Abstract
Acinetobacter baumannii is a pathogen of increasing medical importance with a propensity to be multidrug resistant, thereby making treatment challenging. Little is known of virulence traits in A. baumannii. To identify virulence factors and potential drug targets, random transposon (Tn) mutants derived from the A. baumannii strain AB307-0294 were screened to identify genes essential for growth in human ascites fluid in vitro, an inflammatory exudative fluid. These studies led to the identification of two genes that were predicted to be required for capsule polymerization and assembly. The first, ptk, encodes a putative protein tyrosine kinase (PTK), and the second, epsA, encodes a putative polysaccharide export outer membrane protein (EpsA). Monoclonal antibodies used in flow cytometric and Western analyses confirmed that these genes are required for a capsule-positive phenotype. A capsule-positive phenotype significantly optimized growth in human ascites fluid, survival in human serum, and survival in a rat soft tissue infection model. Importantly, the clearance of the capsule-minus mutants AB307.30 (ptk mutant, capsule minus) and AB307.45 (epsA mutant, capsule minus) was complete and durable. These data demonstrated that the K1 capsule from AB307-0294 was an important protectin. Further, these data suggested that conserved proteins, which contribute to the capsule-positive phenotype, are potential antivirulence drug targets. Therefore, the results from this study have important biologic and translational implications and, to the best of our knowledge, are the first to address the role of capsule in the pathogenesis of A. baumannii infection.
The incidence of Acinetobacter baumannii infection in all venues is increasing worldwide (17, 20, 32, 34). The majority of A. baumannii infections have been acquired in health care facilities. A. baumannii has been reported to account for 1 to 3% of hospital-acquired infections, 2 to 10% of infections in intensive care units, and, from 1995 to 2002, 1.3% of nosocomial bacteremias (18, 34, 42). Both sporadic and epidemic infections occur usually after the first week of hospitalization (4, 17, 18, 25, 44). The respiratory tract, particularly in ventilated patients (6.9% of hospital-acquired pneumonias in 2003 based on NNIS data), the urinary tract, intravascular devices, surgical sites (including in postneurosurgical meningitis patients), and decubitus or diabetic ulcers are favored sites of infection (4, 25, 44). A. baumannii has also been described to cause infections outside the health care setting, namely, severe community-acquired pneumonia (usually in alcoholics) (2, 3, 8) and infections in U.S. service members with war-related injuries (most recently, those injured in the Iraq/Kuwait/Afghanistan regions) (7, 12, 13, 24, 39) and in survivors of the Asian tsunami in 2004 (19, 28). Mortality rates associated with A. baumannii infection range from 19 to 54% (20). Mortality associated with Gram-negative bacteremia was significantly increased in patients with A. baumannii compared to other Gram-negative bacilli (GNB) (23, 35). The changing epidemiology, the increasing incidence of infection, and the significant mortality with A. baumannii establishes it as a pathogen of increasing medical importance.
The propensity for A. baumannii to be multidrug resistant (MDR) compounds the problems with our ability to control this emerging problem. The prevalence of MDR A. baumannii strains is increasing, with some exhibiting resistance to all available antimicrobials (7, 13, 22, 40, 43). Thus, treatment of infections due to A. baumannii has become challenging. Due to the evolution of MDR GNB, we are at risk for entering a postantibiotic era. A. baumannii is the “poster child” for this emerging threat (5). Further, colonization or infection with MDR A. baumannii was associated with an increased mortality compared to that of infection with MDR Pseudomonas aeruginosa (21). Unfortunately, there are virtually no new antimicrobial agents active against GNB in the pharmaceutical “pipeline.” The need to understand the biology of A. baumannii, which may lead to logical drug design or vaccine development, is more pressing than ever.
