Abstract
The dynamic movement of B cells increases the probability of encountering specific antigen and facilitates cell-cell interactions required for mounting a rapid antibody response. B1a and B1b cells are enriched in the coelomic cavity, contribute to T-cell-independent (TI) antibody responses, and increase in number upon antigen exposure. B1 cell movement is largely governed by Cxc ligand 13 (Cxcl13), and mice deficient in this chemokine have a severe reduction in peritoneal B1 cells. In this study, we examined the role of Cxcl13-dependent B cell migration using Borrelia hermsii infection or intraperitoneal immunization with pneumococcal polysaccharide or 4-hydroxy-3-nitrophenyl-acetyl (NP)-Ficoll, all of which induce robust antibody responses from B1b cells. Surprisingly, we found that antibody responses to B. hermsii or to FhbA, an antigenic target of B1b cells, and the resolution of bacteremia were indistinguishable between wild-type and Cxcl13−/− mice. Importantly, we did not observe an expansion of peritoneal B1b cell numbers in Cxcl13−/− mice. Nonetheless, mice that had resolved infection were resistant to reinfection, indicating that the peritoneal B1b cell reservoir is not required for controlling B. hermsii. Furthermore, despite a reduced peritoneal B1b compartment, immunization with pneumococcal polysaccharide vaccine yielded comparable antigen-specific antibody responses in wild-type and Cxcl13−/− mice and conferred protection against Streptococcus pneumoniae. Likewise, immunization with NP-Ficoll elicited similar antibody responses in wild-type and Cxcl13−/− mice. These data demonstrate that homing of B1 cells into the coelomic cavity is not a requirement for generating protective TI antibody responses, even when antigen is initially localized to this anatomical compartment.
Vaccination generates B cell memory, which provides protective antibody responses upon reexposure to the same pathogen. T cells are essential for the development of conventional B cell memory. Many proteinaceous molecules such as tetanus toxin are T-cell-dependent (TD) antigens, and they induce the generation of antigen-specific T cells that help follicular (FO) B cells (49). However, certain bacterial products such as capsular polysaccharides induce antibody responses without T-cell help and are referred to as T-cell-independent (TI) antigens (47). Responses to TI antigens develop significantly more rapidly than TD responses and thus can play a critical role in a number of contexts, such as bacteremia, one of the major causes of death by infectious diseases (39). The B cell subsets primarily involved in TI responses in mice are marginal zone (MZ) B cells in the spleen and B1a (B220+ IgMhi IgDlo CD23− Mac1+ CD5+) and B1b (B220+ IgMhi IgDlo CD23− Mac1+ CD5−) cells, both of which preferentially migrate to and reside in the coelomic cavity (2, 36). A critical marker on B1 cells, Mac1 is expressed only on those B1 cells that reside in the peritoneal or pleural cavities. Thus, B1b cells cannot be definitively identified elsewhere (2).
Using the murine model of B. hermsii infection, we have previously shown that B1b cells generate a novel T cell-independent memory (5). The hallmark of this infection is recurrent episodes of high-level bacteremia (∼108 bacteria/ml blood), each caused by antigenically distinct populations of bacteria generated by DNA rearrangements of the genes encoding the variable major proteins (11). Remarkably, each episode is resolved rapidly within 3 days by a Borrelia hermsii-specific IgM response independently of T-cell help (4, 12, 21), and convalescent-phase T-cell-deficient mice are as resistant as convalescent-phase wild-type mice to reinfection (5). Concurrent with the resolution of bacteremia, B1b cells expand in great numbers in the peritoneal cavity, and purified convalescent-phase B1b cells confer long-lasting immunity to Rag1−/− mice, demonstrating an important role for peritoneal B1b cells in protective immunity (4, 5). Recently, the role of B1b cells in TI immunity has been extended to other bacterial infection systems (26, 29, 32). For instance, Haas et al. have demonstrated that B1a and B1b cells fill independent niches in protecting against Streptococcus pneumoniae (32). B1a cells are important for natural antibodies, while B1b cells mediate protection by generating a specific antibody response to capsular polysaccharide on this bacterium (32).
The dynamic movement of B cells increases the probability of encountering specific antigen and facilitates cell-cell interactions required for mounting a rapid antibody response (19, 23, 41). The omentum, a bilayered sheet of mesothelial cells in the coelomic cavity that connects various organs, such as the stomach and pancreas, plays an important role in the movement of peritoneal B1 cells (8, 14, 15). Upon appropriate stimulus, B1 cells in the peritoneal cavity migrate to the mesenteric lymph nodes (MLNs), where they differentiate into antibody-secreting plasma cells (25, 31, 48). In support of this, we have observed that during infection with B. hermsii, a rapid egress of B1b cells from the peritoneal cavity occurs (2). The magnitude of the migration is directly proportional to the bacterial burden. Interestingly, soon after resolution of bacteremia, B1b cell numbers in the peritoneal cavity are restored (2).
