Abstract
Mice have been extensively employed as an animal model of renal damage caused by Shiga toxins. In this study, we examined the role of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) in the development of toxin-mediated renal disease in mice. Mice pretreated with TNF-α and challenged with Shiga toxin type 1 (Stx1) showed increased survival compared to that of mice treated with Stx1 alone. Conversely, mice treated with Stx1 before TNF-α administration succumbed more quickly than mice given Stx1 alone. Increased lethality in mice treated with Stx1 followed by TNF-α was associated with evidence of glomerular damage and the loss of renal function. No differences in renal histopathology were noted between animals treated with Stx1 alone and the TNF-α pretreatment group, although we noted a sparing of renal function when TNF-α was administered before toxin. Compared to that of treatment with Stx1 alone, treatment with TNF-α after toxin altered the renal cytokine profile so that the expression of proinflammatory cytokines TNF-α and interleukin-1β (IL-1β) increased, and the expression of the anti-inflammatory cytokine IL-10 decreased. Increased lethality in mice treated with Stx1 followed by TNF-α was associated with higher numbers of dUTP-biotin nick end labeling-positive renal tubule cells, suggesting that increased lethality involved enhanced apoptosis. These data suggest that the early administration of TNF-α is a candidate interventional strategy blocking disease progression, while TNF-α production after intoxication exacerbates disease.
Shiga toxins are a family of genetically and functionally related cytotoxic proteins expressed by the enteric pathogens Shigella dysenteriae serotype 1 and certain serotypes of Escherichia coli. Antigenic similarity to Shiga toxin expressed by S. dysenteriae serotype 1 is used to define Shiga toxin type 1 (Stx1) and type 2 (Stx2) expressed by Shiga toxin-producing E. coli (STEC) (44). Shiga toxins consist of a single A subunit in noncovalent association with a pentamer of B subunits. B subunits mediate binding to the neutral glycolipid receptor globotriaosylceramide (Gb3), while the A subunit possesses an N-glycosidase activity (38). Following toxin internalization and routing to the endoplasmic reticulum (ER), a fragment of the toxin A subunit generated by furin or a furin-like protease is translocated across the ER membrane and mediates the cleavage of a single adenine residue (A4256 in the rat) from the 28S rRNA component of ribosomes (39). Stx-induced depurination leads to the disruption of elongation factor-dependent aminoacyl-tRNA binding to nascent polypeptides (30). Thus, Shiga toxins are potent protein synthesis inhibitors, with 50% cytotoxic doses measured in pg/ml amounts for many cell types in vitro. Shiga toxins also activate the ribotoxic and ER stress pathways, which are important in the activation of proinflammatory cytokine/chemokine production and apoptosis (6, 22, 41).
The ingestion of small quantities of Stx-producing bacteria may lead to the development of bloody diarrhea with progression to acute renal failure, designated diarrhea-associated hemolytic uremic syndrome (D+HUS) (33). Epidemiologic studies have shown that the ingestion of STEC strains expressing Stx2 alone or Stx1 and Stx2 are more likely to progress to life-threatening extraintestinal complications (3, 17, 31). D+HUS is a leading cause of pediatric acute renal failure. D+HUS is characterized by rapid-onset oligouria or anuria, azotemia, microangiopathic hemolytic anemia with schistocytosis, and thrombocytopenia (33, 47). The histopathological examination of D+HUS renal tissues showed that glomerular microvascular endothelial cells were frequently swollen and detached from the basement membrane, and glomerular capillary lumina may be occluded with fibrin-rich microthrombi (21, 36).
Numerous animal models have been employed to characterize the role of Stx1 and Stx2 in pathogenesis. Studies utilizing nonhuman primates showed that Shiga toxins are essential virulence determinants in the development of microangiopathic lesions. Fontaine et al. (9) showed that macaque monkeys fed toxigenic strains of S. dysenteriae developed colonic microvascular lesions, while baboons given purified intravenous Stx1 developed acute renal failure (48). The bolus intravenous administration of Stx1 or Stx2 into baboons revealed that the animals were more sensitive to Stx2, although the mean times to death were prolonged in Stx2-treated animals compared to that with Stx1 treatment. Both toxins mediated hematologic changes such as thrombocytopenia and schistocytosis, and both toxins produced renal pathology, but with different presentations. Renal damage caused by Stx1 was characterized by moderate congestion at the cortico-medullary junction, while Stx2-treated animals showed severe medullary congestion with cortical ischemia (42). Mice fed Stx2-producing E. coli or given a single bolus injection of purified Shiga toxins died without the development of glomerular thrombotic microangiopathy (50, 54). However, the administration of multiple low doses of Stx2 allowed the animals to survive initial toxin challenge and develop glomerular lesions characteristic of HUS in humans (40). In addition to the toxins, host response factors may contribute to D+HUS pathogenesis. Prodromal hemorrhagic colitis may alter normal colonic barrier function, and patients with D+HUS may be endotoxemic or show evidence of elevated antibody titers against lipopolysaccharides (LPS) expressed by Stx-producing E. coli (2, 10, 26). LPS elicit the expression of a broad array of pro- and anti-inflammatory cytokines and chemokines (45). In accordance with this, D+HUS patients frequently have increased serum or urinary proinflammatory cytokine and chemokine levels (15, 23). Studies using small-animal models support the hypothesis that additional bacterial and host response factors facilitate the development of renal disease. Keepers et al. (19) demonstrated that the coadministration of Stx2 and LPS to C57BL/6 mice did not produce major changes in lethality but resulted in pathophysiological changes more consistent with disease in humans: intraglomerular platelet and fibrin deposition, decreased renal function, neutrophilia, and lymphocytopenia. Barrett et al. (1) showed that the timing of toxin and LPS challenges were critical in disease outcome. LPS enhanced the lethal effects of purified Stx2 when administered to rabbits or mice after toxin challenge, whereas LPS protected the animals from Stx2 toxicity when administered before the toxin. Palermo et al. (32) showed that the LPS-induced modulation of Stx2 lethality was cytokine time and dose dependent. Mice given low doses of TNF-α or IL-1β 1 h before Stx2 treatment showed increased lethality when treated with Stx2, while mice given higher doses of IL-1β (sufficient to elicit corticosteroid production) were protected from Stx2 lethality.
The proinflammatory cytokines TNF-α and IL-1β sensitize vascular endothelial cells to the cytotoxic action of Shiga toxins in vitro (24, 34, 53) through a mechanism involving the increased expression of genes involved in the biosynthesis of Gb3 (43). Murine and human macrophages or macrophage-like cell lines express proinflammatory cytokines and chemokines when treated with purified Shiga toxins (12, 35, 51). Keepers et al. (18) showed a marked monocytic cell infiltrate into the kidneys of mice given Stx2 and LPS. Collectively, these data suggest that the innate immune response to Shiga toxins, in the presence or absence of LPS, alters the outcome of renal disease. In the present study, we have examined the role of a single proinflammatory cytokine, TNF-α, in pathogenesis using the mouse model of Stx-induced renal damage. Our data suggest that the timing of TNF-α production affects the outcome of disease in that the presence of elevated TNF-α levels prior to toxin challenge protects animals from disease, while high TNF-α levels occurring after toxin administration result in accelerated lethality.
MATERIALS AND METHODS
Mice.
Male C3H/HeN mice weighing approximately 20 g were purchased from Taconic Farms and housed in the College of Medicine Animal Facilities, Texas A&M Health Science Center. All animals were documented to be specific pathogen free by the supplier, and sentinel animals were routinely used to test for the presence of murine pathogens. Animal rooms were temperature controlled and on a 12-h light/12-h dark cycle. Animals were allowed access to food and water ad libitum. All experimental procedures complied with the Guide for Care and Use of Laboratory Animals and were approved by the Texas A&M University Laboratory Animal Care Committee.
Shiga toxins.
Stx1 was expressed from E. coli DH5α(pCKS112), a recombinant strain containing a plasmid encoding the stx1 operon (50). Stx1 in crude bacterial lysates was purified by sequential ion-exchange, chromatofocusing, and immunoaffinity chromatography. Toxin purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with silver staining and by Western blotting using bovine polyclonal Stx1-specific antisera (a kind gift from James Samuel, Texas A&M Health Science Center, College Station, TX). Toxin preparations were passed through ActiClean Etox columns (Sterogene Bioseparations, Carlsbad, CA) to remove trace endotoxin contaminants and were determined to contain less than 0.1 ng of endotoxin per ml by the Limulus amoebocyte lysate assay (Associates of Cape Cod, East Falmouth, MA). Purified Stx2 was obtained from BEI Resources, Manassas, VA.