Multiple bacterial virulence factors are required for the pathogenesis of infections caused by GNB, including A. baumannii. The possession of specialized virulence genes enables pathogens to infect the host efficiently. Currently, a significant gap in our knowledge of the nature of these virulence traits in A. baumannii exists. Surface polysaccharides, such as capsule, have long been recognized to be important virulence traits in GNB. However, our knowledge of the role of capsular polysaccharide in the pathogenesis of A. baumannii infection is nonexistent. Our group has been studying the A. baumannii strain AB307-0294 as a model pathogen. In order to identify virulence factors and potential drug targets, random transposon (Tn) mutants were screened to identify genes essential for growth in human ascites fluid in vitro and subsequently in a rat soft tissue infection model. These studies led to the identification of two genes that were predicted to be required for capsule polymerization and assembly. The first, ptk, encodes a putative protein tyrosine kinase (PTK), and the second, epsA, encodes a putative polysaccharide export outer membrane protein (EpsA) (11). In this report, we establish that these genes are required for a capsule-positive phenotype and describe a major role for capsule in the pathogenesis of A. baumannii infection. These data have important translational implications and support the potential of Ptk and EpsA as drug targets.
MATERIALS AND METHODS
Bacterial strains and media.
A. baumannii strain 307-0294 (blood isolate) is an encapsulated isolate from a patient hospitalized at Erie County Medical Center, Buffalo, NY, in 1994 (38). Capsule serotypes in A. baumannii have not been defined. Therefore, we have designated the capsular serotype present in AB307-0294 K1. Derivatives of the wild-type (wt) strain AB307-0294 include AB307.30 (ptk mutant, capsule minus), AB307.30/pNLAC1 (ptk mutant with the pNLAC1 plasmid without an insert, capsule minus), AB307.30/pNLAC1::ptk (complemented ptk mutant, capsule positive), AB307.45 (epsA mutant, capsule minus), AB307.45/pNLAC1 (epsA mutant with the pNLAC1 plasmid without an insert, capsule minus), AB307.45/pNLAC1::epsA (complemented epsA mutant, capsule positive), AB307::Tn17 (lpsB mutant, deep lipopolysaccharide [LPS] core mutation, capsule positive) (27), AB307::Tn17/pSS11 (complemented lpsB mutant, wt LPS, capsule positive) (27), and AB307::Tn17/pNLAC1 (lpsB mutant with the pNLAC1 plasmid without an insert, deep LPS core mutation, capsule positive). Strains were grown in Luria-Bertani (LB) medium unless stated otherwise and were maintained at −80°C in 50% LB broth and 50% glycerol. Ascites fluid plates were made as described previously (38). For quantitative growth curves, 100% ascites fluid was utilized.
Tn mutagenesis and mutant screening.
Tn mutagenesis and screening for growth defects on ascites fluid plates was performed as described previously (38).
DNA sequencing and analysis.
The locations of the Tn insertion sites in mutant derivatives of AB307-0294 were determined by chromosomal sequencing, with priming off the EZ-Tn5 <Kan-2> Tn, as described previously (38). Sequence comparisons were performed via BLAST analysis of the nonredundant GenBank database.
Cloning of ptk and epsA and construction of complemented derivatives of AB307.30 and AB307.45.
ptk and 336 bases upstream and 474 bases downstream were cloned via PCR-mediated amplification (forward, 5′-ATTTCAGGGCTTATTGGTC-3′; reverse, 5′-TCATAAGCAGCAACGGCAG-3′). Likewise, espA and 348 bases upstream and 74 bases downstream were cloned via PCR-mediated amplification (forward, 5′-ACAAACTTCTTCTGTAGCACC-3′; reverse, 5′-AAAAATACTCTGCCATAGGG-3′). Primers contained capped SacI and AgeI sites to facilitate ligation into the vector pNLAC1 (tetracycline and ampicillin resistant). The cloned ptk and epsA genes were confirmed to be identical to those in strain AB307-0294 by bidirectional DNA sequencing. pNLAC1::ptk and pNLAC1::espA constructs were electroporated into AB307.30 and AB307.45, respectively, generating the complemented strains AB307.30/pNLAC1::ptk and AB307.30/pNLAC1::espA, respectively. In addition, pNLAC1 without the insert was electroporated into AB307.30 and AB307.45 to generate the control strains AB307.30/pNLAC1 and AB307.45/pNLAC1, respectively.
