This raised the possiblity that H-NS may bind and repress the alien gfp gene and thus obscure measurements of promoter activity.
Many variants of GFP have been developed to optimize this versatile reporter for different applications. The alteration of these major characteristics of the protein have typically been achieved by making a very small number of amino acid substitutions involving only minor alterations to the DNA sequence of gfp. For example, the alteration of just one amino acid, arising from a single nucleotide substitution, was required to convert GFP to blue fluorescent protein (BFP) (8). Thus, many of the commonly used variants of GFP are encoded by genes that are ideal targets for H-NS and H-NS-like proteins.
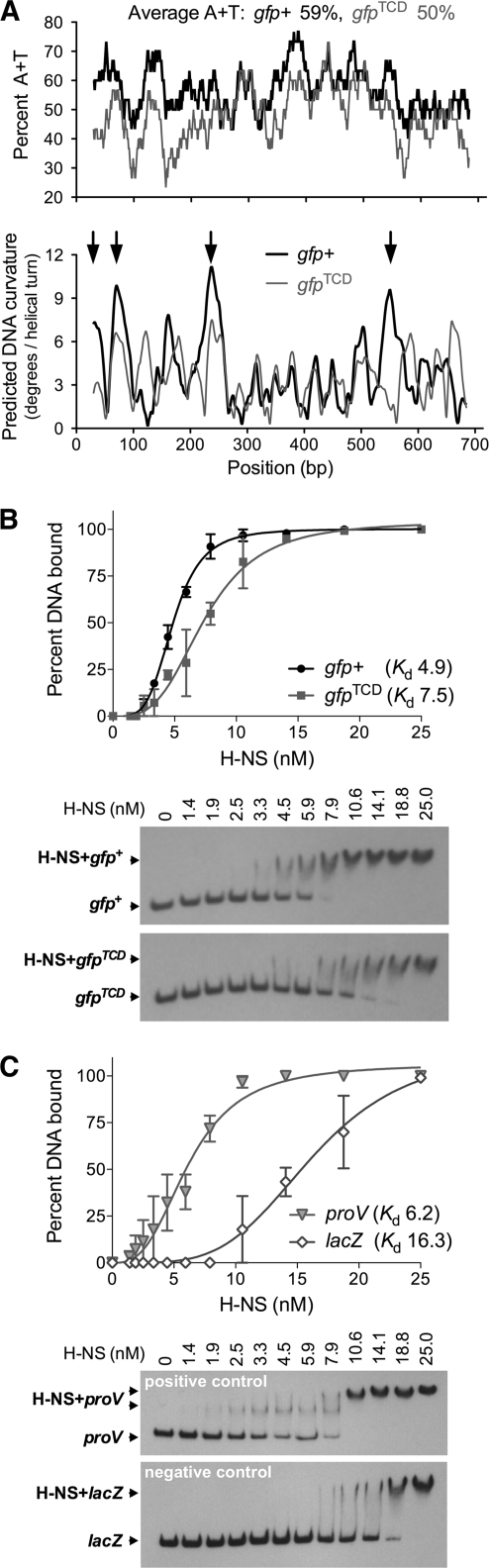
The widely used and highly fluorescent gfp+ (17) has an A+T content of 59% and was predicted by the Bend.it algorithm (21) to contain several regions of curved DNA (Fig. 1 A), making it a likely target for H-NS binding. The gfp+ gene was therefore redesigned to have an A+T content that was similar to that of the E. coli chromosome (49%). Gene Designer (20) was used both to reduce the A+T content of gfp+ and to optimize its codon usage for expression in E. coli. This approach produced iterative, equally optimized sequences that were screened for reduced intrinsic DNA curvature using Bend.it. The preferred sequence differed from gfp+ at 157 positions of the 717-base-pair gene, had 50% A+T content, and showed dramatically reduced predicted DNA curvature (Fig. 1A). This reengineered gene is referred to as gfpTCD (TCD for Trinity College Dublin), and its DNA sequence is provided in Fig. S1 in the supplemental material.
FIG. 1.
