Abstract
This study focuses on the interaction of the three components of the Bacillus cereus Nhe enterotoxin with particular emphasis on the functional roles of NheB and NheC. The results demonstrated that both NheB and NheC were able to bind to Vero cells directly while NheA lacked this ability. It was also shown that Nhe-induced cytotoxicity required a specific binding order of the individual components whereby the presence of NheC in the priming step as well as the presence of NheA in the final incubation step was mandatory. Priming of cells with NheB alone and addition of NheA plus NheC in the second step failed to induce toxic effects. Furthermore, in solution, excess NheC inhibited binding of NheB to Vero cells, whereas priming of cells with excess NheC resulted in full toxicity if unbound NheC was removed before addition of NheB. By using mutated NheC proteins where the two cysteine residues in the predicted β-tongue were replaced with glycine (NheCcys−) or where the entire hydrophobic stretch was deleted (NheChr−), the predicted hydrophobic β-tongue of NheC was found essential for binding to cell membranes but not for interaction with NheB in solution. All data presented here are compatible with the following model. The first step in the mode of action of Nhe is associated with binding of NheC and NheB to the cell surface and probably accompanied by conformational changes. These events allow subsequent binding of NheA, leading to cell lysis.
Bacillus cereus is a spore-forming rod-shaped bacterium, which is commonly present in food. This major food-borne pathogen is known to cause two different types of food poisoning (for reviews, see references 20 and 21), which are characterized by either emesis or diarrhea. As putative agents for the diarrheal type of illness, two different protein complexes, hemolysin BL (Hbl) and nonhemolytic enterotoxin (Nhe) (3, 14), each consisting of three exoproteins, as well as a single protein (cytotoxin K [13]), have been identified. Nhe, described by Lund and Granum (14), contains the protein components NheA (41.0 kDa), NheB (39.8 kDa), and NheC (36.5 kDa). The genes encoding the three components of the Nhe complex have been cloned, and the entire operon has been characterized (10, 11). The significance of these three component toxins of Nhe and Hbl in enterotoxicity remains unclear. However, Moravek et al. (16) have shown by quantitative enterotoxin analyses that Nhe expression levels account for most of the B. cereus-associated cytotoxic activity.
Little is known about the underlying mode of action of Nhe at the cellular level. Lindbäck et al. (11) showed that NheB binds to Vero cells and that the maximum cytotoxic activity in Vero cells was obtained when the molar ratio between NheA, -B, and -C was 10:10:1. Using B. cereus strain NVH 0075/95, it was shown that Nhe acts as a pore-forming toxin inducing cell lysis (9). Based on the crystal structure of the B component of the homologous three-component Hbl toxin (2, 15), sequence identities between all six Hbl and Nhe proteins indicate that NheB and NheC (9) show strong structural similarities to ClyA, a 34-kDa pore-forming hemolysin of several enteropathogenic Gram-negative Enterobacteriaceae (12, 19, 23). They all comprise a 4- to 5-helix bundle connected to a head subdomain with a characteristic hydrophobic β-tongue (24). In addition, functional similarities exist between the Nhe complex and ClyA, namely, formation of large conductance pores as well as cytolytic and hemolytic activity (9). In ClyA significant changes in conformation during membrane insertion and pore assembly have been predicted from electron microscopic measurements (8, 22) and the assembly mechanism of the alpha-helical pore has been recently unraveled (17).
We hypothesized that these recent publications could serve as a framework for understanding Nhe action, particularly for the role of NheB and NheC. There is, however, one major difference, namely, that ClyA is a homo-oligomeric pore former whereas Nhe requires three related proteins for toxicity. Therefore, we dissected the natural working mechanism into single steps. By addition of the single components or combinations of two components in consecutive order, it was possible to demonstrate a specific binding order necessary to achieve toxic activity. In contrast to earlier studies, both NheB and NheC were identified as binding components, whereby the hydrophobic β-tongue of NheC was essential for binding to Vero cells but not necessary in the interaction with NheB. Interaction between NheB and NheC seems to occur mainly in solution, and binding of these two components was a prerequisite to allow action of the third component (NheA), leading to cell lysis.
MATERIALS AND METHODS
B. cereus strains, culture medium, and culture conditions.
Details for the B. cereus strains used are given in Table 1. All strains lacked both hbl and cytK as demonstrated by PCR, immunoassay, and cell culture assay (25). The cytotoxic strain NVH0075/95 was isolated following a large food-poisoning outbreak in Norway (14) and produces all three components of the Nhe complex. The food isolates B. cereus MHI1672 (low cytotoxic) and MHI1761 (not cytotoxic) possess a stop codon in the 5′ end of nheC and nheA, respectively. Cells were grown in CGY medium (4) supplemented with 1% glucose or sucrose. For toxin production a 2% inoculum of an overnight culture was incubated in 50 ml CGY (in a 250-ml flask) at 32°C and shaken at 100 rpm for 5 to 6 h (until cultures reached the transition into stationary phase). Growth curves were indistinguishable between the strains, each yielding 108 CFU ml−1 after 5 to 6 h of incubation at 32°C. To inhibit proteolytic cleavage of the toxins by metalloproteases, EDTA (1 mM) was added at the time of harvesting. Cell-free supernatants (crude toxin preparations) obtained by centrifugation (10,000 × g at 4°C for 20 min) and filtration through 0.2-μm Millipore filters were stored in aliquots at −80°C. These preparations were used for cytotoxicity assays and protein purification and as coating antigens in the indirect enzyme immunoassays (EIAs). For production of recombinant Nhe components, Escherichia coli strains expressing recombinant NheA and NheC (11) were grown in brain heart infusion (BHI) medium supplemented with the desired antibiotic.
TABLE 1.
Characteristics of Bacillus cereus strains used in this studya
| B. cereus strain | Nhe cytotoxin component profile | EIA (antibody used) |
Cytotoxic activity | PCRb |
||||
|---|---|---|---|---|---|---|---|---|
| NheA (1A8) | NheB (1E11) | NheC (pAb) | nheA | nheB | nheC | |||
| MHI1672 | A, B | 640 | 1,280 | −c | 20 | + | + | + |
| MHI1761 | B, C | − | 640 | 80 | − | + | + | + |
| NVH0075/95 | A, B, C | 640 | 1,280 | 160 | 1,280 | + | + | + |
| NVH0075/95d | A, C | 640 | − | 80 | − | |||
All values represent the highest dilution of culture supernatants reacting positively in the respective test system.
According to reference 25.
Negative in the lowest dilution tested (1:5 in the EIA; 1:10 in the cytotoxicity assay).
Cells and antibodies.