Survival studies.
Animals were injected with either Stx1, Stx2, recombinant murine TNF-α (rmTNF-α) (R&D Systems, Minneapolis, MN), or saline, as indicated, via the intraperitoneal (i.p.) route. Treatment groups were randomly assigned and categorized based on the timing of rmTNF-α injection relative to toxin exposure (Fig. 1). Mice pretreated with rmTNF-α prior to Stx1 or Stx2 injection were designated TNF-α pretreatment groups. Briefly, mice were injected with 2.0 μg rmTNF-α at 24, 6, or 1 h before the administration of 9 μg/kg of body weight Stx1 or 10 ng/kg Stx2 and were monitored for survival. Mice receiving Stx1 prior to rmTNF-α injection were designated TNF-α posttreatment groups (Fig. 1). These groups were administered 9 μg/kg of Stx1 and subsequently injected with 2.0 μg rmTNF-α at 1, 6, and 24 h after toxin injection and monitored for survival. All mice were euthanized by CO2 asphyxiation upon signs of morbidity (ruffled fur, lethargy, and hind-limb paralysis). Neutralizing antibodies were used to protect TNF-α posttreated animals from increased lethality. Briefly, mice were given either 4.0 μg anti-rmTNF-α (R&D Systems, Minneapolis, MN) or 60 ng anti-Stx1 (13C4; Hycult Biotechnology, Uden, The Netherlands). One hour later, the mice were challenged with 4.5 μg/kg of Stx1 followed by 2.0 μg rmTNF-α and monitored for survival during a 7-day period. All statistical significance was assessed by the log-rank (Mantel-Cox) test using Graphpad Prism version 5 for Mac OS X (Graphpad Software, San Diego, CA).
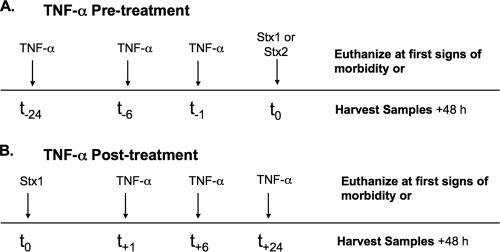
FIG. 1.
TNF-α pre- and posttreatment protocols for survival, histology, and apoptosis studies. (A) Pretreatment. Mice received 2.0 μg rmTNF-α at 24, 6, or 1 h before injection with either 9 μg/kg Stx1 or 10 ng/kg Stx2 at time zero. Subsequently, mice were monitored for morbidity (survival curves) or euthanized 48 h after Stx injection (histology, TUNEL staining). (B) Posttreatment. Mice received 9 μg/kg of Stx1 at time zero. One, 6, and 24 h later, mice were given 2.0 μg rmTNF-α and monitored for morbidity (survival curves) or euthanized 48 h after Stx1 injection (histology, TUNEL staining).
Histopathology studies.
The histopathology protocol of Chen et al. (5) was followed. Briefly, mice were euthanized by CO2 asphyxiation 48 h after Stx1 injection (Fig. 1). Kidney halves (right side) were immediately placed in 4% paraformaldehyde for 24 h. After being rinsed with phosphate-buffered saline (PBS), tissues were placed in 70% ethanol for 24 h and were passed through a series of increasing concentrations of alcohol washes followed by xylene, and finally they were embedded in paraffin wax. Five-μm sections were prepared, and hematoxylin and eosin (H&E) staining was performed. Pathology was evaluated in a blinded manner by a trained veterinary pathologist. Glomerular changes were quantified by randomly counting 10 glomeruli per high-power field for five independent sections from three animals and were scored as hyperemic/occluded versus normal. Total numbers of glomeruli per field also were counted. Statistical significance was assessed by one-way analysis of variance (ANOVA) with Tukey's multiple comparison test using Graphpad Prism version 5 for Mac OS X (Graphpad Software, San Diego, CA).
Blood chemistries.
Mice were treated with Stx1 alone, rmTNF-α 6 h before Stx1 challenge (TNF-α pretreatment group), or rmTNF-α 6 h after Stx1 challenge (TNF-α posttreatment group). On day 2, blood was collected from mice via cardiac puncture in heparin-coated tubes and analyzed at the Clinical Pathology Veterinary Medical Laboratory at Texas A&M University, College Station, TX. White blood cell (WBC) differentials were conducted using a Coulter Z1 particle counter (Beckman Coulter, Fullerton, CA). Blood urea nitrogen (BUN) and serum creatinine levels were analyzed using a Vitros 250 instrument (Ortho Clinical Diagnostics, Rochester, NY). Statistical significance was assessed using the nonparametric one-way ANOVA Kruskal-Wallis test with Dunn's multiple comparison test using Graphpad Prism version 5 (Graphpad Software).
Analysis of cytokine expression.
Kidneys (left side) were harvested from mice at various time points (Fig. 2), and kidney halves were placed in 250 μl radioimmunoprecipitation assay (RIPA) lysis buffer and homogenized. Lysates were centrifuged at 15,000 × g for 30 min. Supernatants were collected and stored at −80°C until analyzed. Levels of IL-1β, IL-2, IL-4, IL-5, IL-10, IFN-γ, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were determined using a Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA) and commercially available kits according to the manufacturer's instructions. A series of eight standards ranging in concentrations from 32,000 to 1.95 pg/ml were included in each assay. Samples were analyzed in duplicate and graphed using GraphPad Prism. Statistical significance was assessed at P < 0.05 by one-way ANOVA with Tukey's multiple comparison test using Graphpad Prism version 5 (Graphpad Software).
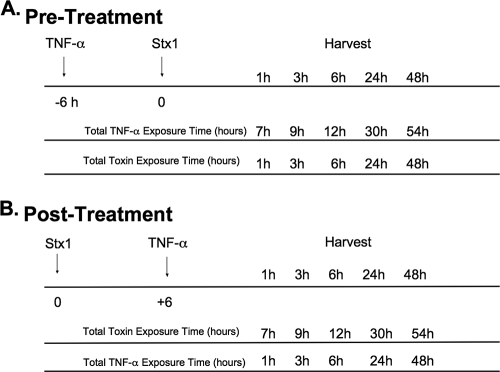
FIG. 2.
TNF-α pre- and posttreatment protocols for cytokine analysis. (A) Pretreatment. Mice were injected with 2.0 μg rmTNF-α, and 6 h later they were injected with 9 μg/kg of Stx1. Kidneys were harvested at various time points and homogenized for cytokine analysis. (B) Posttreatment. Mice were injected with 9 μg/kg of Stx1 at time zero. Six hours later they were given 2.0 μg rmTNF-α, and at various time points kidneys were harvested and homogenized for cytokine analysis.
TUNEL staining.
Kidneys were harvested from animals 48 h after treatment (Fig. 1) and placed in 4% paraformaldehyde for 24 h. After being rinsed with PBS, the tissues were placed in 70% ethanol for 24 h and then embedded in paraffin. Five-μm sections were collected, and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining (Roche, Mannheim, Germany) was performed according to the manufacturer's protocol. Briefly, sections were dewaxed by several washes in undiluted xylene followed by rehydration in ethanol washes (100, 95, 90, 80, and 70%). Slides then were washed with PBS and incubated with a permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 8 min. A TUNEL reaction mixture was added to slides and allowed to incubate in a humidified chamber for 1 h at 37°C. Slides were imaged using a Nikon fluorescent microscope. Images were captured with an excitation wavelength in the range of 450 to 500 nm and detection in the range of 515 to 565 nm. TUNEL-positive cells were quantified by counting stained cells per high-power field from three independent slides from three animals. Statistical significance was assessed at P < 0.05 using one-way ANOVA with Tukey's multiple comparison test using Graphpad Prism version 5 (Graphpad Software).
RESULTS
Treatment of mice with TNF-α alters Stx1 lethality.