Generation of the MAb 13D6, which is specific for the K1 capsule expressed in AB307-0294.
Monoclonal antibodies (MAbs) were developed and screened as described previously (6, 26). MAbs that reacted to proteinase K-resistant, periodate-sensitive bacterial surface epitopes were selected for further analyses. The MAb 13D6 met these criteria and was shown to be directed against the K1 capsule (see “Confirmation that AB307.30 and AB307.45 are capsule-deficient derivatives of AB307-0294” below).
Flow cytometry.
Flow-cytometric assessment of antibody binding was performed as described previously (36). In brief, the appropriate strains were grown in LB medium, diluted to 1 × 106 CFU, washed in 100 μl of 1× phosphate-buffered saline (PBS) (pH 7.2), resuspended in 100 μl of 1:100-diluted hybridoma supernatants (complement inactivated), and incubated while rotating for 60 min at 37°C. Preliminary experiments established that this incubation time and dilution resulted in maximal antibody binding. Postincubation, the bacteria were washed once with 0.5 ml of 1× PBS, resuspended in 100 μl of goat anti-mouse IgG-IgM fluorescein isothiocyanate (FITC)-labeled conjugate (Caltag Laboratories, Burlingame, CA), and incubated while rotating at 37°C for 30 min. The labeled bacteria were washed, resuspended in 500 μl of 1× PBS, and transferred to 5-ml flow cytometry tubes (Falcon 352054). Antibody binding was analyzed by flow cytometry. Ten thousand bacterial events were gated in the forward-scatter versus side-scatter plot, discriminating them from “debris,” and antibody-bound-bacterial events were assessed in the FL-1 channel and are reported as percentages of positive cells or geometric mean fluorescence (GMF). Binding in the absence of antibody (PBS alone) served as the autofluorescence control, and binding with the FITC-labeled conjugate alone served as the nonspecific binding control, to which experimental samples were compared.
Phenotypic analyses of A. baumannii K1 capsule and LPS.
LPS and capsular polysaccharide preparations, obtained by treating whole-cell lysates with proteinase K, were resolved by SDS-16% polyacrylamide gel electrophoresis and subjected to Western blot analyses with MAbs 13D6 and 13C11, as described previously (27).
Rat soft tissue infection model.
The rat soft tissue infection model, approved by the University at Buffalo and Veterans Administration Institutional Animal Care Committee, was used as reported previously (37). The mean starting inocula for AB307-0294, AB307.30, and AB307.45 were 3 × 105, 2.5 × 105, and 1.1 × 105 CFU/ml, respectively.
Serum bactericidal assay.
Complement-mediated bactericidal assays were performed as described previously (37, 38).
Statistical analyses.
Data are presented as means ± standard errors of the mean (SEM). P values of 0.05/n (where n is the number of comparisons) are considered statistically significant based on the Bonferroni correction for multiple comparisons, and P values of >0.05/n but <0.05 are considered as representing a trend. To normalize in vitro and in vivo data, log10-transformed values were utilized, the area under each curve was calculated, and these areas were compared using two-tailed unpaired t tests (Prism 4 for Macintosh; GraphPad Software Inc.).
RESULTS
Identification of AB307.30 (ptk mutant, capsule minus) and AB307.45 (espA mutant, capsule minus).
To identify factors in A. baumannii that are necessary for growth and survival in human infection, we screened random Tn mutants for growth on ascites fluid plates, an ex vivo-modified minimal medium representing a common environment for extracellular bacterial pathogens such as A. baumannii (38). This screen resulted in the identification of AB307.30 and AB307.45. Quantitative growth assays confirmed that these mutants exhibited defective growth in human ascites fluid compared to that of AB307-0294 (wt, capsule positive) (see “Capsule is a critical protectin requisite for optimal growth and survival in human ascites fluid and survival against complement-mediated bactericidal activity in vitro” below).