Vero monkey kidney epithelial cells were grown under standard tissue culture conditions. Basic characteristics of the monoclonal antibody (MAb) 1E11 against NheB and rabbit antiserum (polyclonal antibody [pAb]) against NheC have previously been described (7). At a concentration of 10 μg ml−1, MAb 1E11 was able to neutralize the cytotoxic activity of the Nhe preparations used in this study. For immunofluorescence studies MAb 1E11 was labeled with Alexa 488 Fluor dye according to the manufacturer's instructions (Invitrogen).
Enzyme immunoassays (EIAs).
Nhe production by the B. cereus strains was tested by indirect EIAs as previously described (7). Antigen titers were defined as the reciprocal of the highest dilution of Nhe preparations that gave an absorbance value of ≥1.0.
Cloning of nheC and construction of NheCcys− and NheChr− mutants.
The sequence encoding the mature part of the NheC was PCR amplified from B. cereus NVH0075/95 genomic DNA by using primers nheC-F (GCAGAACAAAACGTAAAAATACAAC) and nheC-R (TTACTTCGCCACACCTTCAT) and cloned into the pCR T7/NT-TOPO vector (Invitrogen) to tag the N terminus with a hexahistidine label. To create NheChr−, in which the 25 amino acids comprising the hydrophobic region (Fig. 1a) were replaced with a single valine, the upstream part of nheC was PCR amplified using primers nheC-F and AACGACGTCATTACTCTTTTTAATCGAATC, encoding an AatII restriction site (underlined), and cloned into pCR T7/NT-TOPO. The downstream part of nheC was PCR amplified using primers CCGACGTCAAAAAAGATATCGCAA (AatII site underlined) and nheC-R, subcloned into pCR 2.1-TOPO (Invitrogen), excised using AatII and EcoRI, and inserted between the corresponding restriction sites in pCR T7/NT-TOPO containing the upstream part of nheC. To create NheCcys−, the two cysteine codons (Fig. 1b) in the hydrophobic segment of the cloned NheC were exchanged with glycine codons using the QuikChange site-directed mutagenesis protocol (Stratagene) and the mutagenesis primers TGGCGTACTTGGCGTAGCTCTAATAACAGGTCTTGCTGGC and CAGCAAGACCTGTTATTAGAGCTACGCCAAGTACGCCACC (mutated bases underlined). Thus, all NheC preparations described in this work are histidine tagged.
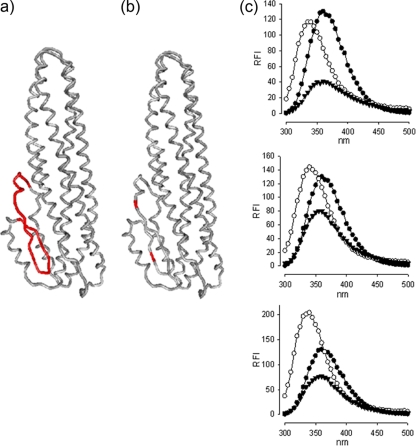
FIG. 1.
Structural models of mutated NheC. (a and b) Locations of the hydrophobic region (shown in red [a]) and the two cysteine residues (b) within NheC that were replaced to create NheChr− and NheCcys−. The structure was modeled on HblB (Protein Data Bank identification 2nrj) using Swiss Expasy and drawn using 3D-mol. (c) Intrinsic tryptophan fluorescence spectra of NheC (top), NheChr− (middle), and NheCcys− (bottom) are shown as relative fluorescence intensity (RFI). Proteins were between 5 and 8 μg ml−1 in 25 mM Tris buffer, pH 7.4, at 25°C, and fluorescence scans are shown before (○) and after (▾) 5 min in 8 M urea. Buffer traces are subtracted. The data points are shown only for every 5 nm to aid clarity. Tryptophan HCl (•, 1 μM) is shown for reference in all traces. The absolute RFI values differ due to the variation in concentration of the different NheC preparations. Note the red shift in all three proteins in 8 M urea as well as the drop in fluorescence intensity.
Intrinsic tryptophan fluorescence.
Intrinsic tryptophan fluorescence spectra of the recombinant NheC, NheChr−, and NheCcys− were measured using a 295-nm excitation wavelength in a Perkin-Elmer LS55 fluorimeter. The proteins, prepared in 25 mM Tris buffer, pH 7.6, were held at 25°C in a thermostatically controlled cuvette. Emission spectra were recorded in the absence and then in the presence of 8 M urea at 200 nm per minute, using 2.5-/4-nm excitation/emission slit widths. Tryptophan HCl (Sigma) at 1 μM was used as a reference. Buffer readings were automatically subtracted.
Purification of Nhe components.
NheB was purified from 5- to 6-h culture supernatants of B. cereus NVH0075/95 and MHI1672 as described previously (7, 11), and purity was documented by SDS-PAGE (7). The three recombinant NheC hexahistidine-tagged proteins were purified as previously described (11). To assess the extent to which such mutations altered protein folding, we monitored tryptophan fluorescence of the three proteins before and after exposure to 8 M urea.
Immunofluorescence microscopy.
Vero cells, cultivated in 8-well Lab-Tek chamber slides (Nunc), were treated with NheB purified by immunoaffinity chromatography (IAC) or cell-free B. cereus supernatants from MHI 1761 containing NheB and NheC for 2 h at 37°C. The cell labeling protocol for immunofluorescence microscopy was as follows: cells were fixed with ice-cold methanol for 10 min, permeabilized with 0.5% Triton X-100, and blocked with 5% inactivated goat serum for 60 min. Then, Alexa Fluor dye-labeled MAb 1E11 against NheB was added at a concentration of 4 μg per well and incubated for 1 h. For all immunoreagents, phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) was used as a diluent. Finally, nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole) and micrographs were taken on a BZ-8000 fluorescence microscope (Keyence).
Binding of NheC to Vero cells.
As the polyclonal antiserum against NheC was not suited for immunofluorescence studies, an indirect approach was chosen to demonstrate the binding of NheC to Vero cells. NheC, Nhecys−, and NheChr−, 350 ng of each, were added to separate wells in a 24-well tray containing Vero cell monolayers and incubated for 60 min at 37°C. The monolayers were washed five times with Eagle minimal essential medium (MEM) and then suspended in 50 μl SDS-PAGE sample buffer. Twenty microliters of each sample was blotted as described below.
Inhibition of binding of NheB to Vero cells in solution by NheC.
Combinations of NheB and recombinant His-NheC protein (shown previously to be fully active [11]) were added to wells in a 24-well tray containing Vero cell monolayers and incubated for 30 min at 37°C. The monolayers were washed five times with MEM and then suspended in 50 μl SDS-PAGE sample buffer. Twenty microliters of each sample was blotted as described below.
SDS-PAGE and immunoblotting.