DNA sequence features of the gfp+ gene are conducive to H-NS binding. (A) A+T content (upper plot) and predicted intrinsic DNA curvature (lower plot) of gfp+ and gfpTCD. Arrows highlight regions of strong predicted curvature in gfp+ that are reduced in gfpTCD. (B and C) EMSA analysis of H-NS binding to gfp+ and gfpTCD (B) and proU and lacZ (C). Binding curves and Kd values are shown in the upper plots; mean values and ranges are plotted. Typical EMSA gels from which binding curves were derived are shown below. See supplemental material for a complete description of the experimental methods.
Electrophoretic mobility shift assays (EMSA) were performed to determine the affinity of H-NS for gfp+ and gfpTCD in vitro. H-NS bound to gfp+ with an apparent dissociation constant (Kd) of 4.9 ± 0.1 nM (Fig. 1B). A narrow protein concentration range (4.5 to 10.55 nM) was required for the transition from initial binding to the fully bound DNA probe that resolved as a single low-mobility complex. In contrast, H-NS displayed a relatively lower affinity for gfpTCD (Kd, 7.5 ± 0.5 nM) (Fig. 1B), resulting in the formation of a poorly resolved H-NS-DNA complex over a wider range of protein concentrations (7.9 to 18.75 nM); the gfpTCD probe resolved as a single H-NS-DNA complex only at protein concentrations above 25 nM.
The E. coli proU transcriptional regulatory region was used as a positive control for H-NS binding because it contains several well-characterized H-NS binding sites (1). As expected, the proU DNA probe was bound by H-NS (Kd, 6.2 ± 0.5 nM) in EMSA where it resolved as two separate H-NS-DNA complexes (Fig. 1C). H-NS has a relatively lower binding affinity for the E. coli lacZ gene (15), so this DNA was used as a negative control; EMSA analysis confirmed that lacZ was indeed more weakly bound by H-NS (Kd, 16.3 ± 1.8 nM) than was proU (Fig. 1C).
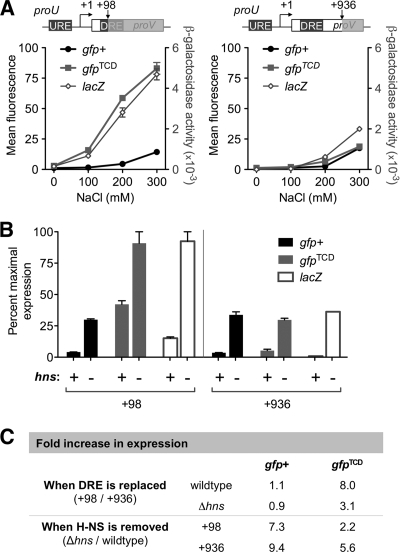
The proU operon was also used to test the effect of H-NS binding to gfp+ on transcription from a nearby promoter. Transcriptional repression of the proU operon in low-osmolarity conditions involves binding by H-NS to the A+T-rich, highly curved DNA located in the upstream and downstream regulatory elements (URE and DRE, respectively) that flank the proU promoter (1, 11, 15). The repressive complex formed by H-NS at proU is disrupted by increasing the osmolarity of the medium (7). While H-NS binding in the DRE is essential for osmoregulation of proU, it has previously been shown that the native DRE can be functionally replaced by an unrelated piece of DNA that is bound by H-NS (15). It was also shown that the lacZ gene cannot functionally replace the DRE and thus its presence in place of the DRE leads to derepression of proU in low-osmolarity conditions (100 mM NaCl) (15). Therefore, we tested the ability of gfp+, gfpTCD, or lacZ to functionally replace the DRE by insertion of each gene within the DRE at +98 bp downstream from the transcriptional start site (Fig. 2 A). Expression of the proU-gfp+(+98) fusion was repressed relative to expression of proU-gfpTCD(+98), indicating that gfp+, but not gfpTCD, functionally replaced the DRE as a binding site for H-NS and thus repressed proU expression. The proU-lacZ(+98) fusion was not repressed at low NaCl concentrations, consistent with earlier observations (15). Reporter fusions that did not disrupt the DRE were generated by insertion of each of the three reporter genes (gfp+, gfpTCD, and lacZ) at +936 bp downstream from the transcriptional start site (Fig. 2A). As predicted, expression of all three proU(+936) fusions was repressed by H-NS binding to the DRE at NaCl concentrations below 200 mM.