Chemokines are not only important for lymphocyte migration into secondary lymphoid organs; they are also critical for development of lymphoid organs (7). Although homing of MZ B cells into the marginal sinus is not affected in mice deficient in Cxcl13 or its receptor Cxcr5, B2-cell-rich primary follicles are severely malformed (9, 27, 35). Germinal centers, while present, are small and ectopic compared to those of wild-type mice, suggesting a role for this chemokine axis in germinal-center dynamics (27). Indeed, Cxcl13 is critical for the migration of B cells into the germinal-center light zone (1). Likewise, the Cxcl13-Cxcr5 axis is also important for B1 cell movement. For example, migration of B1 cells into the coelomic cavity is diminished in the absence of omentum-derived Cxcl13 (8). In studies using mice deficient in Cxcl13 or Cxcr5, it was reported that this chemokine axis is important for antibody responses to bacteria encountered in the peritoneal cavity (8, 31, 33, 40). These studies were focused on B1a-cell-derived antiphosphorylcholine responses (8, 33). In the present study, we endeavored to determine whether Cxcl13-driven migration was essential for stimulating a robust antibody response to antigens that induce an antibody response from peritoneal B1b cells.
MATERIALS AND METHODS
Mice.
The Institutional Animal Care and Use Committee have approved these studies. Mice were housed in microisolator cages with free access to food and water and were maintained in a specific-pathogen-free facility at Thomas Jefferson University. C57BL/6J (wild-type) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). B6.129X1-Cxcl13tm1Cys/J (Cxcl13−/−) mice have been described previously (8, 9).
Infections.
Mice were infected intraperitoneally (i.p.) or intravenously (i.v.) via the tail vein with 5 × 104 bacteria of the fully virulent B. hermsii strain DAH-p1 (from the blood of an infected mouse), and the bacteremia was monitored by dark-field microscopy (4). For pneumococcal infections, 5 × 103 CFU of S. pneumoniae WU2, a type 3 strain (18, 46), were injected i.p. into immunized mice, and survival was monitored for 10 days.
Immunization.
Ten micrograms of 23-valent pneumococcal polysaccharide vaccine (Pneumovax 23; Merck & Co Inc., Whitehouse Station, NJ) (24) or 50 μg of 4-hydroxy-3-nitrophenyl-acetyl conjugated to Ficoll (50NP-aminoethyl carboxymethyl-Ficoll; Biosearch Technologies, Novato, CA) dissolved in 100 μl Dulbecco's phosphate-buffered saline (Mediatech, Herndon, VA) was used to immunize mice i.p. Blood samples were obtained 0, 7, and 14 days following immunization.
ELISA.
IgM or IgG3 levels were measured with enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Bethyl Laboratories, Montgomery, TX). B. hermsii-specific IgM was determined by coating 96-well plates (ICN Biomedicals Inc., Aurora, OH) with in vivo-grown B. hermsii DAH-p1 (105 wet bacteria/well). FhbA-specific IgM was determined by coating 96-well plates with 0.5 μg/ml recombinant FhbA (rFhbA) (20). Pneumovax 23 and pneumococcal polysaccharide type 3 (PPS3)-specific IgM levels were measured by coating 96-well plates with 50 μl of either Pneumovax 23 (5 μg/ml) or PPS3 (5 μg/ml; American Type Culture Collection, Rockville, MD). The hapten NP-specific response was measured by coating the plates with NP-conjugated bovine serum albumin (BSA) (23NP-BSA; Biosearch Technologies). All plates were washed and blocked with 2% BSA in PBS, pH 7.2, for 2 h at room temperature. Blood samples from immunized mice were diluted 1:25, 1:100, or 1:500, samples were centrifuged (16,000 × g for 10 min), and supernatant was used. Bound IgM or IgG3 was measured using horseradish peroxidase (HRP)-conjugated goat anti-mouse IgM or IgG3. Specific antibody levels were interpreted as ng/μl equivalents using IgM or IgG3 standards.
Flow cytometry.
The anti-IgM-fluoroscein isothiocyanate (clone 1B4B1), anti-Mac1-allophycocyanin (clone M1/70) and anti-CD5-peridinin chlorophyll (clone 53-7.3) antibodies were purchased from eBioscience (San Diego, CA); anti-CD23-phycoerythrin (clone B3B4) was from PharMingen (San Diego, CA). 23NP-phycoerythrin was purchased from Biosearch Technologies.
To determine the frequency of B1a and B1b cells, peritoneal cavity cells were harvested from individual mice and the cell concentration was adjusted to 2.5 × 107/ml in staining medium (deficient RPMI 1640 medium [Irvine Scientific, Santa Ana, CA] with 3% new calf serum, 1 mM EDTA). To identify NP-specific B cells in various anatomical compartments, peritoneal cavity cells, spleen tissue, mesenteric lymph nodes, and blood were collected from NP-Ficoll-immunized wild-type and Cxcl13−/− mice. After Fc receptors were blocked with 2.4G2 antibody (1 μg per 106 cells), an aliquot of 25 μl of peritoneal cavity cells was incubated in a microtiter plate with appropriately diluted antibodies. After staining, cells were washed twice with staining medium and the preparations were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA) using CELLQuest software (Becton Dickinson). Data were analyzed using the FlowJo software program (Treestar, San Carlos, CA).
Statistical analysis.
Statistical analyses were performed using the Prism 5 software program (GraphPad Software, Inc., La Jolla, CA). To analyze statistical significance, the unpaired Student's t test (one or two tailed), Mann-Whitney test, or two-way analysis of variance (ANOVA) was used as necessary.
RESULTS
Resolution of B. hermsii bacteremia is not impaired in Cxcl13−/− mice.