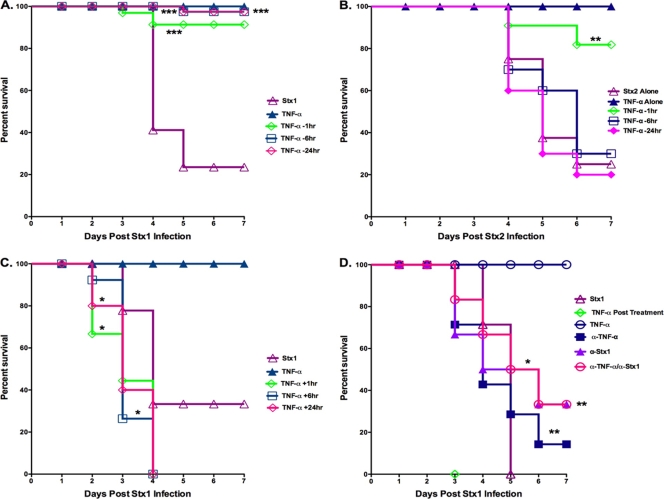
TNF-α has been shown to upregulate the expression of the toxin receptor Gb3 by human endothelial cells in vitro (43). Therefore, we hypothesized that the pretreatment of mice with rmTNF-α followed by Stx1 challenge would sensitize the animals for increased vascular damage, resulting in increased lethality. Mice were treated with Stx1 alone, rmTNF-α alone, or with rmTNF-α followed by the injection of 9 μg/kg Stx1 (TNF-α pretreatment group), and then they were monitored for survival for 7 days (Fig. 3 A). Mice receiving Stx1 alone displayed a mean time to death of 4 days, while the rmTNF-α dose used in this study did not cause lethality. Mice pretreated with rmTNF-α for as little as 1 h before Stx1 administration were protected from the lethal effects of the toxin, with survival rates of 80% during 7 days (P < 0.001). Mice pretreated with rmTNF-α for 6 or 24 h prior to Stx1 treatment had 90% survival rates throughout the monitored time course (P < 0.001). Since HUS in humans is more likely following the ingestion of STEC expressing Stx2, we also examined the effects of rmTNF-α pretreatment on the lethality of mice given 10 ng/kg of Stx2 (Fig. 3B). In accordance with earlier studies (50), we show that lethality is mediated by lower doses of Stx2 compared to that of Stx1, and the mean time to death in Stx2-treated animals was prolonged to approximately 5 days. Pretreatment with rmTNF-α was able to rescue Stx2-challenged mice as well, although the protection was not as effective as that seen in Stx1-treated animals. Mice that were pretreated at 6 and 24 h before Stx2 administration had a survival rate of approximately 30 and 20%, respectively. Mice pretreated with rmTNF-α for 1 h before Stx2 exposure had a survival rate of 80% (P < 0.01). Since rmTNF-α pretreatment manifested increased survival in Stx1- and Stx2-treated mice, we used Stx1 in the remaining experiments.
FIG. 3.
TNF-α pre- and posttreatment alter Shiga toxin 1-mediated lethality. Animals were injected with Stx1, Stx2, rmTNF-α, or saline, as indicated, via the intraperitoneal (i.p.) route. Treatment groups were categorized based on the timing of rmTNF-α injection relative to toxin exposure. (A) Mice pretreated with rmTNF-α prior to Stx1 injection (TNF-α pretreatment group). Mice were injected with 2.0 μg rmTNF-α 24, 6, or 1 h before the administration of 9 μg/kg of Stx1 and monitored for morbidity. The graph represents the results of three independent experiments using a total of 13 mice (n = 13). Three asterisks denotes significance at P < 0.001 compared to Stx1 treatment alone. (B) Mice pretreated with rmTNF-α prior to Stx2 injection (TNF-α pretreatment group). Mice were injected with 2 μg rmTNF-α 24, 6, or 1 h before the administration of 10 ng/kg of Stx2 and monitored for survival. The graph represents results of two independent experiments (n = 10). Two asterisks denotes significance at P < 0.01 compared to Stx2 treatment alone. (C) Mice receiving Stx1 prior to rmTNF-α injection (TNF-α posttreatment group). Mice were given 9 μg/kg of Stx1 and subsequently injected with rmTNF-α 1, 6, and 24 h later and monitored for survival. The graph represents results of two independent experiments (n = 6). The asterisk denotes significance at P < 0.05 compared to Stx1 treatment alone. (D) Neutralizing antibodies were used to protect TNF-α-posttreated animals from increased Stx1 lethality. Briefly, mice were given either 4.0 μg anti-rmTNF-α antibody or 60 ng anti-Stx1 antibody. One hour later, mice were challenged with 4.5 μg/kg of Stx1 followed 1 h later by 2.0 μg rmTNF-α and were monitored for survival during a 7-day period. The graph represents results of two independent experiments (n = 10). An asterisk denotes significance at P < 0.05, and a double asterisk denotes significance at P < 0.01 compared to TNF-α-posttreated animals. All statistical significance was assessed by the log-rank (Mantel-Cox) test using Graphpad Prism version 5 for Mac OS X (Graphpad Software, San Diego, CA).
We also examined the effects on the lethality of mice given rmTNF-α after exposure to Stx1 (Fig. 3C, TNF-α posttreatment group). Mice given Stx1 before rmTNF-α showed modest decreases in mean times to death, with 50% of the animals receiving rmTNF-α 1 h after Stx1 dying by day 2 (P < 0.05). By day 4, all mice that received rmTNF-α after toxin exposure were dead. We attempted to rescue TNF-α-posttreated animals from death using anti-Stx1 and anti-rmTNF-α neutralizing antibodies (Fig. 3D). We were unable to protect mice from the heightened lethality we observed when mice were treated with rmTNF-α after 9 μg/kg of Stx1 (data not shown). Therefore, antibody neutralization experiments were done using 4.5 μg/kg of Stx1. Mice exposed to this dose of Stx1 displayed a mean time to death of approximately 5 days, while mice that were TNF-α posttreated all died by day 3 (P < 0.001). The administration of anti-Stx1 or anti-rmTNF-α to TNF-α-posttreated animals resulted in increased survival times for both groups (P < 0.01 and P < 0.05, respectively). However, animals treated with anti-Stx1 displayed a greater percentage of survival than mice treated with anti-TNF-α (40 and 20% survival, respectively) during the 7-day monitoring period. When anti-TNF-α and anti-Stx1 antibodies were coadministered, 40% of the mice survived Stx1 and TNF-α posttreatment challenge (P < 0.01). All mice survived treatment with anti-TNF-α and anti-Stx1 alone (data not shown). Collectively, these data suggest that the timing of TNF-α exposure alters the pathogenesis of disease caused by Stx1, as evidenced by changes in lethality. Pretreatment with rmTNF-α protects mice from lethality caused by Stx1 and Stx2, while rmTNF-α treatment after Stx1 challenge slightly increases lethality. Mice were only partially protected from increased lethality in the TNF-α-posttreated animals by neutralizing both TNF-α and Stx1. Finally, the in vivo results do not correlate with previous reports demonstrating the ability of TNF-α to upregulate Gb3 expression by endothelial cells in vitro.
Treatment of mice with TNF-α alters Stx1-induced renal histopathology.