FIG. 2.
gfp+ and gfpTCD have different effects on transcription and translation. (A) The effect of increasing NaCl concentrations on the expression of reporter gene fusions inserted at +98 and +936 bp from the proU transcriptional start site. Mean values and ranges are plotted. Diagrams not to scale. (B) The effect of removing H-NS on fusion gene expression in 100 mM NaCl. Mean values and ranges are plotted. (C) Fold increases in fusion gene expression caused by changes in H-NS occupancy. See supplemental material for a complete description of the experimental methods.
To confirm that H-NS binding to gfp+ accounted for repression of proU-gfp+(+98) in vivo, the expression of all proU fusions was tested in a Δhns background. Cells were cultured in the repressive conditions of 100 mM NaCl, and the data were expressed as a percentage of maximal derepressed expression to facilitate comparisons between GFP fluorescence and β-galactosidase activity (Fig. 2B). For all three fusions, proU expression was elevated in the absence of H-NS. This revealed that even in the absence of the DRE, H-NS continued to bind the URE and repress the proU-gfpTCD(+98) and proU-lacZ(+98) fusions. These data allowed an assessment of the relative effects of replacing only the DRE compared to those of eliminating H-NS protein from the cell (Fig. 2C). Replacing the DRE with gfpTCD resulted in an 8-fold increase in expression relative to expression from the proU-gfpTCD(+936) fusion; a similar comparison between proU-gfp+ at positions +98 and +936 showed that gfp+ inserted in the DRE maintained full repression. Consequently, eliminating H-NS genetically resulted in a 7.3- to 9.4-fold increase in expression from the gfp+ fusions while the expression of proU-gfpTCD(+98) improved only 2.2-fold upon removal of H-NS (Fig. 2C). This also confirmed that H-NS-mediated repression of proU occurs primarily through binding to the DRE (Fig. 2C).
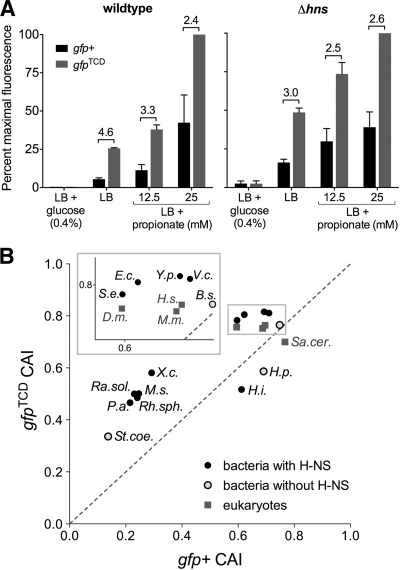
Even in the absence of H-NS, proU-gfpTCD(+98) produced 3-fold-higher levels of GFP than proU-gfp+(+98) (Fig. 2C). The gfpTCD gene is codon optimized for translation in E. coli, and these data suggest that gfpTCD is translated 3-fold more efficiently than gfp+. Improved translation efficiency was confirmed by cloning gfp+ and gfpTCD in the pPro vector under the control of the propionate-inducible prpBCDE promoter (9), for which there is no evidence of H-NS binding or repression (3). In pPro, gfpTCD produced on average 3.5-fold more GFP in a wild-type background and 2.7-fold more GFP in a Δhns background than gfp+ (Fig. 3 A). The codon adaptation index (CAI) is a standard means to calculate the effects of species-specific codon biases on translation (18); Fig. 3B shows that gfpTCD is predicted to have improved translation efficiency in both bacterial and eukaryotic model organisms. gfpTCD therefore provides an ideal template for the directed evolution of novel gfp variants.