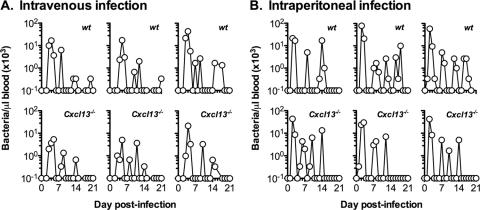
B1a cells recognize phosphorylcholine on the surface of S. pneumoniae bacteria (32). Mice lacking either Cxcl13 or its receptor Cxcr5 have impaired B1a cell migration into the peritoneal cavity and thus respond poorly to phosphorylcholine after intraperitoneal but not intravenous immunization with nonencapsulated S. pneumoniae (8, 33). In the murine model of B. hermsii infection, we have previously shown that B1b cells in the peritoneal cavity play a central role in protection (5). Furthermore, Toll-like receptor 2 (TLR2) stimulation contributes to a rapid IgM response required for the resolution of bacteremia (3, 17). Like B1a cells, B1b cells are highly chemotactic toward Cxcl13 (8). Interestingly, Borrelia-infected mice have increased production of Cxcl13 mediated by TLR2 (28, 42), suggesting a link between B. hermsii infection and B1b cell migration. To understand whether Cxcl13-mediated B cell migration is critical for protective immunity to B. hermsii, we studied B. hermsii infection in Cxcl13−/− mice. Surprisingly, both wild-type and Cxcl13−/− mice resolved intravenous infection indistinguishably, and the levels of bacteremia were similar in wild-type and knockout mice (Fig. 1A). For example, there was no significant difference in bacterial burden during the first wave of bacteremia between wild-type and Cxcl13−/− mice (52,800 ± 29,200 bacteria per μl of blood in wild-type mice versus 36,800 ± 12,200 bacteria per μl of blood in Cxcl13−/− mice). Since Cxcl13−/− mice are deficient in peritoneal B1 cells (8), we examined B. hermsii bacteremia after intraperitoneal infection. Similar to the results with i.v. infection, both wild-type and Cxcl13−/− mice resolved infection indistinguishably after i.p. infection with B. hermsii (Fig. 1B). In fact, when the initial wave of infection was measured, there was a significantly lower (P = 0.0492) bacterial burden in Cxcl13−/− mice than in wild-type mice (28,700 ± 11,000 bacteria per μl of blood in wild-type mice versus 16,000 ± 11,200 bacteria per μl of blood in Cxcl13−/− mice). These results demonstrate that Cxcl13-mediated B cell migration is dispensable for clearance of B. hermsii.
FIG. 1.
Resolution of B. hermsii bacteremia in the absence of Cxcl13-mediated migration. Wild-type (n = 5 or 6) or Cxcl13−/− (n = 7 to 9) mice were infected intravenously (A) or intraperitoneally (B) with 5 × 104 B. hermsii bacteria (strain DAH-p1), and bacteremia was monitored by dark-field microscopy. Each plot represents data from an individual mouse. For brevity, results for three representative mice from each group are shown.
Functional B. hermsii-specific IgM responses in Cxcl13−/− mice.
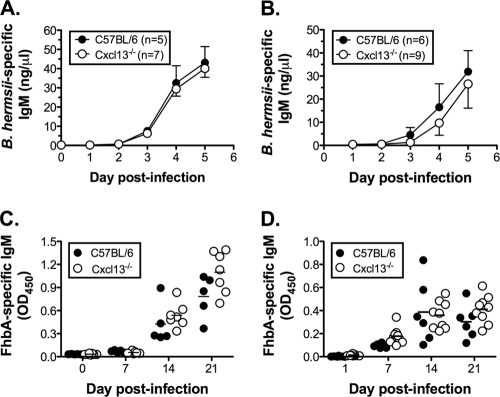
IgM is the essential and sufficient immunoglobulin isotype required for controlling B. hermsii (4). Since the correlate of protection during B. hermsii infection is the generation of specific IgM (5, 12, 22), we measured IgM responses in wild-type and Cxcl13−/− mice. Regardless of the route of infection, the IgM response in Cxcl13−/− mice to intact B. hermsii bacteria was indistinguishable from that of wild-type mice (Fig. 2A and B). Furthermore, wild-type and Cxcl13−/− mice produced comparable levels of IgM specific for FhbA, a B. hermsii-expressed antigenic target of B1b cells (Fig. 2C and D). These results demonstrate that Cxcl13-mediated B cell migration is not required for the generation of B. hermsii-specific IgM responses and the concurrent resolution of bacteremia.
FIG. 2.
Comparable B. hermsii-specific IgM responses in wild-type and Cxcl13−/− mice. B. hermsii-specific IgM generated during intravenous (A) or intraperitoneal (B) infection was measured by ELISA. Means ± standard deviations (SD) are shown. FhbA-specific IgM generated during intravenous (C) or intraperitoneal (D) infection was measured by ELISA. Data points in panels C and D represent individual animals, and horizontal bars represent means of results for each group. Statistical significance of the genotype was measured by two-way ANOVA with significance reached at a P of <0.05. The differences between the two genotypes were not statistically significant.
Despite impaired B1b cell expansion, convalescent-phase Cxcl13−/− mice are resistant to reinfection.