Samples were applied to 4 to 12% gradient SDS-PAGE gels (Invitrogen) and transferred to nitrocellulose membranes (Bio-Rad) by Western immunoblotting. NheB was detected on Western blots using MAb 1E11. NheC was detected using polyclonal antisera (7) from a rabbit immunized with a peptide sequence derived from NheC, at a dilution of 1:500. Biotin-conjugated anti-mouse or anti-rabbit antibodies (Amersham Biosciences) were used as secondary antibodies (1:3,000). A complex of streptavidin (Bio-Rad) and biotinylated alkaline phosphatase (Bio-Rad) was used at a dilution of 1:3,000, prior to development with nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (Bio-Rad).
Cytotoxicity assays.
Cytotoxic activity of Nhe preparations and B. cereus culture supernatants was determined as an endpoint titer under simultaneous incubation conditions (Fig. 2) using Vero cells as previously described (6). Briefly, serial dilutions of the single components or supernatants were placed into microtiter plates (0.1 ml per well) and Vero cell suspensions (0.1 ml; 2 × 104 cells per well) were added immediately afterwards. The growth medium and diluent consisted of MEM (Biochrom KG) with Earle salts supplemented with 1% fetal calf serum and 2 mmol glutamine liter−1. The test mixture was incubated for 24 h at 37°C in a 5% CO2 atmosphere, and then the mitochondrial activity of viable cells was determined at 450 nm by using the tetrazolium salt WST-1. The resulting dose-response curve was used to calculate the 50% inhibitory concentration (expressed as the reciprocal dilution that resulted in 50% loss of mitochondrial activity) by linear interpolation. Endpoint titers were defined as the highest dilution of Nhe-containing preparations that inhibited cell proliferation by more than 50%.
FIG. 2.
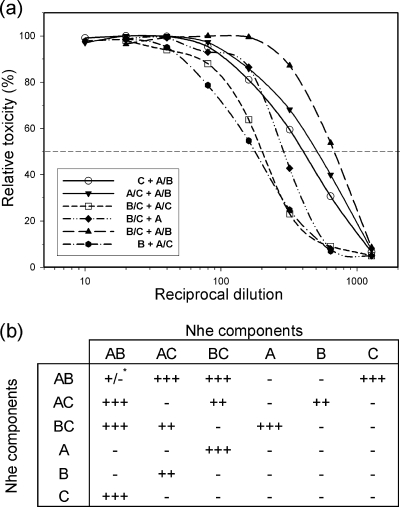
Simultaneous incubation of different combinations of Nhe components with Vero cells. (a) Dose-response curves obtained by adding serial dilutions of the indicated mixtures of Nhe components to the cells at the same time (at least three independent experiments were performed for each combination; a representative curve is shown for toxic combinations). (b) Table showing semiquantitative results based on the 50% levels of the dose-response curves (horizontal dashed line in panel a). Reciprocal cytotoxicity titers were classified as follows: −, titers of ≤10; +/−, 11 to 40; +, 41 to 100; ++, 101 to 250; +++, ≥250. *, reciprocal cytotoxicity titer of 20 as indicated in Table 1 (curve not depicted).
In order to address the question of whether Nhe components bind independently to cells or whether one binds via the other, the Vero cell assay was modified (consecutive testing order). At first, cells were primed for 24 h with serial dilutions of single components or supernatants containing two Nhe components (details are in Fig. 3). Following the 24-h incubation step, the cells were washed four times with cell culture medium to remove unbound toxin components. Then, 0.1 ml of fixed dilutions of Nhe components (alone or as a mixture) was added and incubated for a further 2 h at 37°C. Cell viability was subsequently determined by addition of WST-1.
FIG. 3.
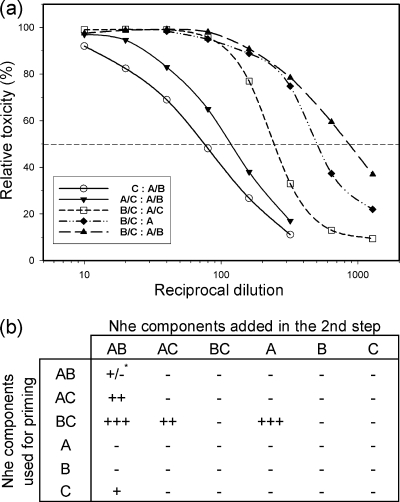
Consecutive incubation of different combinations of Nhe components with Vero cells. (a) Dose-response curves obtained by priming cells with serial dilutions of the Nhe preparations as stated in the table (b) (priming step), washing them, and reexposing them to single or combined components in fixed concentrations as stated in the table (b) (second incubation step). At least three independent experiments were performed for each combination; a representative curve is shown for toxic combinations. (b) Table showing semiquantitative results based on the 50% levels of the dose-response curves (horizontal dashed line in panel a). Reciprocal cytotoxicity titers were classified as follows: −, titers of ≤10; +/−, 11 to 40; +, 41 to 100; ++, 101 to 250; +++, ≥250. *, reciprocal cytotoxicity titer of 20 as indicated in Table 1 (curve not depicted).
To test the short-term effects of Nhe preparations on Vero cells, lactate dehydrogenase (LDH) cytotoxicity assays were used. All experiments were carried out in the following extracellular bathing solution (referred to as EC buffer) containing 135 mM NaCl, 15 mM HEPES, 1 mM MgCl2, 1 mM CaCl2, and 10 mM glucose adjusted to pH 7.2 with Tris. LDH release from confluent Vero cells grown in 6-well trays was measured as described previously (9). Briefly, tissue culture medium was removed and cells were bathed in prewarmed EC buffer for no less than 15 min to allow the cells to equilibrate to the buffer conditions. Buffer was replaced by various concentrations of B. cereus culture supernatants and/or NheC in a final volume of 2 ml of EC buffer, and cells were incubated for up to 30 min at 37°C. LDH in the EC buffer was measured at timed intervals. Aliquots of each well were collected and centrifuged briefly to deposit the cell debris, following which samples were analyzed for LDH concentration on an Advia 1650 autoanalyzer (Bayer AG, Leverkusen, Germany). To measure total cell monolayer LDH content, cells were lysed with a 1% solution of Triton X-100. A sample was also taken from a blank well as a negative control to measure spontaneous LDH release.
Coimmunoprecipitation (co-IP) of NheB and NheC.
To demonstrate the interaction of NheB and NheC in solution, two concentrations of NheC (1 μg/ml or 0.1 μg/ml) were incubated for 1 h with a fixed amount of NheB (1 μg/ml) in a total volume of 0.4 ml PBS. The complex was then captured for 1 h at room temperature by adding 40 μl of Sepharose-bound MAb 1E11 against NheB to the solution. The gel had been prepared according to the instructions of the manufacturer by addition of 2.8 mg of antibody per ml of CNBr-activated Sepharose 4B (GE Healthcare). The gel was collected by centrifugation and washed with PBS. Finally, the pellets were resuspended in 100 μl of electrophoresis loading buffer, separated by SDS-PAGE (12% Bis-Tris Criterion XT gel; Bio-Rad), and analyzed by Western immunoblotting using the polyclonal antiserum (1:500) against NheC (7) and MAb 1E11 (2 μg/ml) against NheB. Primary antibody binding was visualized with secondary peroxidase-labeled antibodies (1:2,000; Cell Signaling) and chemiluminescence using SuperSignal West Femto maximum-sensitivity substrate (Thermo Fisher Scientific).