FIG. 3.
gfpTCD has improved translation efficiency. (A) Expression of gfp+ and gfpTCD from the prpBCDE promoter on the plasmid pPro in wild-type and Δhns cells. The prpBCDE promoter is repressed by glucose and induced by propionate. Mean values and ranges are plotted; fold differences between gfp+ and gfpTCD fluorescence levels are shown above the bars. (B) Codon adaptation index (CAI) values for gfpTCD and gfp+ in various organisms. Organisms listed above the dashed line will translate gfpTCD more efficiently than gfp+ (and wild-type gfp). Organism classification and abbreviations are as follows. Alphaproteobacteria: Rh.sph., Rhodobacter sphaeroides. Betaproteobacteria: Ra.sol., Ralstonia solanacearum. Epsilonproteobacteria: H.p., Helicobacter pylori. Gammaproteobacteria: E.c., Escherichia coli; H.i., Haemophilus influenzae; P.a., Pseudomonas aeruginosa; S.e., Salmonella enterica; V.c., Vibrio cholerae; X.c., Xanthomonas campestris; Y.p., Yersinia pestis. Actinobacteria: M.s., Mycobacterium smegmatis; St.coe., Streptomyces coelicolor. Firmicutes: B.s., Bacillus subtilis. Eukaryota: D.m., Drosophila melanogaster; H.s., Homo sapiens; M.m., Mus musculus; Sa.cer., Saccharomyces cerevisiae. See supplemental material for a complete description of the experimental methods.
In many bacteria, including E. coli, H-NS selectively targets horizontally acquired genes of high A+T content preventing transcription of the potentially deleterious foreign DNA (5). The introduction of A+T-rich plasmid DNA can thus titrate H-NS away from native chromosomal locations, leading to a mild Δhns phenotype with pleiotropic effects, including reduced virulence and motility (3, 6). We have shown that H-NS binds strongly in the gfp+ gene, perhaps explaining a recent report of reduced invasiveness of Salmonella due solely to the presence of a promoterless gfp+ gene on a multicopy plasmid (2). Over the years, many bacterial studies have relied on plasmid-based gfp fusions, and some of these studies may have experienced unrecognized pleiotropic effects due to H-NS titration. The gfp gene has been used in conjunction with plasmid-based high-throughput genetic screens in many landmark studies, including the identification of promoters involved in the intracellular survival of Salmonella enterica serovar Typhimurium (19) and the adaptation by E. coli to metabolisms of different carbon sources (23).
Since nucleoid-associated proteins with a preference for A+T-rich DNA are found in most cell types, including archaea and eukaryotes (4), our discovery of H-NS binding and repression of gfp may have far-reaching implications for the use of gfp in all domains of life. The development of gfpTCD, which is both a poor target for H-NS and a more accurate reporter of transcription that avoids H-NS-mediated downregulation of the promoter under investigation, provides a new tool for future studies free from artifacts caused by H-NS interference.
Supplementary Material
Acknowledgments
We thank Matthew McCusker and Shane Dillon for helpful suggestions and Jay Hinton for providing purified H-NS.
This work was supported by a grant from Science Foundation Ireland.