Naïve mice suffer recurrent B. hermsii bacteremia, whereas convalescent-phase mice are resistant to reinfection. We have previously shown that the resolution of B. hermsii is concurrent with a specific expansion of B1b cells in the peritoneal cavity, and these expanded B cells confer long-lasting immunity (4, 5). Peritoneal B1b cells of convalescent-phase mice, when adoptively transferred into Rag1−/− mice, persist for more than 2 months and remain in a quiescent state similar to conventional TD memory B cells. However, upon challenge with B. hermsii, these B1b-cell-reconstituted mice rapidly generate B. hermsii-specific IgM (5). Therefore, the peritoneal cavity is a reservoir for apparent memory B1b cells. Since Cxcl13−/− mice are deficient in peritoneal B1b cells, we sought to determine whether convalescent-phase Cxcl13−/− mice are resistant to reinfection. To our surprise, Cxcl13−/− mice were as resistant to reinfection as wild-type mice (see Fig. S1 in the supplemental material), demonstrating that peritoneal memory-like B1b cells are not required for resistance to B. hermsii reinfection.
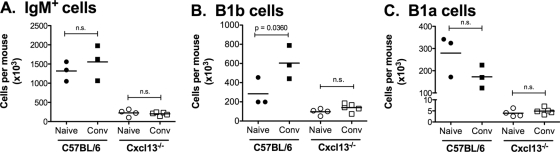
Despite a deficiency in peritoneal B1b cells, X-linked immunodeficient (xid) mice control B. hermsii bacteremia (3, 4). This resolution of bacteremic episodes is coincident with a remarkable de novo expansion of B1b cells, possibly due to an engagement of a variety of inflammatory pathways, in particular TLR signaling triggered by B. hermsii infection (3). Since Cxcl13−/− mice were resistant to reinfection, we considered the possibility that a similar expansion occurred in Cxcl13−/− mice. As reported previously (4, 5), we observed a significant expansion of B1b, but not B1a or B2, cell numbers in wild-type mice (Fig. 3). On the contrary, we did not observe an expansion of peritoneal B1b cell numbers in Cxcl13−/− mice concurrent with the resolution of infection (Fig. 3B). Since B1b cells do not express Mac1 outside the peritoneal cavity and thus cannot be definitively identified elsewhere, we did not attempt to measure B1b subset expansion in other compartments such as the spleen or lymph nodes. These results demonstrate that peritoneal B1b cells are not required for controlling either primary or secondary B. hermsii infection.
FIG. 3.
Lack of an expansion of peritoneal B1b cells in B. hermsii-infected Cxcl13−/− mice. Peritoneal cells from naïve and convalescent-phase (at 35 days postinfection) wild-type and Cxcl13−/− mice were harvested and stained with monoclonal antibodies specific for IgM, CD23, CD5, and Mac1. The absolute cell counts of IgM+ B cells (A), B1b cells (IgM+ CD23− Mac1+ CD5−) (B), and B1a cells (IgM+ CD23− Mac1+ CD5+) (C) are shown. Data points represent cell counts from individual animals. Statistical significance was determined by a one-tailed unpaired Student's t test, with significance reached at a P of <0.05. n.s., differences not statistically significant.
Pneumococcal polysaccharide vaccination elicits a protective antibody response in the absence of Cxcl13-mediated B cell migration.
Phosphorylcholine is a common antigen found on a variety of bacterial species, including S. pneumoniae (37), and in mice it is recognized by MZ B cells as well as splenic and peritoneal B1a cells (38). The participation of B1a cells from these two niches is related to the route of antigen exposure (38). Consistent with the lack of peritoneal B1a cells, intraperitoneal exposure to heat-killed nonencapsulated S. pneumoniae does not induce a robust antibody response in Cxcl13−/− mice (8).
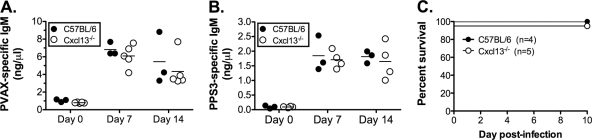
Peritoneal B1b cells have been shown to generate a significant antibody response to a serotype-specific pneumococcal polysaccharide, PPS3 (32). Since the presence of B1b cells in anatomic sites other than the peritoneal cavity has not been definitively shown, and since Cxcl13−/− mice have a paucity of peritoneal B1b cells, we expected that intraperitoneal immunization of Cxcl13−/− mice with PPS would not generate a specific antibody response. To test this, we subjected wild-type and Cxcl13−/− mice to intraperitoneal immunization with Pneumovax 23, a multivalent pneumococcal vaccine composed of a mixture of 23 serotype-specific polysaccharides, including PPS3 and PPS14. To our surprise, Cxcl13−/− mice exhibited no impairment in the IgM response to the multivalent vaccine (Fig. 4A), purified PPS3 (Fig. 4B), or purified PPS14 (data not shown). PPS-specific IgG3 responses were comparable between knockout and wild-type mice (data not shown). To determine if the antibody response to PPS3 was indeed a protective response, we challenged Pneumovax 23-immunized mice with a lethal dose (5,000 CFU) of the PPS3-expressing S. pneumoniae strain WU2. Wild-type and Cxcl13−/− mice were completely protected by the vaccine (Fig. 4C), indicating that Cxcl13-mediated B1 cell migration is not required for the development of a protective antibody response.