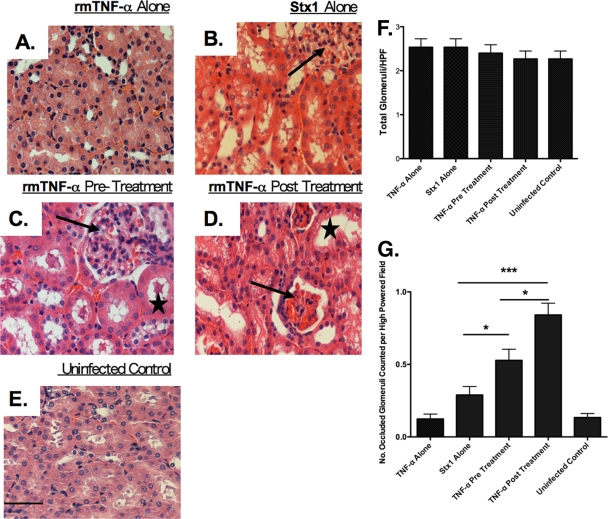
A major shortcoming of murine models of Stx-mediated renal damage is the failure to fully recapitulate the pathogenesis of human D+HUS, in which pathology is primarily localized to renal glomeruli. In mice, toxin-mediated damage appears to be localized to renal tubules (50, 54). Mice do, however, develop hallmark signs of D+HUS, including thrombocytopenia, microangiopathic hemolytic anemia, and acute renal failure, when Stx2 is coadministered with LPS, suggesting that proinflammatory cytokine expression affects the localization of renal tissue damage (19). We therefore examined renal pathology in mice administered Stx1 alone or treated with rmTNF-α 24 h before or after toxin challenge. It is important to note that histopathology at the 1- and 6-h pre- and posttreatment time points also was examined; however, for simplicity, only the 24-h time point is shown (Fig. 4). The damage seen at earlier treatment times was consistent with damage elicited by Shiga toxin exposure (data not shown). Animals treated with saline were used as controls for normal renal structure (Fig. 4E). The treatment of mice with rmTNF-α did not cause renal damage (Fig. 4A). Consistent with earlier studies, the administration of Stx1 alone resulted in tubular necrosis (Fig. 4B), and in all Stx1-plus-TNF-α treatment groups there was damage to tubules, as evidenced by tubular swelling (Fig. 4D, star) and the sloughing of cells into the lumen (Fig. 4C, star). The treatment of mice with rmTNF-α before toxin challenge (Fig. 4C), which is associated with increased survival, did not result in significant changes in renal pathology compared to that of animals treated with Stx1 alone (Fig. 4B). In contrast, the treatment of mice with rmTNF-α after toxin treatment exacerbated pathology in the kidney. Glomeruli in the TNF-α posttreatment group appeared to be hyperemic or occluded and were smaller in size with increased Bowman's capsule space (Fig. 4D, arrow) compared to glomeruli in Stx1-treated animals (Fig. 4B, arrow) or the TNF-α pretreatment group (Fig. 4C, arrow). The data are further supported by the quantification of hyperemic/occluded versus normal glomeruli by light microscopy. Total glomeruli were counted per high-power field to show that equal numbers of glomeruli were counted in each experimental group (Fig. 4F). Animals treated with Stx1 before TNF-α exposure showed a significant increase in hyperemic or occluded glomeruli (P < 0.05) compared to that of TNF-α-pretreated mice and mice treated with Stx1 alone (Fig. 4G). Thus, renal pathology occurring in mice treated with rmTNF-α after toxin challenge was greater in severity and involved glomeruli to a greater extent than mice injected with Stx1 alone or treated with rmTNF-α before toxin challenge. The expansion of renal compartments damaged in the TNF-α posttreatment group may contribute to the accelerated mean time to death seen in this group.
FIG. 4.
TNF-α pre- and posttreatment alter Shiga toxin 1-mediated renal histopathology. Mice were treated with rmTNF-α alone (A), 9 μg/kg Stx1 alone (B), pretreated with rmTNF-α 24 h before toxin (C), posttreated with rmTNF-α 24 h after toxin challenge (D), or treated with saline (E). Kidney halves were harvested 48 h after toxin administration and processed for H&E analysis. Slides were blinded, examined, and quantified by a veterinary pathologist. Arrows in panels B, C, and D point to glomeruli; stars in C and D denote renal tubule damage. The magnification of all images is ×40 (bar = 500 μm). Glomerular quantification was done blinded by counting total glomeruli (F) versus hyperemic glomeruli (G) per high-power field (10 fields were counted per slide). The experiment was repeated twice with a total of six mice. Statistical significance was assessed by one-way ANOVA and Tukey's multiple comparison post hoc test. An asterisk denotes significance at P < 0.05, and three asterisks denotes significance at P < 0.001.
Treatment of mice with TNF-α alters Stx1-induced changes in renal function and blood chemistries.
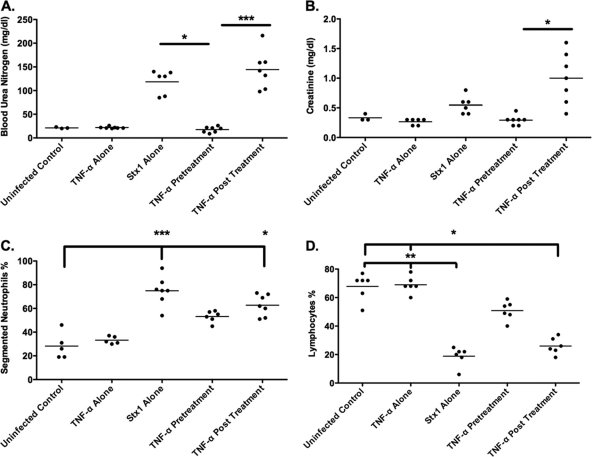
D+HUS is defined, in part, by the loss of renal function. Therefore, we examined indicators of renal function, blood urea nitrogen (BUN), and serum creatinine in control mice, mice treated with rmTNF-α or Stx1 alone, or mice pre- or posttreated with rmTNF-α and Stx1 (Fig. 5). Because of the complexity of the experimental protocol, we have chosen one TNF-α treatment time in which there was both significant survival and a significant increase in lethality. The survival curves (Fig. 3A and C) show that there was 90% survival by pretreating the mice with TNF-α 6 h before Stx1 administration. Additionally, when mice were treated with TNF-α 6 h after Stx1, there was 100% lethality. Therefore, these experiments were conducted using the 6-h pre- and posttreatment timeline. BUN levels of animals infected with Stx1 alone rose to approximately 118 mg/dl, while uninfected control and TNF-α-only levels remained within normal limits of 21.33 and 21.86 mg/dl, respectively (Fig. 5A). Mice treated with rmTNF-α before Stx1 exposure also remained within normal limits, approximately 25 mg/dl. Mice treated with rmTNF-α after Stx1 exposure had the greatest increase in mean BUN levels, at approximately 150 mg/dl. This same trend was seen with serum creatinine levels, with significant increases in Stx1 alone and rmTNF-α-posttreated animals and no significant changes in the TNF-α-pretreated animals (Fig. 5B). The administration of Shiga toxins may alter other clinical factors in mice, such as numbers of circulating neutrophils and lymphocytes (19). Therefore, complete blood counts were conducted on samples collected from control and treatment groups. There were significant increases in segmented neutrophils in both Stx1 alone and TNF-α-posttreated animals compared to the levels for control animals (P < 0.001 and P < 0.05, respectively) (Fig. 5C). Both groups treated with Stx1 alone or posttreated with TNF-α also presented with lymphocytopenia more often than the controls (P < 0.05); however, the TNF-α pretreatment and posttreatment groups were not significantly different from each other (Fig. 5D). Animals pretreated with rmTNF-α showed a slight decrease in circulating lymphocyte numbers, which were not statistically significant compared to those of uninfected controls or rmTNF-α-treated animals.
FIG. 5.
Treatment of mice with TNF-α alters Shiga toxin 1-induced changes in renal function and blood chemistries. Mice were treated with saline, 9 μg/kg Stx1 alone, rmTNF-α alone, pretreated with rmTNF-α 6 h prior to Stx1 exposure (TNF-α pretreatment), or posttreated with rmTNF-α 6 h after Stx1 exposure (TNF-α posttreatment). Forty-eight hours later, all groups were euthanized. Sera were collected and analyzed for BUN (A) and creatinine levels (B), and the percentages of lymphocytes (C) and segmented neutrophils (D) were determined. The scatter plots represent results of two independent experiments (n = 6). Statistical significance between all groups was assessed using the Kruskal-Wallis test with Dunn's multiple comparison post test. An asterisk denotes significance at P < 0.05, and three asterisks denotes significance at P < 0.001.
Treatment of mice with TNF-α alters Stx1-induced cytokine profiles.