RESULTS
B. cereus strains and recombinant proteins.
Comprehensive screening of more than 500 B. cereus isolates for Nhe expression identified two strains which showed, despite positive PCR results, no or weak reactivity in the EIA of either NheA or NheC (Table 1). By sequencing of the respective genes, it was found that the strains possessed a stop codon in the 5′ end of nheA (strain MHI1761; EMBL accession no. FN825684) or nheC (strain MHI1672; EMBL accession no. FN825685) accompanied by incomplete expression of the Nhe complex. As a consequence, culture supernatants of these strains showed no (MHI1761) or only weak (MHI1672; about 2% of full activity) cytotoxic activities (Table 1). These isolates were used to elucidate the Nhe-associated mode of action together with recently developed antibodies and purified (NheB) and recombinant (NheA and NheC) proteins. In addition, NheC mutants were created in which the 25 amino acids of the β-tongue of NheC were totally replaced with valine (NheChr−) or the two cysteines were replaced by glycine (Fig. 1a and b). It is assumed that any alterations to the normal folding of the recombinant proteins will be restricted to the β-tongue since the intrinsic fluorescence of the tryptophan residues located in the helical stretches of all three NheC preparations was reduced and redshifted following denaturation with 8 M urea (Fig. 1c).
Nhe cytotoxicity requires a specific cell binding sequence.
To address whether Nhe components show a compulsory binding order, we conducted a series of Vero cell assays using different incubation conditions (Fig. 2 and 3). NheB purified from B. cereus strain MHI1672 by immunoaffinity chromatography was used for these experiments alongside recombinant NheA and NheC as well as unpurified supernatants from B. cereus mutants lacking NheA (MHI1761) or NheC (MHI1672). Supernatant of strain NVH0075/95 from which NheB had been removed by immunoaffinity chromatography (Table 1) was used as an NheA- plus -C-containing preparation. Since it had been shown previously that an approximately 10:10:1 concentration ratio between NheA, NheB, and NheC, respectively, was optimal for generating toxicity (11), the starting dilutions contained approximately 1 μg/ml of NheA, 1 μg/ml of NheB, and 0.1 μg/ml of NheC. To test for actual toxicity, Vero cells were exposed to serial dilutions of combinations of these preparations added at the same time (simultaneous incubation; Fig. 2). The summary of the results (Fig. 2b) clearly shows that preparations containing only one or two of the Nhe components lacked toxicity, except for MHI1672 supernatants, which showed about 2% of wild-type toxicity in the Vero cell assay.
For the consecutive approach (Fig. 3), cells were primed with serial dilutions of single components of Nhe or combinations of two components, washed, and reexposed to single or combined components in fixed concentrations as indicated in Fig. 3b. Applying MHI1672 supernatants in the priming and second incubation steps resulted in low toxicity as observed under simultaneous conditions. However, significant cytotoxic effects could be induced only if the cells were primed with preparations containing either NheB plus -C or NheC alone (Fig. 3b). Moreover, by priming cells with a combination of NheB plus -C and addition of NheA or any preparation containing NheA after washing, cytotoxic activities with titers comparable to those for simultaneous incubation with all three components were observed. Under the experimental conditions used (ratio of NheC to NheB, 1:10), priming cells with only NheC resulted in lower cytotoxicity titers than did NheB plus -C priming of Vero cells. However, as shown below, applying inhibiting concentrations of NheC in the first step and removing excess NheC by washing resulted in toxicity comparable to that under optimized simultaneous conditions. Finally, priming of cells with NheB alone and addition of NheA plus -C in the second step failed to induce toxic effects. These results indicate that NheC binding to cells was essential for full toxicity. Surprisingly, priming with NheB did not induce toxic effects, despite earlier observations in which binding of this component to Vero cell membranes was recorded (11). To induce toxicity, the presence of NheA in the priming step was not necessary but was mandatory in the final incubation step, and binding of NheA alone could not be shown.
NheB and NheC bind to Vero cells, and binding of NheC is dependent on the β-tongue.
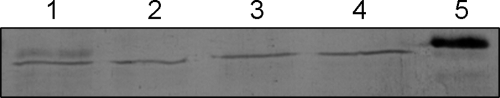
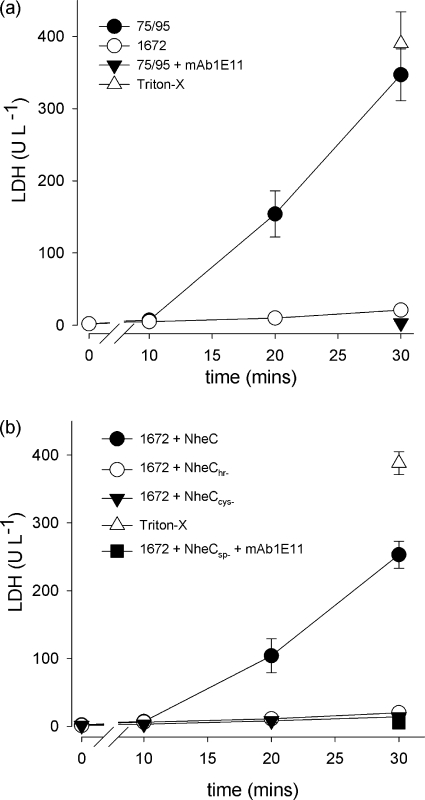
In order to reassess the question whether NheB is able to bind directly to Vero cells, NheB obtained from culture supernatants of strain MHI1672 (not producing NheC) and purified by immunoaffinity chromatography as well as culture supernatants of B. cereus MHI1761 (not producing NheA) was added to Vero cells and stained with fluorescence-labeled antibody 1E11 (Fig. 4). The staining patterns were similar for MHI1761 supernatant and purified NheB. Since the rabbit polyclonal antiserum against NheC was not suitable for immunofluorescence, we used an indirect assay to detect the binding of NheC to Vero cells. The recombinant NheC proteins were incubated with Vero cell monolayers and washed, and then the cells were solubilized in SDS-PAGE buffer directly before Western blotting. Figure 5 shows the presence of an appropriately sized band for NheC in the Vero cells exposed to the wild-type protein but not with the two mutated NheC proteins lacking either the entire hydrophobic region (NheChr−) or with the two cysteines (NheCcys−) replaced by glycine (Fig. 1). Also, addition of either of the mutated NheC proteins to culture supernatants (before addition to Vero cells) of MHI1672 did not induce LDH release (Fig. 6b). These data indicate that NheB and NheC bind directly to Vero cell membranes and that the β-tongue is essential for NheC binding.