FIG. 4.
Pneumovax 23 (PVAX) elicits a protective antibody response in Cxcl13−/− mice. Wild-type and Cxcl13−/− mice were immunized intraperitoneally with 10 μg of Pneumovax 23. Blood samples were obtained on 0, 7, and 14 days postimmunization and Pneumovax 23 (A)- or PPS3 (B)-specific IgM levels were determined by ELISA. Data points represent individual animals, and horizontal bars represent means of results for each group. The statistical significance of genotypes was measured by two-way ANOVA, with significance reached at a P of <0.05. (C) Four weeks following Pneumovax 23 immunization, mice were challenged with 5,000 CFU of S. pneumoniae WU2 (serotype 3) and survival was monitored for 10 days. The differences between the results for the two genotypes were not statistically significant.
Anti-NP-Ficoll responses are not impaired in Cxcl13−/− mice.
NP-Ficoll is a widely used model antigen for studying TI antibody responses. In mice, MZ B (30) and B1b cells respond to NP-Ficoll (34). Since intravenous immunization will target MZ B cells (38), we examined whether a deficiency in peritoneal B1b cells predisposes Cxcl13−/− mice to an impaired response to intraperitoneal exposure of NP-Ficoll. We found that wild-type and Cxcl13−/− mice elicited comparable NP-specific IgM responses (Fig. 5). A possible explanation for the comparable NP-specific IgM responses between wild-type and Cxcl13−/− mice after NP-Ficoll immunization could be the participation of other B cell subsets (45) or B1b cells outside the peritoneal cavity. Indeed, we found that Cxcl13−/− mice had a higher frequency of Mac1-negative NP-specific B cells in the blood, MLNs, and spleen than did wild-type mice (Table 1). Since Mac1 is not expressed on B1b cells outside the peritoneal cavity, it is unclear whether the NP-specific B cells in the blood, MLNs, and spleen were B1b cells. Taken together, these data demonstrate that B cell migration in response to Cxcl13 is not required for TI responses to intraperitoneal antigens.
FIG. 5.
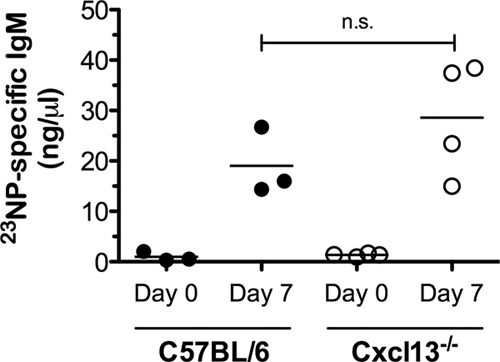
NP-specific IgM responses in Cxcl13−/− mice. Wild-type and Cxcl13−/− mice were immunized intraperitoneally with 50 μg of 50NP-Ficoll. Blood samples were obtained on 0 (preimmune) or 7 (immune) days following the immunization, and 23NP-specific IgM levels were determined by ELISA. Data points represent individual mice, and horizontal bars represent means of results for each group. Statistical significance of the response to immunization was determined by a two-tailed unpaired Student's t test. n.s., not statistically significant.
TABLE 1.
Frequencies of NP-specific B cells in various anatomic compartmentsa
| Compartment | Frequency of IgM+ NP+ cells inb: |
Significance (P) | |
|---|---|---|---|
| C57BL/6 mice (n = 5) | Cxcl13−/− mice (n = 6) | ||
| Peritoneal cavity | 0.40 ± 0.19 | 0.22 ± 0.16 | n.s.c |
| Blood | 0.063 ± 0.016 | 0.16 ± 0.041 | 0.0043 |
| MLNs | 0.043 ± 0.019 | 0.088 ± 0.030 | 0.0303 |
| Spleen | 0.11 ± 0.030 | 0.16 ± 0.028 | 0.0441 |
Wild-type (n = 5) and Cxcl13−/− (n = 6) mice were immunized i.p. with NP-Ficoll. Five to 6 days postimmunization, cells were harvested from various compartments of mice and stained with NP-phycoerythrin and anti-IgM-FITC.
Means ± SDs of IgM+ NP+ cell frequencies are given.
n.s., not significantly different.
DISCUSSION
A variety of murine models deficient in B1 cells respond poorly to TI antigens (16). Most of these murine models have mutations in positive regulators of B-cell antigen receptor (BCR) signaling. Since BCR-mediated signaling is a critical requirement for the antibody responses to TI type 2 antigens such as PPS and NP-Ficoll, these murine models complicate the evaluation of the role of peritoneal B1 cells in TI antibody responses. For instance, xid mice have a point mutation in Btk, a kinase critical for BCR signaling, and do not respond to PPS and NP-Ficoll (2). Interestingly, during B. hermsii infection in xid mice, we observed a specific TI response likely due to the induction of TLR signaling (3). The resolution of the infection in xid mice coincides with a remarkable de novo expansion of B1b cells (4), which stands in contrast to the observation that there is an absolute requirement for peritoneal B1 cells in immunity to B. hermsii. In the present study using Cxcl13−/− mice, which have a diminished number of B1a and B1b cells in the peritoneal cavity, we have demonstrated that TI responses do not require the peritoneal B1 cell compartment (Fig. 2, 4, and 5). Naïve but not PPS-immunized mice succumb to S. pneumoniae infection. Similarly, naïve mice suffer recurrent episodes of B. hermsii bacteremia whereas convalescent-phase mice are resistant (5). The acquired resistance to these infections has been attributed to an expansion of antigen-specific B1b cells that migrate to the coelomic cavity (4, 5). Interestingly, we did not observe any expansion of peritoneal B1b cell numbers in Cxcl13−/− mice (Fig. 3), yet these were resistant to reinfection. Furthermore, Cxcl13−/− mice immunized with Pneumovax 23 are completely protected from lethal challenge with S. pneumoniae (Fig. 4). These results demonstrate that the peritoneal cavity is not an essential reservoir for these expanded B1b cells.