Chromosomal sequencing determined that the Tn insertion within AB307.30 was in ptk (locus tag ABBFA003461, between nucleotides [nt] 3688640 and 3688641) and within AB307.45 was in epsA (locus tag ABBFA003459, between nt 3687340 and 3687341) (1) (Fig. 1). BLAST analysis of the deduced protein sequence generated from these open reading frames identified Wcz as the Escherichia coli ortholog of PTK and Wza as the E. coli ortholog of EpsA (11). Both of these genes encode proteins that facilitate the polymerization and transport of capsular polysaccharide. PTK and EpsA consist of 727 amino acids (aa) and 333 aa, have predicted molecular masses of 81,639 kDa and 40,512 kDa, and have pIs of 6.39 and 6.91, respectively. Reverse transcription-PCR (RT-PCR) analyses confirmed that ptk and epsA were not cotranscribed with other genes (data not shown). Nonetheless, to establish that the Tn insertion within these genes did not result in a polar effect, complementation studies were performed and were confirmatory.
FIG. 1.
Genetic organization of an A. baumannii capsule cluster containing polymerization and transport genes. Eps I polysaccharide export outer membrane protein (epsA; locus tag ABBFA003459; E. coli ortholog, wza). Low-molecular-weight protein tyrosine phosphatase (ptp; locus tag ABBFA003460; E. coli ortholog, wzb). Protein tyrosine kinase (ptk; locus tag ABBFA003461; E. coli ortholog wzc). An FK506-binding protein-type peptidyl-prolyl cis-trans isomerase (fkpA) is outside the capsule gene cluster. Ruler numbering indicates the genomic coordinates (NCBI accession number CP001172) (1).
Confirmation that AB307.30 and AB307.45 are capsule-deficient derivatives of AB307-0294.
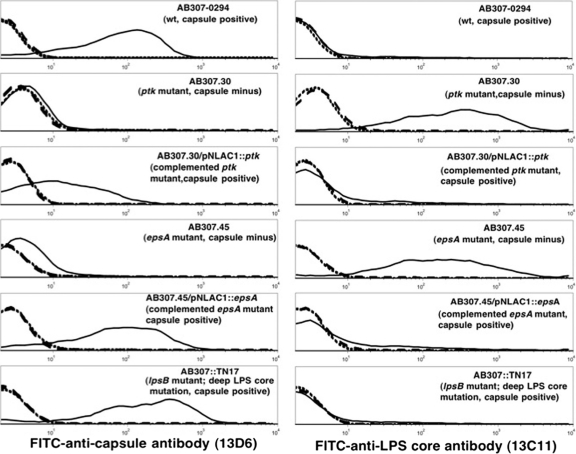
To confirm that both mutants were capsule minus, flow cytometry studies were performed comparing the reactivity of MAb 13D6 on live AB307-0294 to its reactivities on AB307.30, AB307.45, and their respective complemented derivatives, AB307.30/pNLAC1::ptk and AB307.45/pNLAC1::epsA (Fig. 2; Table 1). As predicted, MAb 13D6 bound AB307-0294 and the complemented mutants AB307.30/pNLAC1::ptk and AB307.45/pNLAC1::espA but not AB307.30 or AB307.45. Antibody binding was nearly restored to the degree observed for the wild type for AB307.45/pNLAC1::espA, but complementation did not completely restore antibody binding for AB307.30/pNLAC1::ptk. To further confirm that AB307.30 and AB307.45 were defective in capsule expression and not lipopolysaccharide (LPS), we performed additional studies with the recently described MAb 13C11 and LPS core mutant AB307::Tn17 (27). MAb 13C11 reacts to an LPS core epitope expressed on the surface of AB307-0294 (27). Figure 2 shows that MAb 13D6 reacts to AB307::Tn17 but that MAb 13C11 does not react to this LPS mutant, which excludes the possibility that MAb 13D6 recognized the O-antigen moiety of LPS. Further, MAb 13C11 bound AB307.30 and AB307.45, as predicted, but did not react to AB307-0294, AB307.30/pNLAC1::ptk, or AB307.45/pNLAC1::espA, presumably due to the steric hindrance of capsule (Fig. 2, right, and Table 1).