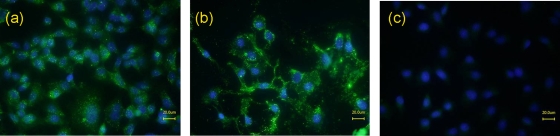
FIG. 4.
Binding of NheB to Vero cells. (a and b) Cells were treated with culture supernatants of B. cereus strain MHI1761 (diluted 1:30; containing NheB and NheC) (a) or IAC-purified NheB (2 μg/ml) (b) and stained with Alexa 488-labeled anti-NheB MAb 1E11. (c) Negative control (NheB replaced by buffer).
FIG. 5.
Binding of NheC to Vero cells. Immunoblot of Vero cells exposed to NheC and the two mutated NheC proteins. Lane 1 shows an appropriate-size band of NheC consistent with NheC binding to Vero cells, whereas lanes 2 (NheCcys−) and 3 (NheChr−) do not. Lane 4 is the Vero cell preparation without added NheC, and lane 5 shows the NheC alone. Note that the lower-molecular-weight band in lanes 1 to 4 is a cross-reacting protein present in Vero cells.
FIG. 6.
LDH release from Vero cell monolayers by Nhe. (a) Extracellular LDH measured over time following exposure of 106 Vero cell monolayers to culture supernatants of NVH0075/95 (•, 25 μl) and MHI1672 (○, 50 μl). The effect of 10 μg ml−1 MAb 1E11 on NVH0075/95 is shown after 30 min only (▾). Maximum LDH release induced by 1% (vol/vol) Triton X-100 is indicated by ▵. Values are means ± standard errors from between 3 and 7 separate wells. (b) Extracellular LDH measured over time following exposure to culture supernatants of MHI1672 (50 μl in 2 ml EC buffer) supplemented by addition of 5 ng NheC (•), NheChr− (○), and NheCcys− (▾). The effect of 10 μg ml−1 MAb 1E11 on MHI1672 plus NheC is shown after 30 min only (▪). Maximum LDH release induced by 1% (vol/vol) Triton X-100 is indicated by ▵. Values are means ± standard errors from between 3 and 7 separate wells.
Maximum LDH release of Vero cells depends on the concentration of NheC.
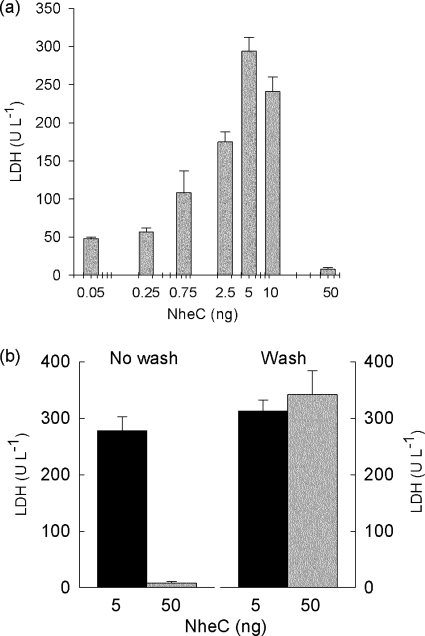
To measure the time course of cell lysis induced by Nhe, we used LDH release as a marker of increased plasma membrane permeability to further elucidate the role of NheC. A 1:80 dilution of a culture supernatant of B. cereus NVH0075/95 induced LDH release when exposed to Vero cell monolayers after 10 to 20 min (Fig. 6a), whereas a 2-fold-higher concentration of B. cereus MHI1672, a strain that does not produce NheC, failed to produce toxic effects within 30 min (Fig. 6a). The dependence on all three Nhe components to induce LDH release was examined by supplementing culture supernatants of MHI1672 with recombinant NheC (before addition to Vero cells). Figure 6b shows the increased LDH level in Vero cell suspensions when recombinant NheC (approximately 5 ng) was added to MHI1672 culture supernatants. This effect was abolished by exposure to MAb 1E11 raised against NheB. In addition, with use of supernatants from strain MHI1672 (NheC negative), restoration of cytotoxicity by NheC was dose dependent up to a threshold concentration (Fig. 7a). Using 50 ng NheC failed to induce significant LDH release from Vero cells.
FIG. 7.
Threshold inhibition of LDH release by NheC. (a) Concentration-dependent increase in LDH release from 106 Vero cells following exposure to increasing amounts of NheC added to 50 μl MHI1672 culture supernatants in 2-ml final volumes (mean ± standard error, n = 3 for each concentration). (b) LDH release from Vero cells after exposure to 5 ng (black bars) or 50 ng (gray bars) for 15 min before addition of AB (culture supernatant of MHI1672). The right-hand bars show LDH release when cells were washed before addition of AB, while the left-hand bars show LDH release when AB was added to the Vero cell monolayer without removal of excess C (mean ± standard error, n = 3 for each data set).
If, however, Vero cells were exposed to NheC alone (50 ng) and unbound NheC was removed before subsequent addition of NheA plus -B in the form of MHI1672 culture supernatants, toxic activity was maintained. Figure 7b shows that despite the addition of “excess” amounts of NheC (50 ng) full cytotoxicity was observed, if the cell monolayers were washed with buffer (“wash”) before NheA and -B were added. In contrast, cytotoxicity was negligible if excess NheC was left in the bathing solution (Fig. 7b, “No wash”). These results indicate that the level of NheC in relation to NheB is critical in solution, but that is not the case when cells are saturated with NheC and unbound NheC is subsequently removed.
Excess NheC inhibits binding of NheB to Vero cells.
To indicate at which step excess NheC is able to inhibit cytotoxicity, we tested the effect of NheC on binding of NheB. NheB and NheC were incubated with Vero cell monolayers, and then the cells were washed and solubilized in SDS-PAGE buffer directly before Western blotting. Comparison of the immunoblots of NheB binding to Vero cells in the presence of “cytotoxic” (5 ng) and “excess” (50 ng) NheC indicates that binding of NheB to Vero cells is impaired when NheC is mixed with purified NheB at a high concentration (Fig. 8), before addition to the Vero cell monolayer. The reduced intensity of the NheB bands was also seen when NheB was mixed with NheChr− and NheCcys−. Thus, inhibition of binding of NheB to Vero cells by NheC is not dependent on the β-tongue region of NheC. Again, inhibition of binding of NheB is not observed if excess NheC is washed away from the cell monolayer before addition of NheB (Fig. 8, lane 4).
FIG. 8.