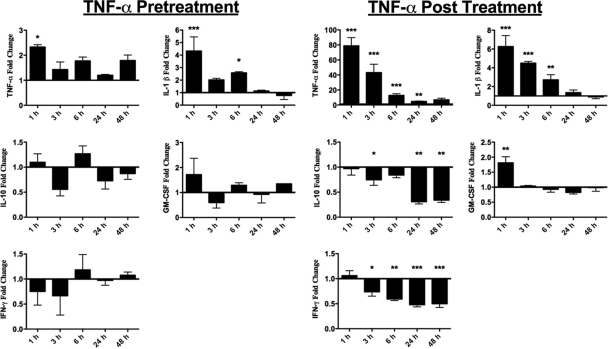
Cytokines are signaling proteins mediating a wide range of physiological responses, including the activation of innate and adaptive immunity, inflammation, and hematopoiesis. Many cytokines made early in response to infectious challenges, like TNF-α, trigger the expression of multiple downstream cytokines. According to our data, the time at which the cytokine TNF-α initiates signaling cascades may play an important role in the mortality and pathogenesis of renal disease caused by Shiga toxins. To address the role of cytokines potentially induced by Stx1 and TNF-α in the pathogenesis of Stx1-induced renal failure, cytokine levels were measured in renal tissue homogenates prepared from animals treated with Stx1 alone and in TNF-α pretreatment and posttreatment groups. Because of the complexity of the experimental protocol, we have chosen one TNF-α treatment time at which there was both significant survival and a significant increase in lethality. The survival curves (Fig. 3A and C) show that there was 90% survival by pretreating the mice with TNF-α 6 h before Stx1 administration. Additionally, when mice were treated with TNF-α 6 h after Stx1, there was 100% lethality. Therefore, these experiments were conducted using the 6-h pre- and posttreatment timeline. A comparison of renal cytokine expression in mice treated with toxin alone versus the TNF-α pretreatment group showed a rapid, transient fold increase in TNF-α and IL-1β expression in renal tissues of the pretreatment group (Fig. 6); however, there were no statistically significant effects on the other assayed cytokines. TNF-α posttreatment animals showed multiple differences in cytokine profiles (Fig. 6). The extensive fold change in TNF-α levels at the first time point probably was due to the exogenous administration of rmTNF-α 1 h prior to kidney harvesting; however, TNF-α expression remained significantly elevated up to 24 h after rmTNF-α injection. Injected rmTNF-α appeared to induce IL-1β production in the TNF-α posttreatment group, which remained significantly elevated up to 6 h posttreatment. Interestingly, the levels of the anti-inflammatory cytokine IL-10 were significantly downregulated in the TNF-α posttreatment group beginning 24 h after rmTNF-α injection. A similar pattern of IFN-γ expression was apparent; that is, IFN-γ expression began to decrease 6 h after toxin treatment and remained downregulated throughout the course of the experiment. Finally, in contrast to the rmTNF-α-pretreated animals, significant differences in GM-CSF expression were detected 1 h after rmTNF-α injection. These data suggest that skewing the cytokine response to a heightened proinflammatory and a lowered anti-inflammatory state contribute to the increased mortality and glomerular pathology seen in animals administered rmTNF-α after toxin exposure.
FIG. 6.
Comparison of renal cytokine expression in TNF-α-pretreated and TNF-α-posttreated animals. Mice were treated with either 9 μg/kg Stx1 alone, with Stx1 6 h after rmTNF-α exposure (TNF-α pretreatment group), or with rmTNF-α 6 h after Stx1 exposure (TNF-α posttreatment group). At various time points, kidneys were harvested and the expression of the cytokines TNF-α, IL-1β, IL-10, IFN-γ, and GM-CSF in renal tissue homogenates was analyzed as described in Materials and Methods. The data shown are expressed as the fold change of the rmTNF-α pretreatment group or the fold change of the rmTNF-α posttreatment group from results for Stx1 alone at each time point. The experiment was repeated twice with a total of six mice in each group. Statistical significance between cytokine expression by Stx1-treated cells versus treatment groups at each time point was assessed using one-way ANOVA with Tukey's multiple comparison post test. An asterisk denotes significance at P < 0.05, two asterisks denotes significance at P < 0.01, and three asterisks denotes significance at P < 0.001 between the TNF-α treatment group and Stx1 treatment only.
Treatment with TNF-α alters Stx1-mediated tubular apoptosis in the murine kidney.
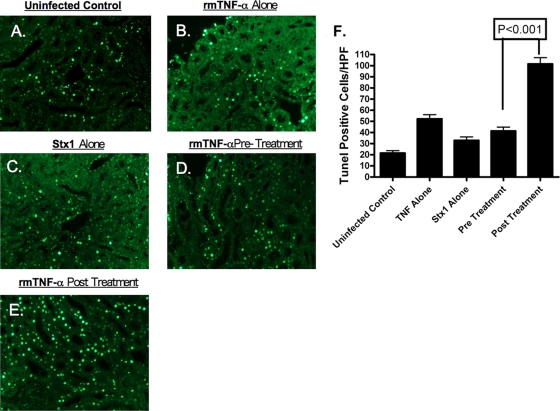
Stx1 and Stx2 induce programmed cell death or apoptosis in vitro and in vivo (49). Therefore, we examined the capacity of Stx1 to elicit apoptosis in renal tissues isolated from mice treated with Stx1 and in TNF-α pre- and posttreatment groups. It is important to note that TUNEL staining at the 1- and 6-h pre- and posttreatment time points also was examined; however, for simplicity, only the 24-h time point is shown (Fig. 7). Mice administered Stx1 or rmTNF-α alone (Fig. 7 B and C) showed low levels of apoptotic cells in TUNEL-stained renal sections, which were not significantly different from saline control tissues (Fig. 7A). TUNEL-positive cells appeared to be localized to renal tubules. The levels of apoptotic cells in the TNF-α-pretreated animals remained relatively low (Fig. 7D). Mice treated with rmTNF-α after initial toxin exposure (Fig. 7E) appeared to have higher numbers of apoptotic cells compared to mice treated with Stx1 alone or the TNF-α pretreatment group. Data were quantified by counting the number of TUNEL-positive cells in blinded tissue sections for all treatment groups (Fig. 7F). When mice were exposed to Stx1 alone or rmTNF-α alone or were pretreated with rmTNF-α, the average number of TUNEL-positive cells per high-power field remained relatively constant, whereas mice posttreated with rmTNF-α had a significant increase (P < 0.001) in TUNEL-positive cells.
FIG. 7.
Treatment with TNF-α alters Shiga toxin 1-mediated apoptosis in the murine kidney. Mice were treated with saline (A), rmTNF-α alone (B), Stx1 alone (C), rmTNF-α 24 h before Stx1 exposure (pretreatment group) (D), or rmTNF-α 24 h after Stx1 injection (posttreatment group) (E). Kidneys were harvested 48 h after toxin administration and stained for the presence of apoptosis. (F) Apoptosis was quantified by counting the numbers of TUNEL-positive cells per high-power field, with three fields per treatment group. Statistical significance between the groups was assessed using one-way ANOVA and Tukey's multiple comparison post test.
DISCUSSION
While advancements in our understanding of the host innate immune response in D+HUS pathogenesis have been made using cultured cells, the corroboration of these results using animal models has proven problematic, since many small-animal models do not fully reproduce the pathology seen in humans with D+HUS. Unlike humans, where the primary renal vascular lesion is glomerular and may take weeks to develop, mice develop damage to tubule epithelia and die within days of the injection of purified Stx1 or Stx2 (50). Exposure to multiple low doses of Stx2, or the injection of LPS with Stx2 into mice, results in glomerular pathology and thrombocytopenia that more closely mimic HUS in humans (19, 40). Thus, bacterial products known to induce TNF-α expression alter pathogenesis in the mouse model. Based on earlier studies showing that TNF-α upregulates toxin receptor expression on endothelial cells in vitro, thereby sensitizing the cells to the lethal action of the toxins (24, 34, 53), we predicted that the pretreatment of mice with rmTNF-α would manifest the toxin sensitization phenomenon in vivo. Surprisingly, we found that animals pretreated with rmTNF-α displayed decreased lethality when challenged with Stx1 or Stx2, while animals that received exogenous rmTNF-α after toxin exposure died faster than animals given Stx1 alone. Increased lethality in the TNF-α posttreatment group was partially neutralized by the administration of anti-Stx1 and anti-rmTNF-α antibodies. The partial protection seen with neutralizing antibodies might be due to insufficient dosing or the timing of antibody administration. The data suggest that the timing of exposure to TNF-α relative to toxin exposure is a critical determinant in the outcome of disease in mice.