FIG. 2.
Confirmation that MAb 13D6 recognizes the K1 capsular serotype by flow cytometry. Binding of MAb 13D6 (left) and MAb 13C11 (right) to the indicated strains. MAB 13C11 recognizes a deep LPS core epitope. Small dashed back line, PBS control; large dashed gray line, FITC control; solid line, bacteria.
TABLE 1.
Percentages of bacterial cells bound by MAb 13C11 (anti-LPS core) and 13D6 (anti-K1 capsule) as assessed by flow cytometry
| Exptl parameter | % of cells bounda |
|||||
|---|---|---|---|---|---|---|
| AB307 | AB307.30 | AB307.30/pNLAC1::ptk | AB307.45 | AB307.45/pNLAC1::epsA | AB307::Tn17 | |
| PBS (autofluorescence control) | 0.2 ± 0.08 | 1.8 ± 0.8 | 0.6 ± 0.2 | 0.9 ± 0.2 | 0.5 ± 0.1 | 0.4 ± 0.07 |
| FITC alone (conjugate control) | 1.1 ± 0.3 | 2.7 ± 0.5 | 1.6 ± 0.2 | 3.3 ± 0.2 | 2.0 ± 0.4 | 2.1 ± 0.3 |
| 13C11 (anti-LPS core) | 7.3 ± 0.2* | 87.1 ± 1.1* | 10.4 ± 1.1** | 86.0 ± 1.8* | 21.7 ± 1.4* | 11.4 ± 1.3*** |
| 13D6 (anti-K1 capsule) | 86.8 ± 0.8* | 4.0 ± 0.1 | 44.6 ± 3.6* | 11.3 ± 0.8**** | 73.3 ± 1.5* | 88.0 ± 0.6* |
AB307 (wt, capsule positive), AB307.30 (ptk mutant, capsule minus), AB307.30/pNLAC1::ptk (complemented ptk mutant, capsule positive), AB307.45 (epsA mutant, capsule minus), AB307.45/pNLAC1::epsA (complemented epsA mutant, capsule positive), AB307::Tn17 (lpsB mutant, deep LPS core mutation, capsule positive). Positivity is relative to that of the FITC control. *, P < 0.0001; **, P = 0.0009; ***, P = 0.0010; ****, P = 0.0002; compared to bacteria stained with FITC conjugate alone. Values are means ± SEM (n = 6).
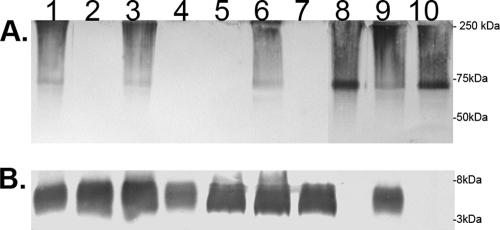
To further confirm that AB307.30 and AB307.45 were unable to express capsule, proteinase K-treated whole-cell lysates were subjected to Western analysis using MAb 13D6 and MAb 13C11 (Fig. 3). MAb 13D6-probed immunoblots indicated that this antibody reacted to the high-molecular-mass capsular polysaccharide produced by AB307-0294 and the LPS-deficient mutant AB307::Tn17 but exhibited no reactivity to AB307.30 or AB307.45 (Fig. 3A). MAb 13D6 reactivity was restored by in trans complementation for both Tn mutants. Corresponding immunoblots were probed with MAb 13C11, which reacts only to wild-type LPS (Fig. 3B), and all strains except AB307::Tn17 were reactive as expected. Taken together, these data demonstrate that the loss of functional PTK and EpsA activities specifically affected the production of capsular polysaccharide by AB307-0294 and did not alter the production of LPS. The fact that AB307::Tn17, which produces a deeply truncated LPS glycoform consisting only of KDO2-lipid A (27), maintains MAb 13D6 reactivity further demonstrated that the AB307-0294 capsular polysaccharide is distinct from the LPS molecule produced by this strain. Moreover, these data confirm that the poor binding of MAb 13C11 observed in the flow cytometric studies was due to steric hindrance (29, 41).