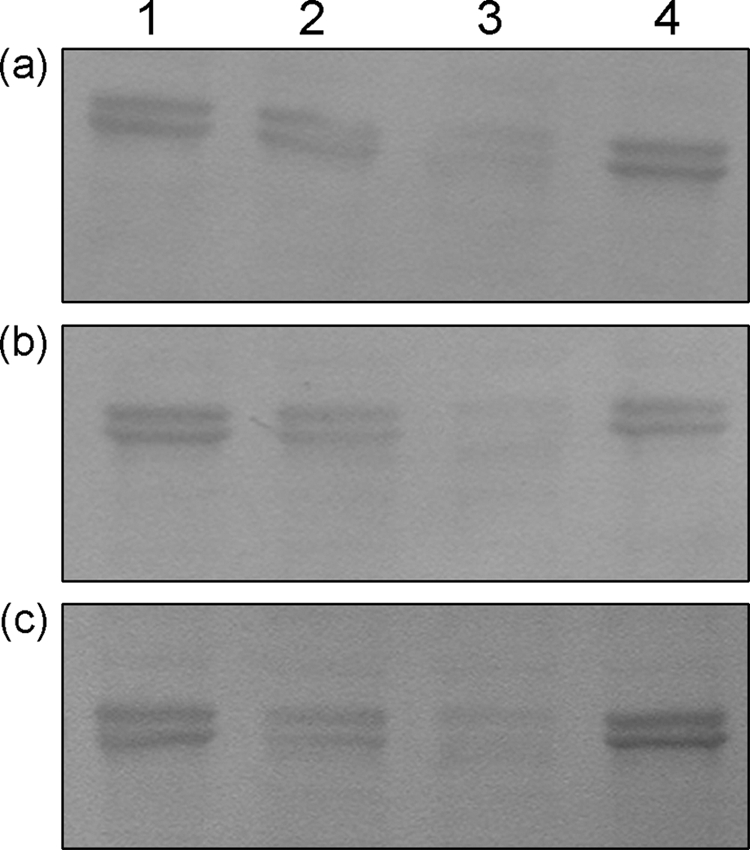
NheC inhibits binding of NheB to Vero cells. Immunoblots against NheB were developed from Vero cells incubated with NheB alone (lane 1). Lanes 2 and 3 show the effect of preincubating optimal amounts of NheC (lane 2) or excess amounts of NheC (lane 3) with NheB for 10 min at 37°C before addition to Vero cell monolayers. Lane 4 shows that the effect of excess NheC incubated with the Vero monolayers can be removed by washing prior to addition of NheB. In all experiments the NheB concentration was approximately 10 nM and recombinant NheC protein concentrations were either 1 nM (optimal) or 10 to 20 nM (excess). (a) Results obtained using NheC. (b) Results obtained using NheChr−. (c) Results obtained using NheCcys−. With all three NheC proteins the NheB signal was reduced when excess NheC was mixed with NheB in solution prior to incubation on the Vero cell monolayers. Note that the lower-molecular-weight band in lanes 1 to 4 is an N-terminal nicked form of NheB (14).
To provide direct evidence for interaction between NheB and NheC in solution, a co-IP experiment was carried out (Fig. 9). NheB and NheC were mixed in an approximately equimolar ratio as well as in a 10:1 ratio. The resulting NheB/C complexes were pulled down by MAb 1E11 (against NheB) immobilized on Sepharose. The presence of captured NheC and NheB in the immunoprecipitate was detected by Western blotting using polyclonal antiserum against the C-terminal part of NheC and MAb 1E11, respectively. The intensities of the NheC bands (Fig. 9a) after co-IP indicate that NheC at low as well as at high concentrations was almost completely immunoprecipitated together with NheB (Fig. 9b). The interaction of the two components did not result in any degradation, indicating that neither NheC nor NheB possesses proteolytic activity. The double band seen for NheC resulted from a mixture of N-terminally His-tagged (upper band) and His-tag-cleaved NheC present in the recombinant NheC preparation, where the two seem to bind equally well to NheB in solution.
FIG. 9.
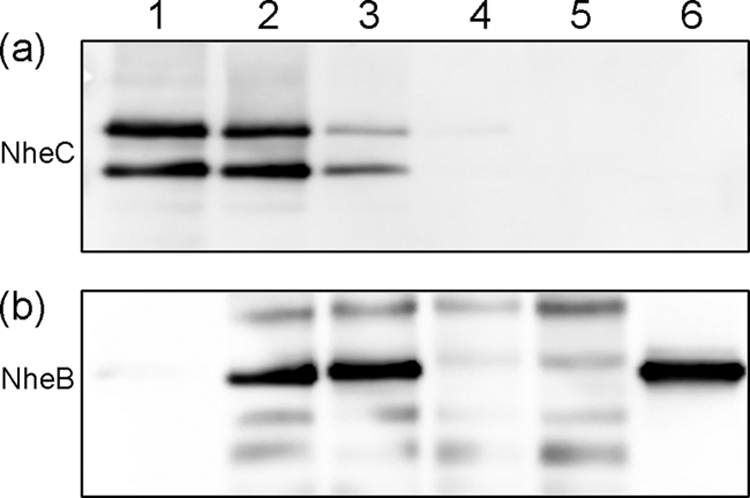
Coimmunoprecipitation of NheB and NheC. NheB and NheC were incubated at a 1:1 (lane 2) and a 10:1 (lane 3) ratio for 1 h and then immunoprecipitated by adding Sepharose-bound MAb 1E11 against NheB. Lanes 1 and 6 show the NheC and NheB preparations used for this experiment. Trials without NheB (lane 4) and those without NheB and NheC (lane 5) served as controls. (a) Immunoprecipitated NheC visualized by immunoblotting after SDS-PAGE using polyclonal rabbit antiserum against NheC. (b) Immunoprecipitated NheB visualized by immunoblotting after SDS-PAGE using MAb 1E11 against NheB. Minor bands represent degradation products of the mouse monoclonal antibody used for IP reacting with the secondary anti-mouse antibody.
DISCUSSION
Among the several hundred bacterial toxins described, the B. cereus Hbl and Nhe cytolysins are unique in their composition of three distinct proteins necessary to induce toxic activity on Vero and Caco-2 cells. The mechanism by which these complexes exhibit their biological activity is not yet clear, although some theories have been proposed. For Hbl a membrane attack complex rather than an enzymatic degradation of the membrane of erythrocytes has been suggested (5). Recently published data indicate that Nhe is a pore-forming toxin, and both NheB and NheC are mostly α-helical molecules showing structural similarities to ClyA (9). Nhe requires a defined ratio of the individual components for maximum activity, and NheB was thought to be the binding component (11). In-depth studies on the mode of action have so far been hindered by the lack of a full set of biologically active recombinant proteins; in particular, all efforts to express active NheB from E. coli failed. Therefore, the strategy used here was to combine recombinant proteins (NheA and NheC) with mutant strains effectively lacking either NheA (MHI1761) or NheC (MHI1672). In addition, when necessary, NheB purified from supernatants of strain MHI1672 was used to avoid biased results due to traces of copurified NheC. These tools enabled us to examine the role of NheB and NheC in toxicity to Vero cells in more detail and to propose a sequence of events necessary for toxic activity which was clearly due to Nhe and not other contaminating proteins for the following reasons: (i) cell-free supernatants of MHI1761 and MHI1672 showed no or negligible toxicity; (ii) a monoclonal antibody specific to NheB completely abolished toxicity; (iii) when unpurified culture supernatants of these mutants were supplemented (before addition to Vero cells) with the missing components (recombinant NheA or NheC), cytotoxic activity could be restored; (iv) the site-directed deletion and cysteine mutants of NheC were markedly impaired in their ability to induce cytotoxicity when added to the other two components; and (v) purified NheB and recombinant NheA and NheC alone were not toxic.