Stx1 and Stx2 share general structural and functional properties and are roughly comparable in terms of in vitro cytotoxicity when using toxin-sensitive cell lines such as Vero cells or human umbilical vein endothelial cells. However, infections with Stx2- or Stx1- and Stx2-expressing STEC are more likely to progress to extraintestinal complications, and in vivo studies using mice, gnotobiotic piglets, and baboons demonstrated that the animals are more sensitive to lethality caused by Stx2 than that by Stx1 (7, 42, 50). A number of mechanisms may contribute to this difference in lethality. Both toxin types bind the glycolipid receptor Gb3 but with different dissociation constants, so that Stx1 binds more avidly than Stx2 (28). Stx1 and Stx2 have been shown to differentially translocate across polarized intestinal epithelial cell monolayers (14), suggesting that differences in toxin uptake in the gut contribute to differences in lethality. Rutjes et al. (37) demonstrated the differential distribution of Stx1 and Stx2 in mice. Stx2 accumulated to a greater extent in the murine kidney, while Stx1 was found to be associated with the bone marrow of the spine and long bones, nasal turbinates, and lungs. Furthermore, the serum clearance rate of 125I-Stx2 was much slower than that of 125I-Stx1 in mice. Human renal microvascular endothelial cells were reported to be more sensitive to the cytotoxic action of Stx2 than that of Stx1 (25). Suzuki et al. (46) showed that the A subunit of Stx2, but not that of Stx1, contained a sequence capable of binding the prosurvival molecule Bcl-2, which may sensitize host cells to apoptosis induced by Stx2. However, we have found, using human macrophage-like cells, that the apoptosis-inducing capacities of Stx1 and Stx2 are roughly comparable (unpublished data). Finally, the cytotoxic activity of Stx2, but not that of Stx1, was reported to be neutralized by binding to the acute-phase protein human serum amyloid P (HuSAP) (20). However, while HuSAP inhibits the binding of Stx2 to Gb3-expressing murine neutrophils, it actually increases Stx2 binding to Gb3-negative human neutrophils (11). Despite these differences in toxin translocation and distribution, we show here that the rates of survival in mice and baboons given Stx1 or Stx2 are similar; i.e., the animals are more sensitive to the lethal action of Stx2, but the mean times to death are prolonged compared to that for Stx1 (42). The delayed progression of lethality in Stx2-treated baboons is associated with differences in renal pathology. Comparative studies exploring renal pathology in Stx1- versus Stx2-treated mice are planned. The phenomenon of protection provided by pretreatment with TNF-α before toxin challenge manifested in mice given Stx1 or Stx2, suggesting that the early administration of TNF-α is a candidate for further study as an immunotherapeutic agent to block disease progression.
All animals receiving purified Stx1 alone or Stx1 with rmTNF-α pretreatment showed signs of acute tubular necrosis (Fig. 4B, C, and D) with evidence of tubular epithelial necrosis and the sloughing of cells into tubule lumina (Fig. 4C, star), while glomeruli were largely spared vascular damage (Fig. 4B and 4C). Animals that received rmTNF-α after toxin treatment showed evidence of tubular damage but also showed occasional glomerular pathology characterized by intraglomerular hyperemia and glomerular hypertrophy (Fig. 4D). Thus, the increased lethality we observed in animals that received rmTNF-α after toxin exposure was associated with increased glomerular damage (Fig. 4G). The postmortem autolysis of tissue specimens is a significant concern in histopathological studies. It is important to note, therefore, that the renal tubule pathology consistently detected in all animals receiving Stx1 in the presence or absence of rmTNF-α was not detected in saline-treated controls, nor was it detected in renal specimens collected from animals receiving rmTNF-α alone. The fact that the animals were sacrificed and kidney tissues were harvested and processed in an identical manner strongly supports the concept that renal tubular necrosis is toxin mediated and not the result of postmortem autolysis or fixation artifacts, since one would expect these changes to be present in all treatment groups. The protection of animals by the administration of rmTNF-α before toxin challenge does not appear to involve major differences in renal histopathology, i.e., tubular damage is similar in animals given toxin alone and in the rmTNF-α pretreatment group. While differences in lethality noted as a function of the timing of TNF-α exposure were not correlated with striking differences in renal pathology, it is possible that differences in renal function manifest in the absence of gross histopathological changes. Our data indicate clear differences in renal function (BUN and serum creatinine levels) among the treatment groups. Renal function was preserved in animals receiving rmTNF-α prior to intoxication, whereas decreased renal function was evident in mice treated with Stx1 or Stx1 followed by rmTNF-α. Lymphocytopenia and neutrophilia previously described in mice treated with Stx2 and LPS (19) were noted in toxin-only groups; however, the changes in the TNF-α treatment groups were not statistically significant. rmTNF-α pretreatment prevented lymphocytopenia, although elevated numbers of segmented neutrophils were detected in rmTNF-α-pretreated animals. Recently, Stearns-Kurosawa et al. (42) showed that Stx1, but not Stx2, increased the expression of granulocyte-colony stimulating factor (G-CSF) in baboons. It would be interesting to determine if a similar differential induction in G-CSF exists in Stx1- and Stx2-treated mice and whether differences in granulocyte production account for the difference in toxin lethal doses seen in mice. The maintenance of functional, circulating lymphocytes may be a mechanism by which TNF-α pretreatment increases survival after Stx1 challenge.
Shiga toxins elicit cytokine and chemokine expression from myeloid (12, 35, 51), epithelial (52, 55), and endothelial cells (4, 27) in vitro, and clinical studies have reported elevated cytokine levels in patients with HUS (15, 23). TNF-α is known to be an effective inducer of a number of cytokines, including the autoinduction of its own expression (45). Taken together, these data suggest that differences in renal cytokine expression in animals receiving Stx1 alone compared to that with Stx1 and rmTNF-α contribute to differences in lethality. Fold changes in cytokine expression in renal homogenates prepared from mice treated with rmTNF-α prior to Stx1 (TNF-α pretreatment group) revealed transient increases in the proinflammatory cytokines TNF-α and IL-1β at early time points; however, no significant changes were noted in the other assayed cytokines. In animals intoxicated prior to receiving rmTNF-α (TNF-α posttreatment group), we detected sustained higher fold changes of proinflammatory cytokines, while the expression of IL-10 and gamma interferon (IFN-γ) showed significant decreases compared to the levels at early time points. Thus, increased lethality and glomerular histopathology detected in animals receiving Stx1 prior to rmTNF-α treatment are associated with the increased expression of proinflammatory cytokines and the decreased expression of the anti-inflammatory cytokine IL-10 and the macrophage-activating cytokine IFN-γ.
Karpman et al. (16) used TUNEL staining to present evidence of apoptosis in human renal tissue sections isolated from HUS cases and in murine renal sections derived from animals treated with Shiga toxins. We examined the capacity of rmTNF-α pre- or posttreatment to modulate Stx1-induced renal cell apoptosis. Mice treated with Stx1 alone or pretreated with rmTNF-α prior to toxin showed relatively low numbers of apoptotic cells, primarily in renal tubules. These data suggest that the protective mechanism triggered by the early exposure to rmTNF-α does not alter the degree of apoptotic cell death in renal tissues. When animals were treated with Stx1 before rmTNF-α, however, we detected many more apoptotic cells in renal sections, suggesting that increased lethality correlates with increased renal apoptosis.