B1 cell subsets, in particular B1a cells, have a limited BCR repertoire and produce natural antibodies. These antibodies recognize evolutionarily conserved antigens, and positive selection for B1a cells has been shown to be driven by self-antigens (37). T15 idiotype-positive (T15+) B1a cells recognize phosphorylcholine, an antigenic determinant commonly found on the surface of many bacteria, including nonencapsulated S. pneumoniae (37). Interestingly, T15+ IgM also cross-reacts with an epitope on oxidized low-density lipoprotein, indicating that interaction with a self-antigen is a mechanism for positive selection of phosphorylcholine-reactive B1a cell clones (43, 44). Unlike B1a cells, B1b cells appear to recognize a variety of structurally heterogeneous antigens, such as NP-Ficoll (34), the bacterial outer membrane proteins FhbA (20) and OmpD (29) of B. hermsii and Salmonella enterica, respectively, and specific polysaccharides of S. pneumoniae and Enterobacter cloacae (26, 32). Previous studies using nonencapsulated S. pneumoniae (strain R36A), which expresses phosphorylcholine, an antigenic target for B1a cells, have shown that mice deficient in Cxcl13 or its receptor Cxcr5 are impaired in the antiphosphorylcholine response when the bacterium is introduced intraperitoneally (8, 33). Interestingly, Cxcl13−/− mice were not impaired in their antiphosphorylcholine response after intravenous administration of the same bacterial strain (8). This is because splenic T15+ B1a cells and MZ B cells, including the M167 idiotype, are the dominant contributors to the antibody response to phosphorylcholine encountered via blood (38). These findings led to the conclusion that Cxcl13-mediated homing is critical for body cavity immunity (8, 33). In contrast to these findings, in the present study we were unable to observe any impairment in Cxcl13−/− antibody responses to intraperitoneal introduction of either bacterial antigens (B. hermsii or PPS) or a synthetic antigen (NP-Ficoll), all of which are known to induce a response from peritoneal B1b cells.
Despite certain key differences between B1a and B1b cells, such as those in BCR repertoire and responses to cytokines, they share a number of similarities, including self-renewal, rapid antibody responses, and anatomical localization (2). Since Cxcl13−/− mice have normal or greater numbers of B1a cells in the blood and spleen (8), it is likely that B1b cells could also exist in other compartments. Although B1b cells cannot be definitively identified in any anatomical site apart from the coelomic cavity, the lymph node is known to contain a small population of B220+ IgMhi IgDlo CD5− cells, a phenotype characteristic of B1b cells. Moreover, studies have shown that lymph node cells, when adoptively transferred into the peritoneal cavity of Rag1−/− mice, can give rise to B1b cells (34). These findings suggest that B1b cells or their precursors are present in lymph nodes and could possibly be responsible for the TI responses in Cxcl13−/− mice. Although Cxcl13−/− mice exhibit many defects in lymph node development, their MLNs appear to be normal (9). MLNs drain peritoneal antigens and support the differentiation of B1 cells into antibody-secreting cells (25, 48). In support of this, we detected NP-specific B cells in the MLNs, and they were also present in the blood and spleen. Thus, it is possible that the TI responses in these mice might have emanated from B1b cells present in MLNs, as described for B1a cells (25, 48).
B. hermsii gains rapid access to the vasculature; therefore, B cell subsets such as MZ B cells, which are strategically located in the spleen for encountering blood-borne antigens, could also play an important role in controlling the B. hermsii bacteremia. Indeed, using CD1d-deficient mice, Belperron et al. have elucidated a role for MZ B cells in B. hermsii infection (13). Consistent with these findings, we have previously shown that splenectomized mice are impaired in controlling B. hermsii infection during high-level primary bacteremic episodes, suggesting that splenic B cells, including MZ B cells, play an important role in B. hermsii-specific responses (4, 6). The fact that splenectomized mice efficiently control subsequent relapses and moderate primary bacteremic episodes (4) suggests that other B cell subsets such as B1b cells play an important role during low-level bacteremia. In fact, Martin et al. have shown cooperation between B1a cells and MZ B cells depending on the blood-borne particular antigen load (38). Additionally, they have also demonstrated that other cells, such as blood dendritic cells, are responsible for transporting blood-borne bacteria to the spleen to be engaged by MZ B cells (10). The relative contribution of peritoneal B1a cells and MZ B cells in response to TI antigens is also dependent on the route of immunization. Intravenous immunization predominantly engages MZ B cells, whereas intraperitoneal immunization predominantly engages peritoneal B1 cells (38). However, MZ B cells and splenic B1a cells can contribute to intraperitoneal immunization if the antigen dose is high (38), likely due to diffusion of the antigen into other compartments, such as blood. Therefore, it is possible that in the absence of Cxcl13-mediated B1 cell migration, diffusion of antigen to various sites in the body can be accountable for the observed TI antibody responses. Indeed, we have identified antigen-specific B cells in various sites after intraperitoneal immunization with NP-Ficoll (Table 1). These data suggest that in the absence of peritoneal B1b cells, other B cell subsets elsewhere contribute to TI antigen-specific immune responses.