FIG. 3.
Confirmation that AB307.30 and AB307.45 are capsule deficient by Western analysis. Composite Western blotting was performed on proteinase K-treated whole-cell lysates; the lysates were then probed with MAb 13D6, which recognizes the capsular polysaccharide epitope, and MAb 13C11, which recognizes a deep LPS core epitope. Binding of MAb 13D6 (A) and of MAb 13C11 (B) to AB307-0294 (lane 1), AB307.30 (lane 2), AB307.30/pNLAC1::ptk (lane 3), AB307.30/pNLAC1 (ptk mutant with the pNLAC1 plasmid without the insert, capsule minus) (lane 4), AB307.45 (lane 5), AB307.45/pNLAC1::epsA (lane 6), AB307.45/pNLAC1 (epsA mutant with the pNLAC1 plasmid without the insert, capsule minus) (lane 7), AB307::Tn17 (lane 8), AB307::Tn17/pSS11 (complemented lpsB mutant, wt LPS, capsule positive) (lane 9), and AB307::Tn17/pNLAC1 (lpsB mutant with the pNLAC1 plasmid without the insert, deep LPS core mutation, capsule positive) (lane 10). The positions of molecular mass standards are shown to the right.
Taken together, these data demonstrate that MAb 13D6 recognizes the K1 capsule. Further, these data also establish that AB307.30 and AB307.45 are defective in capsule expression. Lastly, these data confirm that PTK and EpsA are essential for a capsule-positive phenotype but do not contribute to LPS synthesis.
Capsule is a critical protectin requisite for optimal growth and survival in human ascites fluid and survival against complement-mediated bactericidal activity in vitro.
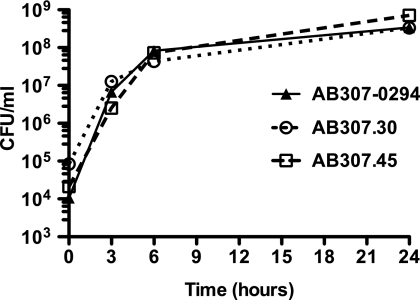
It was important to confirm that the observed growth difference of AB307.30 and AB307.45 on ascites fluid plates was not due to an innate growth defect. Therefore, the ability of AB307-0294 and those of its mutant derivatives AB307.30 and AB307.45 to grow in laboratory medium (Luria-Bertani broth) were assessed and found to be similar (P = 0.13 and P = 0.26 for AB307.30 and AB307.45, respectively, compared to AB307-0294) (Fig. 4).
FIG. 4.
The levels of growth of AB307-0294 (wt), AB307.30 (ptk mutant, capsule minus), and AB307.45 (epsA mutant, capsule minus) in laboratory medium were similar. The levels of growth of AB307-0294 (n = 3), AB307.30 (n = 3), and AB307.45 (n = 3) were assessed at 0, 3, 6, and 24 h in Luria-Bertani medium and were similar (P = 0.13 and P = 0.26 for AB307.30 and AB307.45 compared to AB307-0294, respectively) (two-tailed unpaired t test). Data are means ± SEM.