Currently, there are no effective vaccines to prevent infections with Stx-producing bacteria, and there are no effective therapeutic strategies to prevent the progression of disease from the prodromal diarrheal phase to D+HUS. We showed that mice treated with a single dose of rmTNF-α before challenge with purified Stx1 or Stx2 were protected from lethality. Protection was associated with the maintenance of renal function and the preservation of normal numbers of circulating lymphocytes. Protection did not correlate with major differences in renal histopathology, renal expression of cytokines, neutrophilia, or differences in the degree of renal apoptosis. We speculate that pretreatment with rmTNF-α before Stx1 exposure rendered target cells more resistant to the effects of Shiga toxins. One possible explanation of protection is a phenomenon known as acquired cytoresistance. Zager et al. (56) demonstrated that renal proximal tubule cells, when subjected to an initial insult, such as the injection of LPS, may “prime” the kidney and protect against a second insult. Honda et al. (13) and Nath et al. (29) demonstrated that when the kidney is preexposed to LPS, it can become resistant to nephrotoxin-induced renal failure. Collectively, these studies are in accordance with our findings and suggest that pretreating mice with rmTNF-α before toxin exposure protects the kidney from further insult and injury. Mechanisms of cytoresistance may involve the upregulated expression of stress proteins and changes in membrane cholesterol levels (56). We have shown that Shiga toxins will activate the stress-associated protein kinases JNK and p38 (6), and the maintenance of lipid rafts has been shown to be essential for toxin uptake and retrograde transport (8). Another possible mechanism of protection afforded by rmTNF-α pretreatment is the modulation of toxin receptor expression on the surface of target cells. Our preliminary studies suggest that rmTNF-α downregulates membrane Gb3 expression on renal tubular epithelial cells by affecting the expression of enzymes involved in Gb3 biosynthesis (unpublished data). In marked contrast to the protective effect of early rmTNF-α exposure, animals intoxicated first, followed by rmTNF-α administration, showed increased sensitivity to the lethal action of Stx1 with a decreased mean time to death. Increased lethality was associated with increased glomerular damage, decreased renal function, increased proinflammatory cytokine expression, the decreased expression of an anti-inflammatory and a macrophage-activating cytokine, and increased renal apoptosis. In summary, the data suggest that the early administration of TNF-α is a candidate interventional strategy meriting further study for the amelioration of renal disease caused by Shiga toxins. However, caution must be taken in any immunomodulatory approach to the treatment of diseases caused by Shiga toxins, since our data suggest that the timing of TNF-α administration relative to intoxication is a critical determinant in disease outcome.
Acknowledgments
This work was supported by the National Institutes of Health grant RO1 AI34530-14.
In accordance with HHS Select Agents and Toxins requirements, Vernon Tesh's laboratory is registered with the Centers for Disease Control and Prevention and has obtained approval for the possession and use of a select agent (registration no. 20060605-0489).
We thank Alan Parrish for his assistance in the interpretation of the findings and for the careful reading of the manuscript. The assistance of Dinorah Leyva-Illades, Moo-Seung Lee, and Christine McFarland with animal handling and monitoring is gratefully acknowledged.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 6 July 2010.
REFERENCES
- 1.Barrett, T. J., M. E. Potter, and I. K. Wachsmuth. 1989. Bacterial endotoxin both enhances and inhibits the toxicity of Shiga-like toxin II in rabbits and mice. Infect. Immun. 57:3434-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitzan, M., E. Moebius, K. Ludwig, D. E. Müller-Wiefel, J. Heesemann, and H. Karch. 1991. High incidence of serum antibodies to Escherichia coli O157 lipopolysaccharide in children with hemolytic-uremic syndrome. J. Pediatr. 119:380-385. [DOI] [PubMed] [Google Scholar]
- 3.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brigotti, M., D. Carnicelli, E. Ravanelli, A. G. Vara, C. Martinelli, R. R. Alfieri, P. G. Petronini, and P. Sestili. 2007. Molecular damage and induction of proinflammatory cytokines in human endothelial cells exposed to Shiga toxin 1, Shiga toxin 2, and alpha-sarcin. Infect. Immun. 75:2201-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, G., E. A. Bridenbaugh, A. D. Akintola, J. M. Catania, V. S. Vaidya, J. V. Bonventre, A. C. Dearman, H. W. Sampson, D. C. Zawieja, R. C. Burghardt, and A. R. Parrish. 2007. Increased susceptibility of aging kidney to ischemic injury: identification of candidate genes changed during aging, but corrected by caloric restriction. Am. J. Physiol. Renal Physiol. 293:F1272-F1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherla, R. P., S.-Y. Lee, P. L. Mees, and V. L. Tesh. 2006. Shiga toxin 1-induced cytokine production is mediated by MAP kinase pathways and translation initiation factor eIF4E in the macrophage-like THP-1 cell line. J. Leukoc. Biol. 79:397-407. [DOI] [PubMed] [Google Scholar]
- 7.Donohue-Rolfe, A., I. Kondova, S. Oswald, D. Hutto, and S. Tzipori. 2000. Escherichia coli O157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for gnotobiotic piglets than are isotypes producing only Stx1 or both Stx1 and Stx2. J. Infect. Dis. 181:1825-1829. [DOI] [PubMed] [Google Scholar]
- 8.Falguières, T., F. Mallard, C. Baron, D. Hanau, C. Lingwood, B. Goud, J. Salamero, and L. Johannes. 2001. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 12:2453-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontaine, A., J. Arondel, and P. J. Sansonetti. 1988. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox− mutant of Shigella dysenteriae 1. Infect. Immun. 56:3099-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greatorex, J. S., and G. M. Thorne. 1994. Humoral immune responses to Shiga-like toxins and Escherichia coli 0157 lipopolysaccharide in hemolytic-uremic syndrome patients and healthy subjects. J. Clin. Microbiol. 32:1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griener, T. P., G. L. Mulvey, P. Marcato, and G. D. Armstrong. 2007. Differential binding of Shiga toxin 2 to human and murine neutrophils. J. Med. Microbiol. 56:1423-1430. [DOI] [PubMed] [Google Scholar]
- 12.Harrison, L. M., W. C. E. van Haaften, and V. L. Tesh. 2004. Regulation of proinflammatory cytokine expression by Shiga toxin 1 and/or lipopolysaccharides in the human monocytic cell line THP-1. Infect. Immun. 72:2618-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honda, N., A. Hishida, K. Ikuma, and K. Yonemura. 1987. Acquired resistance to acute renal failure. Kidney Int. 31:1233-1238. [DOI] [PubMed] [Google Scholar]
- 14.Hurley, B. P., M. Jacewicz, C. M. Thorpe, L. L. Lincicome, A. J. King, G. T. Keusch, and D. W. K. Acheson. 1999. Shiga toxins 1 and 2 translocate differently across polarized intestinal epithelial cells. Infect. Immun. 67:6670-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpman, D., A. Andreasson, H. Thysell, B. S. Kaplan, and C. Svanborg. 1995. Cytokines in childhood hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Pediatr. Nephrol. 9:694-699. [DOI] [PubMed] [Google Scholar]
- 16.Karpman, D., A. Håkansson, M.-T. R. Perez, C. Isaksson, E. Carlemalm, A. Caprioli, and C. Svanborg. 1998. Apoptosis of renal cortical cells in the hemolytic-uremic syndrome: in vivo and in vitro studies. Infect. Immun. 66:636-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawano, K., M. Okada, T. Haga, K. Maeda, and Y. Goto. 2008. Relationship between pathogenicity for humans and stx genotype in Shiga toxin-producing Escherichia coli serotype O157:H7. Eur. J. Clin. Microbiol. Infect. Dis. 27:227-231. [DOI] [PubMed] [Google Scholar]
- 18.Keepers, T. R., L. K. Gross, and T. G. Obrig. 2007. Monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha, and RANTES recruit macrophages to the kidney in a mouse model of hemolytic-uremic syndrome. Infect. Immun. 75:1229-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keepers, T. R., M. A. Psotka, L. K. Gross, and T. G. Obrig. 2006. A murine model of HUS: Shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. J. Am. Soc. Nephrol. 17:3404-3414. [DOI] [PubMed] [Google Scholar]