Supplementary Material
Acknowledgments
We thank Jason Cyster for providing Cxcl13−/− mice and discussion and Tim Manser for critical review of the manuscript.
This work was supported by NIH grant R01 AI065750 to K.R.A.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 28 June 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Allen, C. D., K. M. Ansel, C. Low, R. Lesley, H. Tamamura, N. Fujii, and J. G. Cyster. 2004. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nature Immunology 5:943-952. [DOI] [PubMed] [Google Scholar]
- 2.Alugupalli, K. R. 2008. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr. Top. Microbiol. Immunol. 319:105-130. [DOI] [PubMed] [Google Scholar]
- 3.Alugupalli, K. R., S. Akira, E. Lien, and J. M. Leong. 2007. MyD88- and Bruton's tyrosine kinase-mediated signals are essential for T cell-independent pathogen-specific IgM responses. J. Immunol. 178:3740-3749. [DOI] [PubMed] [Google Scholar]
- 4.Alugupalli, K. R., R. M. Gerstein, J. Chen, E. Szomolanyi-Tsuda, R. T. Woodland, and J. M. Leong. 2003. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170:3819-3827. [DOI] [PubMed] [Google Scholar]
- 5.Alugupalli, K. R., J. M. Leong, R. T. Woodland, M. Muramatsu, T. Honjo, and R. M. Gerstein. 2004. B1b Lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379-390. [DOI] [PubMed] [Google Scholar]
- 6.Alugupalli, K. R., A. D. Michelson, I. Joris, T. G. Schwan, K. Hodivala-Dilke, R. O. Hynes, and J. M. Leong. 2003. Spirochete-platelet attachment and thrombocytopenia in murine relapsing fever borreliosis. Blood 102:2843-2850. [DOI] [PubMed] [Google Scholar]
- 7.Ansel, K. M., and J. G. Cyster. 2001. Chemokines in lymphopoiesis and lymphoid organ development. Curr. Opin. Immunol. 13:172-179. [DOI] [PubMed] [Google Scholar]
- 8.Ansel, K. M., R. B. Harris, and J. G. Cyster. 2002. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16:67-76. [DOI] [PubMed] [Google Scholar]
- 9.Ansel, K. M., V. N. Ngo, P. L. Hyman, S. A. Luther, R. Forster, J. D. Sedgwick, J. L. Browning, M. Lipp, and J. G. Cyster. 2000. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406:309-314. [DOI] [PubMed] [Google Scholar]
- 10.Balazs, M., F. Martin, T. Zhou, and J. F. Kearney. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17:341-352. [DOI] [PubMed] [Google Scholar]
- 11.Barbour, A. G. 1990. Antigenic variation of a relapsing fever Borrelia species. Annu. Rev. Microbiol. 44:155-171. [DOI] [PubMed] [Google Scholar]
- 12.Barbour, A. G., and V. Bundoc. 2001. In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect. Immun. 69:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belperron, A. A., C. M. Dailey, and L. K. Bockenstedt. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681-5686. [DOI] [PubMed] [Google Scholar]
- 14.Berberich, S., S. Dahne, A. Schippers, T. Peters, W. Muller, E. Kremmer, R. Forster, and O. Pabst. 2008. Differential molecular and anatomical basis for B cell migration into the peritoneal cavity and omental milky spots. J. Immunol. 180:2196-2203. [DOI] [PubMed] [Google Scholar]
- 15.Berberich, S., R. Forster, and O. Pabst. 2007. The peritoneal micromilieu commits B cells to home to body cavities and the small intestine. Blood 109:4627-4634. [DOI] [PubMed] [Google Scholar]
- 16.Berland, R., and H. H. Wortis. 2002. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20:253-300. [DOI] [PubMed] [Google Scholar]
- 17.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, J. H. Weis, T. G. Schwan, and J. J. Weis. 2006. Dual role of MyD88 in rapid clearance of relapsing fever Borrelia spp. Infect. Immun. 74:6750-6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briles, D. E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Anti-phosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153:694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell, D. J., C. H. Kim, and E. C. Butcher. 2003. Chemokines in the systemic organization of immunity. Immunol. Rev. 195:58-71. [DOI] [PubMed] [Google Scholar]
- 20.Colombo, M. J., and K. R. Alugupalli. 2008. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J. Immunol. 180:4858-4864. [DOI] [PubMed] [Google Scholar]
- 21.Connolly, S. E., and J. L. Benach. 2001. The spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J. Immunol. 167:3029-3032. [DOI] [PubMed] [Google Scholar]
- 22.Connolly, S. E., D. G. Thanassi, and J. L. Benach. 2004. Generation of a complement-independent bactericidal IgM against a relapsing fever Borrelia. J. Immunol. 172:1191-1197. [DOI] [PubMed] [Google Scholar]
- 23.Cyster, J. G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098-2102. [DOI] [PubMed] [Google Scholar]
- 24.Do, R. K., E. Hatada, H. Lee, M. R. Tourigny, D. Hilbert, and S. Chen-Kiang. 2000. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J. Exp. Med. 192:953-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagarasan, S., and T. Honjo. 2000. T-Independent immune response: new aspects of B cell biology. Science 290:89-92. [DOI] [PubMed] [Google Scholar]