From two-component toxins, such as staphylococcal gamma-hemolysin and the related leukocidins, it is known that the components show a compulsory cell-binding order (1). In order to address this question in terms of Nhe compounds, we conducted a Vero cell assay using consecutive incubation conditions. From the data shown in Fig. 3, it appears that toxic effects comparable to the wild-type toxicity were observable only if cells were primed either with NheC alone or with a mixture of NheB and NheC. This unexpected finding prompted us to reassess binding of NheB and to verify binding of NheC to Vero cells. Fluorescently labeled antibodies against NheB permitted the first microscopical images of NheB attached to Vero cells, using either purified protein or supernatant of MHI1761 containing both NheB and NheC (Fig. 4). Fluorescence microscopy did not show a significant difference between cells incubated with NheB alone and cells incubated with NheB and NheC simultaneously, although Nhe exhibited cytotoxicity only if either NheB plus -C or NheC was already attached to the cell surface before addition of the other components (Fig. 3b). NheC binding to Vero cells could be verified by immunoblotting (Fig. 5). Interestingly, incubation with only NheA and NheB (supernatant of MHI1672) gave low but detectable toxicity in the WST assay (Table 1; Fig. 2 and 3), which could be neutralized by MAb 1E11 (data not shown). This finding indicates that NheA and NheB alone may be sufficient for some (about 2% of wild-type toxicity) toxic activity (Fig. 2b and 3b), which is greatly enhanced by the presence of NheC. It is important to note that all experiments were done with Vero cells, and it currently cannot be excluded that NheA and NheB alone could be effective in other cell lines. In Vero cells, however, both NheB and NheC can be considered binding components, whereas binding of NheA to the cell surface cannot be shown. Obviously there must be some interaction between the single components either in solution or after membrane association.
Our data show that the presence of NheC in the first step as well as the presence of NheA in the final step was mandatory for cytotoxicity in Vero cells (Fig. 3b). Furthermore, priming Vero cells with NheB and subsequent addition of NheA and NheC in the second step did not induce cytotoxicity. According to the ClyA model (17), NheB may undergo a substantial change in tertiary structure during cell binding which could prevent association with NheC. If so, this conformational change does not disrupt the epitope for binding of MAb 1E11 (Fig. 4). While it cannot be ruled out that NheB alone may be internalized by the cells, our data suggest that Nhe complex formation between the two components can occur in solution before cell binding as well as by association of the two compounds on the cell surface provided that NheC is already attached. Evidence for complex formation in solution has been provided by immunoblotting experiments on native gels, where the individual Nhe bands disappeared when NheB and NheC were combined (11), whereas interaction between NheA and NheB could not be shown. Thus, it was suggested that NheC functions as a “catalyst,” either by bringing NheA and NheB together (after binding of NheB to the target cells) or by enhancing conformational changes in another component(s) (11). However, data presented here show that we found no significant toxicity when cells were primed with NheA plus -B or NheA plus -C and the respective third component was added in a second step. Also, no toxic action could be provoked if cells were primed with NheB and exposed to both NheC and NheA in the second step. These observations cannot be explained if NheC acts as a “transporter” to carry NheA to NheB.
Previously, it was found that a concentration of NheC higher than about 10% of that of NheA and NheB inhibited toxic activity (11). This inhibitory effect by NheC resembles the “paradoxical zone phenomenon” observed with Hbl (5) in which the inhibitory effect of HblB on hemolysis by the three Hbl components occurred only when HblB exceeded a critical “threshold concentration.” The new finding here is that NheC exerts its threshold inhibition by interacting with NheB only in solution before either protein has bound to the Vero cell membranes (Fig. 7 and 9).
These results prompted us to further elucidate the role of NheC in formation of an active toxin complex, and the data presented here indicate a number of properties of NheC. The nontoxic mutant proteins show that the hydrophobic stretch predicted to show a β-tongue fold is essential for binding to Vero cells but not necessary in the interaction of NheC with NheB in solution. Based on common structural and functional properties, it was recently proposed that ClyA and the Hbl and Nhe toxin family constitute a new superfamily of pore-forming toxins (9). The β-tongue in ClyA is proposed to be the region of the toxin necessary for the initial membrane binding event for the ClyA monomers (17), and thus, it is tempting to suggest a similar function for NheC. As expected from earlier studies on ClyA (18, 24), the two mutated proteins could not support cytotoxicity. The finding that NheCcys− was markedly impaired in restoring cytotoxicity indicates that the two cysteines are functionally important within the predicted β-tongue. The two cysteines in ClyA are located on two distinct helices (αB2 and αG) whereas the two cysteines in NheC are in a CXXXXXC motif, and the function remains undefined in both proteins. It is possible that the replacement of cysteines with glycine abolished cytotoxicity through simple structural disfiguration of NheC rather than the cysteines being the two critical residues necessary for cytotoxic activity. If so, such changes are likely to be limited to the predicted β-tongue, as we could not detect alterations in the fluorescence of the tryptophans located in the helical stretches (Fig. 1c). Furthermore, the mutant NheC proteins retained the ability to interact with NheB (Fig. 8), a finding which is in line with the results shown in Fig. 7b, indicating that the inhibitory threshold effect of NheC occurs before the proteins are bound to the plasma membrane. Therefore, co-IP experiments were carried out to provide direct evidence for interaction of NheC with NheB in solution. The results presented in Fig. 9 show that NheC binds to NheB and that even at a one-to-one ratio of the two components nearly all of the NheC is pulled down. From that, it could be speculated that the mechanism behind the inhibitory effect of excess NheC is the formation of NheB plus -C complexes with a high NheC content which are substantially impaired in cell binding.