Footnotes
Published ahead of print on 16 July 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Bouffartigues, E., M. Buckle, C. Badaut, A. Travers, and S. Rimsky. 2007. H-NS cooperative binding to high-affinity sites in a regulatory element results in transcriptional silencing. Nat. Struct. Mol. Biol. 14:441-448. [DOI] [PubMed] [Google Scholar]
- 2.Clark, L., I. Martinez-Argudo, T. J. Humphrey, and M. A. Jepson. 2009. GFP plasmid-induced defects in Salmonella invasion depend on plasmid architecture, not protein expression. Microbiology 155:461-467. [DOI] [PubMed] [Google Scholar]
- 3.Dillon, S. C., A. D. S. Cameron, K. Hokamp, S. Lucchini, J. C. Hinton, and C. J. Dorman. 2010. Genome-wide analysis of the H-NS and Sfh regulatory networks in Salmonella Typhimurium identifies a plasmid-encoded transcription silencing mechanism. Mol. Microbiol. 76:1250-1265. [DOI] [PubMed] [Google Scholar]
- 4.Dillon, S. C., and C. J. Dorman. 2010. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 8:185-195. [DOI] [PubMed] [Google Scholar]
- 5.Dorman, C. J. 2007. H-NS, the genome sentinel. Nat. Rev. Microbiol. 5:157-161. [DOI] [PubMed] [Google Scholar]
- 6.Doyle, M., M. Fookes, A. Ivens, M. W. Mangan, J. Wain, and C. J. Dorman. 2007. An H-NS-like stealth protein aids horizontal DNA transmission in bacteria. Science 315:251-252. [DOI] [PubMed] [Google Scholar]
- 7.Gowrishankar, J. 1985. Identification of osmoresponsive genes in Escherichia coli: evidence for participation of potassium and proline transport systems in osmoregulation. J. Bacteriol. 164:434-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heim, R., D. C. Prasher, and R. Y. Tsien. 1994. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. U. S. A. 91:12501-12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, S. K., and J. D. Keasling. 2005. A propionate-inducible expression system for enteric bacteria. Appl. Environ. Microbiol. 71:6856-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucchini, S., G. Rowley, M. D. Goldberg, D. Hurd, M. Harrison, and J. C. Hinton. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucht, J. M., P. Dersch, B. Kempf, and E. Bremer. 1994. Interactions of the nucleoid-associated DNA-binding protein H-NS with the regulatory region of the osmotically controlled proU operon of Escherichia coli. J. Biol. Chem. 269:6578-6586. [PubMed] [Google Scholar]
- 12.Navarre, W. W., S. Porwollik, Y. Wang, M. McClelland, H. Rosen, S. J. Libby, and F. C. Fang. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313:236-238. [DOI] [PubMed] [Google Scholar]
- 13.Noom, M. C., W. W. Navarre, T. Oshima, G. J. Wuite, and R. T. Dame. 2007. H-NS promotes looped domain formation in the bacterial chromosome. Curr. Biol. 17:R913-914. [DOI] [PubMed] [Google Scholar]
- 14.Oshima, T., S. Ishikawa, K. Kurokawa, H. Aiba, and N. Ogasawara. 2006. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 13:141-553. [DOI] [PubMed] [Google Scholar]
- 15.Owen-Hughes, T. A., G. D. Pavitt, D. S. Santos, J. M. Sidebotham, C. S. Hulton, J. C. Hinton, and C. F. Higgins. 1992. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell 71:255-265. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo, M. A., M. W. Davidson, and D. W. Piston. 1 December 2009, posting date. Fluorescent protein tracking and detection: fluorescent protein structure and color variants. Cold Spring Harb. Protoc. doi: 10.1101/pdb.top63. [DOI] [PubMed]
- 17.Scholz, O., A. Thiel, W. Hillen, and M. Niederweis. 2000. Quantitative analysis of gene expression with an improved green fluorescent protein. Eur. J. Biochem. 267:1565-1570. [DOI] [PubMed] [Google Scholar]
- 18.Sharp, P. M., and W. H. Li. 1987. The codon adaptation index—a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15:1281-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 20.Villalobos, A., J. E. Ness, C. Gustafsson, J. Minshull, and S. Govindarajan. 2006. Gene Designer: a synthetic biology tool for constructing artificial DNA segments. BMC Bioinformatics 7:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlahovicek, K., L. Kajan, and S. Pongor. 2003. DNA analysis servers: plot.it, bend.it, model.it and IS. Nucleic Acids Res. 31:3686-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada, H., S. Muramatsu, and T. Mizuno. 1990. An Escherichia coli protein that preferentially binds to sharply curved DNA. J. Biochem. 108:420-425. [DOI] [PubMed] [Google Scholar]
- 23.Zaslaver, A., A. Bren, M. Ronen, S. Itzkovitz, I. Kikoin, S. Shavit, W. Liebermeister, M. G. Surette, and U. Alon. 2006. A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat. Methods 3:623-628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.