- 26.Foote, J. B., and J. F. Kearney. 2009. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J. Immunol. 183:6359-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forster, R., A. E. Mattis, E. Kremmer, E. Wolf, G. Brem, and M. Lipp. 1996. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87:1037-1047. [DOI] [PubMed] [Google Scholar]
- 28.Gelderblom, H., D. Londono, Y. Bai, E. S. Cabral, J. Quandt, R. Hornung, R. Martin, A. Marques, and D. Cadavid. 2007. High production of CXCL13 in blood and brain during persistent infection with the relapsing fever spirochete Borrelia turicatae. J. Neuropathol Exp. Neurol. 66:208-217. [DOI] [PubMed] [Google Scholar]
- 29.Gil-Cruz, C., S. Bobat, J. L. Marshall, R. A. Kingsley, E. A. Ross, I. R. Henderson, D. L. Leyton, R. E. Coughlan, M. Khan, K. T. Jensen, C. D. Buckley, G. Dougan, I. C. MacLennan, C. Lopez-Macias, and A. F. Cunningham. 2009. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc. Natl. Acad. Sci. U. S. A. 106:9803-9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guinamard, R., M. Okigaki, J. Schlessinger, and J. V. Ravetch. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31-36. [DOI] [PubMed] [Google Scholar]
- 31.Ha, S. A., M. Tsuji, K. Suzuki, B. Meek, N. Yasuda, T. Kaisho, and S. Fagarasan. 2006. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 203:2541-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas, K. M., J. C. Poe, D. A. Steeber, and T. F. Tedder. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23:7-18. [DOI] [PubMed] [Google Scholar]
- 33.Hopken, U. E., A. H. Achtman, K. Kruger, and M. Lipp. 2004. Distinct and overlapping roles of CXCR5 and CCR7 in B-1 cell homing and early immunity against bacterial pathogens. J. Leukoc. Biol. 76:709-718. [DOI] [PubMed] [Google Scholar]
- 34.Hsu, M. C., K. M. Toellner, C. G. Vinuesa, and I. C. Maclennan. 2006. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc. Natl. Acad. Sci. U. S. A. 103:5905-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu, T. T., and J. G. Cyster. 2002. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297:409-412. [DOI] [PubMed] [Google Scholar]
- 36.Martin, F., and J. F. Kearney. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13:195-201. [DOI] [PubMed] [Google Scholar]
- 37.Martin, F., and J. F. Kearney. 2000. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory.” Immunol. Rev. 175:70-79. [PubMed] [Google Scholar]
- 38.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T- independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 39.Mokdad, A. H., J. S. Marks, D. F. Stroup, and J. L. Gerberding. 2004. Actual causes of death in the United States, 2000. JAMA 291:1238-1245. [DOI] [PubMed] [Google Scholar]
- 40.Muller, G., U. E. Hopken, and M. Lipp. 2003. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol. Rev. 195:117-135. [DOI] [PubMed] [Google Scholar]
- 41.Okada, T., and J. G. Cyster. 2006. B cell migration and interactions in the early phase of antibody responses. Curr. Opin. Immunol. 18:278-285. [DOI] [PubMed] [Google Scholar]
- 42.Rupprecht, T. A., C. J. Kirschning, B. Popp, S. Kastenbauer, V. Fingerle, H. W. Pfister, and U. Koedel. 2007. Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infect. Immun. 75:4351-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw, P. X., C. S. Goodyear, M. K. Chang, J. L. Witztum, and G. J. Silverman. 2003. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J. Immunol. 170:6151-6157. [DOI] [PubMed] [Google Scholar]
- 44.Shaw, P. X., S. Horkko, M. K. Chang, L. K. Curtiss, W. Palinski, G. J. Silverman, and J. L. Witztum. 2000. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105:1731-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shriner, A. K., H. Liu, G. Sun, M. Guimond, and A. K. R.2010. Interleukin-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J. Immunol. 185:525-531. [DOI] [PubMed] [Google Scholar]
- 46.Swiatlo, E., J. King, G. S. Nabors, B. Mathews, and D. E. Briles. 2003. Pneumococcal surface protein A is expressed in vivo, and antibodies to PspA are effective for therapy in a murine model of pneumococcal sepsis. Infect. Immun. 71:7149-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vos, Q., A. Lees, Z. Wu, C. M. Snapper, and J. J. Mond. 2000. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 176:154-170. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe, N., K. Ikuta, S. Fagarasan, S. Yazumi, T. Chiba, and T. Honjo. 2000. Migration and differentiation of autoreactive B-1 cells induced by activated gamma/delta T cells in antierythrocyte immunoglobulin transgenic mice. J. Exp. Med. 192:1577-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zinkernagel, R. M. 2000. What is missing in immunology to understand immunity? Nat. Immunol. 1:181-185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.