In conclusion we have been able to identify roles for both NheB and NheC in the mode of action of Nhe, i.e., membrane binding and complex formation, whereas NheA seems to trigger toxicity by an unknown mechanism. The experimental data provide evidence that NheB and NheC are able to attach directly to Vero cells and that the predicted hydrophobic β-tongue of NheC is essential for this activity. It was also shown that Nhe-induced cytotoxicity requires a specific binding order of the individual components whereby the presence of NheB and NheC in the priming step as well as the presence of NheA in the final incubation step was mandatory. Priming of cells with NheB alone and addition of NheA plus -C in the second step failed to induce toxic effects. In addition, it was found that in solution excess NheC forms NheB plus -C complexes which impair binding of NheB to Vero cells. On the other hand, priming of cells with excess NheC resulted in full toxicity if unbound NheC was removed before addition of NheB. These results indicate a key role of NheC during toxic activity. In sum these data would suggest that the first step in the mode of action of the nonhemolytic enterotoxin is attachment of NheB and NheC to the cell surface, which does not need the presence of NheA. This step seems to require hetero-oligomer formation between NheB and NheC at a defined ratio to mediate ordered binding. According to the ClyA model, these events are likely to be accompanied by conformational changes, which allow subsequent binding of NheA and pore formation.
Acknowledgments
Both research groups (Oslo and Munich) contributed equally to this work.
We thank Brunhilde Minich, Kristin O'Sullivan, Franziska Witzko, and Diana Hermann for excellent technical assistance.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 12 July 2010.
REFERENCES
- 1.Alouf, J. E. 2003. Molecular features of the cytolytic pore-forming bacterial protein toxins. Folia Microbiol. 48:5-16. [DOI] [PubMed] [Google Scholar]
- 2.Beecher, D. J., and J. D. Macmillan. 1991. Characterization of the components of hemolysin BL from Bacillus cereus. Infect. Immun. 59:1778-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beecher, D. J., J. L. Schoeni, and A. C. Wong. 1995. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 63:4423-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beecher, D. J., and A. C. L. Wong. 1994. Improved purification and characterization of hemolysin BL, a hemolytic dermonecrotic vascular-permeability factor from Bacillus cereus. Infect. Immun. 62:980-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beecher, D. J., and A. C. Wong. 1997. Tripartite hemolysin BL from Bacillus cereus. Hemolytic analysis of component interactions and a model for its characteristic paradoxical zone phenomenon. J. Biol. Chem. 272:233-239. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich, R., C. Fella, S. Strich, and E. Märtlbauer. 1999. Production and characterization of monoclonal antibodies against the hemolysin BL enterotoxin complex produced by Bacillus cereus. Appl. Environ. Microbiol. 65:4470-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietrich, R., M. Moravek, C. Bürk, P. E. Granum, and E. Märtlbauer. 2005. Production and characterization of antibodies against each of the three subunits of the Bacillus cereus nonhemolytic enterotoxin complex. Appl. Environ. Microbiol. 71:8214-8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eifler, N., M. Vetsch, M. Gregorini, P. Ringler, M. Chami, A. Philippsen, A. Fritz, S. A. Müller, R. Glockshuber, A. Engel, and U. Grauschopf. 2006. Cytotoxin ClyA from Escherichia coli assembles to a 13-meric pore independent of its redox-state. EMBO J. 25:2652-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagerlund, A., T. Lindbäck, A. K. Storset, P. E. Granum, and S. P. Hardy. 2008. Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HIyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 154:693-704. [DOI] [PubMed] [Google Scholar]
- 10.Granum, P. E., K. O'Sullivan, and T. Lund. 1999. The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol. Lett. 177:225-229. [DOI] [PubMed] [Google Scholar]
- 11.Lindbäck, T., A. Fagerlund, M. S. Rodland, and P. E. Granum. 2004. Characterization of the Bacillus cereus Nhe enterotoxin. Microbiology 150:3959-3967. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig, A., C. von Rhein, S. Bauer, C. Hüttinger, and W. Goebel. 2004. Molecular analysis of cytolysin A (ClyA) in pathogenic Escherichia coli strains. J. Bacteriol. 186:5311-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund, T., M. L. De Buyser, and P. E. Granum. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38:254-261. [DOI] [PubMed] [Google Scholar]
- 14.Lund, T., and P. E. Granum. 1996. Characterisation of a non-haemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbiol. Lett. 141:151-156. [DOI] [PubMed] [Google Scholar]
- 15.Madegowda, M., S. Eswaramoorthy, S. K. Burley, and S. Swaminathan. 2008. X-ray crystal structure of the B component of hemolysin BL from Bacillus cereus. Proteins 71:534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moravek, M., R. Dietrich, C. Buerk, V. Broussolle, M. H. Guinebretiere, P. E. Granum, C. Nguyen-The, and E. Märtlbauer. 2006. Determination of the toxic potential of Bacillus cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol. Lett. 257:293-298. [DOI] [PubMed] [Google Scholar]
- 17.Mueller, M., U. Grauschopf, T. Maier, R. Glockshuber, and N. Ban. 2009. The structure of a cytolytic α-helical toxin pore reveals its assembly mechanism. Nature 459:726-730. [DOI] [PubMed] [Google Scholar]
- 18.Oscarsson, J., Y. Mizunoe, L. Li, X. H. Lai, A. Wieslander, and B. E. Uhlin. 1999. Molecular analysis of the cytolytic protein ClyA (SheA) from Escherichia coli. Mol. Microbiol. 32:1226-1238. [DOI] [PubMed] [Google Scholar]
- 19.Oscarsson, J., M. Westermark, S. Löfdahl, B. Olsen, H. Palmgren, Y. Mizunoe, S. N. Wai, and B. E. Uhlin. 2002. Characterization of a pore-forming cytotoxin expressed by Salmonella enterica serovars Typhi and Paratyphi A. Infect. Immun. 70:5759-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoeni, J. L., and A. C. L. Wong. 2005. Bacillus cereus food poisoning and its toxins. J. Food Prot. 68:636-648. [DOI] [PubMed] [Google Scholar]
- 21.Stenfors Arnesen, L. P., A. Fagerlund, and P. E. Granum. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579-606. [DOI] [PubMed] [Google Scholar]
- 22.Tzokov, S. B., N. R. Wyborn, T. J. Stillman, S. Jamieson, N. Czudnochowski, P. J. Artymiuk, J. Green, and P. A. Bullough. 2006. Structure of the hemolysin E (HlyE, ClyA, and SheA) channel in its membrane-bound form. J. Biol. Chem. 281:23042-23049. [DOI] [PubMed] [Google Scholar]
- 23.von Rhein, C., K. P. Hunfeld, and A. Ludwig. 2006. Serologic evidence for effective production of cytolysin A in Salmonella enterica serovars Typhi and Paratyphi A during human infection. Infect. Immun. 74:6505-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace, A. J., T. J. Stillman, A. Atkins, S. J. Jamieson, P. A. Bullough, J. Green, and P. J. Artymiuk. 2000. E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell 100:265-276. [DOI] [PubMed] [Google Scholar]
- 25.Wehrle, E., M. Moravek, R. Dietrich, C. Bürk, A. Didier, and E. Märtlbauer. 2009. Comparison of multiplex PCR, enzyme immunoassay and cell culture methods for the detection of enterotoxinogenic Bacillus cereus. J. Microbiol. Methods 78:265-270. [DOI] [PubMed] [Google Scholar]