- 20.Kimura, T., S. Tani, Y. Matsumoto, and T. Takeda. 2001. Serum amyloid P component is the Shiga toxin 2-neutralizing factor in human blood. J. Biol. Chem. 276:41576-41579. [DOI] [PubMed] [Google Scholar]
- 21.Koster, F. T., V. Boonpucknavig, S. Sujaho, R. H. Gilman, and M. M. Rahaman. 1984. Renal histopathology in the hemolytic-uremic syndrome following shigellosis. Clin. Nephrol. 21:126-133. [PubMed] [Google Scholar]
- 22.Lee, S.-Y., M.-S. Lee, R. P. Cherla, and V. L. Tesh. 2008. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol. 10:770-780. [DOI] [PubMed] [Google Scholar]
- 23.López, E. L., M. M. Contrini, S. Devoto, M. F. de Rosa, M. G. Graña, M. H. Genero, C. Canepa, H. F. Gomez, and T. G. Cleary. 1995. Tumor necrosis factor concentrations in hemolytic uremic syndrome patients and children with bloody diarrhea in Argentina. Pediatr. Infect. Dis. J. 14:594-598. [DOI] [PubMed] [Google Scholar]
- 24.Louise, C. B., and T. G. Obrig. 1991. Shiga toxin-associated hemolytic-uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1 beta, and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect. Immun. 59:4173-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louise, C. B., and T. G. Obrig. 1995. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. J. Infect. Dis. 172:139-1401. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig, K., M. Bitzan, S. Zimmermann, M. Kloth, H. Ruder, and D. E. Müller-Wiefel. 1996. Immune response to non-O157 Vero toxin-producing Escherichia coli in patients with hemolytic uremic syndrome. J. Infect. Dis. 174:1028-1039. [DOI] [PubMed] [Google Scholar]
- 27.Matussek, A., J. Lauber, A. Bergau, W. Hansen, M. Rohde, K. E. Dittmar, M. Gunzer, M. Mengel, P. Gatzlaff, M. Hartmann, J. Buer, and F. Gunzer. 2003. Molecular and functional analysis of Shiga toxin-induced response patterns in human vascular endothelial cells. Blood 102:1323-1332. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima, H., N. Kiyokawa, Y. U. Katagiri, T. Taguchi, T. Suzuki, T. Sekino, K. Mimori, T. Ebata, M. Saito, H. Nakao, T. Takeda, and J. Fujimoto. 2001. Kinetic analysis of binding between Shiga toxin and receptor glycolipid Gb3Cer by surface plasmon resonance. J. Biol. Chem. 276:42915-42922. [DOI] [PubMed] [Google Scholar]
- 29.Nath, K. A., G. Balla, G. M. Vercellotti, J. Balla, H. S. Jacob, M. D. Levitt, and M. E. Rosenberg. 1992. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Investig. 90:267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obrig, T. G., T. P. Moran, and J. E. Brown. 1987. The mode of action of Shiga toxin on peptide elongation of eukaryotic protein synthesis. Biochem. J. 244:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orth, D., K. Grif, A. B. Khan, A. Naim, M. P. Dierich, and R. Würzner. 2007. The Shiga toxin genotype rather than the amount of Shiga toxin or cytotoxicity of Shiga toxin in vitro correlates with the appearance of the hemolytic uremic syndrome. Diagn. Microbiol. Infect. Dis. 59:235-242. [DOI] [PubMed] [Google Scholar]
- 32.Palermo, M., F. Alves-Rosa, C. Rubel, G. C. Fernández, G. Fernández-Alonso, F. Alberto, M. Rivas, and M. Isturiz. 2000. Pretreatment of mice with lipopolysaccharide (LPS) or IL-1beta exerts dose-dependent opposite effects on Shiga toxin-2 lethality. Clin. Exp. Immunol. 119:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proulx, F., E. G. Seidman, and D. Karpman. 2001. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 50:163-171. [DOI] [PubMed] [Google Scholar]
- 34.Ramegowda, B., J. E. Samuel, and V. L. Tesh. 1999. Interaction of Shiga toxins with human brain microvascular endothelial cells: cytokines as sensitizing agents. J. Infect. Dis. 180:1205-1213. [DOI] [PubMed] [Google Scholar]
- 35.Ramegowda, B., and V. L. Tesh. 1996. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 64:1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson, S. E., M. A. Karmali, L. E. Becker, and C. R. Smith. 1988. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum. Pathol. 19:1102-1108. [DOI] [PubMed] [Google Scholar]
- 37.Rutjes, N. W. P., B. A. Binnington, C. R. Smith, M. D. Maloney, and C. A. Lingwood. 2002. Differential tissue targeting and pathogenesis of verotoxins 1 and 2 in the mouse model. Kidney Int. 62:832-845. [DOI] [PubMed] [Google Scholar]
- 38.Sandvig, K. 2001. Shiga toxins. Toxicon 39:1629-1635. [DOI] [PubMed] [Google Scholar]
- 39.Sandvig, K., S. Grimmer, S. U. Lauvrak, M. L. Torgersen, G. Skretting, B. van Deurs, and T. G. Iversen. 2002. Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell Biol. 117:131-141. [DOI] [PubMed] [Google Scholar]
- 40.Sauter, K. A., A. R. Melton-Celsa, K. Larkin, M. L. Troxell, A. D. O'Brien, and B. E. Magun. 2008. Mouse model of hemolytic-uremic syndrome caused by endotoxin-free Shiga toxin 2 (Stx2) and protection from lethal outcome by anti-Stx2 antibody. Infect. Immun. 76:4469-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, W. E., A. V. Kane, S. T. Campbell, D. W. Acheson, B. H. Cochran, and C. M. Thorpe. 2003. Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells. Infect. Immun. 71:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stearns-Kurosawa, D. J., V. Collins, S. Freeman, V. L. Tesh, and S. Kurosawa. 2010. Distinct physiologic and inflammatory responses elicited in baboons after challenge with Shiga toxin type 1 or 2 from enterohemorrhagic Escherichia coli. Infect. Immun. 78:2497-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stricklett, P. K., A. K. Hughes, Z. Ergonul, and D. E. Kohan. 2002. Molecular basis for up-regulation by inflammatory cytokines of Shiga toxin 1 cytotoxicity and globotriaosylceramide expression. J. Infect. Dis. 186:976-982. [DOI] [PubMed] [Google Scholar]
- 44.Strockbine, N. A., L. R. M. Marques, J. W. Newland, H. Williams-Smith, R. K. Holmes, and A. D. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suffredini, A. F., and N. P. O'Grady. 1999. Pathophysiological responses to endotoxins in humans, p. 817-830. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (ed.), Endotoxin in health and disease. Marcel Dekker, Inc., New York, NY.
- 46.Suzuki, A., H. Doi, F. Matsuzawa, S. Aikawa, K. Takiguchi, H. Kawano, M. Hayashida, and S. Ohno. 2000. Bcl-2 antiapoptotic protein mediates verotoxin II-induced cell death: possible association between Bcl-2 and tissue failure by E. coli O157:H7. Genes Dev. 14:1734-1740. [PMC free article] [PubMed] [Google Scholar]
- 47.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073-1086. [DOI] [PubMed] [Google Scholar]
- 48.Taylor, F. B., Jr., V. L. Tesh, L. DeBault, A. Li, A. C. Chang, S. D. Kosanke, T. J. Pysher, and R. L. Siegler. 1999. Characterization of the baboon responses to Shiga-like toxin: descriptive study of a new primate model of toxic responses to Stx-1. Am. J. Pathol. 154:1285-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tesh, V. L. 2010. Induction of apoptosis by Shiga toxins. Fut. Microbiol. 5:431-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, A. D. O'Brien, and J. E. Samuel. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesh, V. L., B. Ramegowda, and J. E. Samuel. 1994. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine peritoneal macrophages. Infect. Immun. 62:5085-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorpe, C. M., B. P. Hurley, L. L. Lincicome, M. S. Jacewicz, G. T. Keusch, and D. W. Acheson. 1999. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect. Immun. 67:5985-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Setten, P. A., V. W. M. van Hinsbergh, T. J. A. N. van der Velden, N. C. A. J. van de Kar, M. Vermeer, J. D. Mahan, K. J. M. Assmann, L. P. W. J. van den Heuvel, and L. A. H. Monnens. 1997. Effects of TNF alpha on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int. 51:1245-1256. [DOI] [PubMed] [Google Scholar]
- 54.Wadolkowski, E. A., L. M. Sung, J. A. Burris, J. E. Samuel, and A. D. O'Brien. 1990. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect. Immun. 58:3959-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamasaki, C., Y. Natori, X. T. Zeng, M. Ohmura, S. Yamasaki, and Y. Takeda. 1999. Induction of cytokines in a human colon epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by non-toxic mutant Stx1 which lacks N-glycosidase activity. FEBS Lett. 442:231-234. [DOI] [PubMed] [Google Scholar]
- 56.Zager, R. A., A. C. M. Johnson, S. Y. Hanson, and S. Lund. 2006. Acute nephrotoxic and obstructive injury primes the kidney to endotoxin-driven cytokine/chemokine production. Kidney Int. 69:1181-1188. [DOI] [PubMed] [Google Scholar]