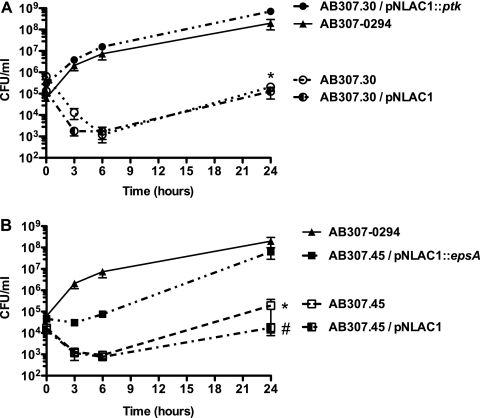
Next, comparative quantitative growth assays in human ascites fluid were performed. As expected, AB307-0294, AB307.30/pNLAC1::ptk, and AB307.45/pNLAC1::epsA grew in ascites fluid, whereas the overall growth and survival of AB307.30, AB307.30/pNLAC1 (ptk mutant with the pNLAC1 plasmid without the insert, capsule minus), AB307.45, and AB307.45/pNLAC1 (espA mutant with the pNLAC1 plasmid without the insert, capsule minus) were significantly less (P < 0.0001, P < 0.0001, P = 0.0002, and P = 0.0005, respectively, compared to AB307-0294) (Fig. 5 A and B). These data demonstrated that a capsule-positive phenotype was necessary for optimal growth and survival in this ex vivo human body fluid. Importantly, the complemented mutants confirmed that PTK and EpsA are essential for optimal growth in ascites fluid.
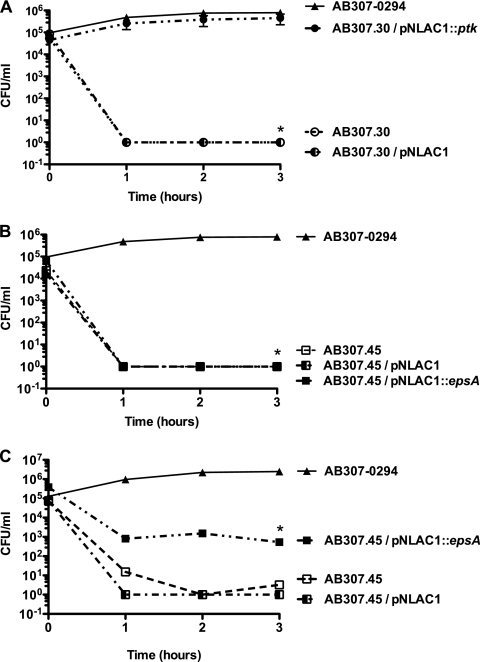
FIG. 5.
AB307.30 (ptk mutant, capsule minus) and AB307.45 (epsA mutant, capsule minus) were significantly killed in human ascites fluid compared to their wild-type parent AB307-0294. (A) The growth of AB307-0294 (n = 6), AB307.30 (n = 6), AB307.30/pNLAC1 (ptk mutant with the pNLAC1 plasmid without the insert, capsule minus) (n = 5), and AB307.30/pNLAC1::ptk (n = 4) was assessed at 0, 3, 6, and 24 h. Data are means ± SEM. The survival of AB307.30 and AB307.30/pNLAC1 was significantly decreased in ascites fluid compared to that of AB307-0294 (*, P < 0.0001, two-tailed unpaired t test). The levels of growth of the wild-type strain AB307-0294 and the complemented ptk mutant AB307.30/pNLAC1::ptk were similar (P = 0.61). (B) The growth of AB307-0294 (n = 6), AB307.45 (n = 4), AB307.45/pNLAC1 (epsA mutant with the pNLAC1 plasmid without the insert, capsule minus) (n = 4), and AB307.45/pNLAC1::epsA (n = 4) was assessed at 0, 3, 6, and 24 h. The survival of AB307.45 and AB307.45/pNLAC1 was significantly decreased in ascites fluid compared to that of AB307-0294 (*, P = 0.0002 for AB307.45; #, P = 0.0005 for AB307.45/pNLAC1). The levels of growth of the wild-type strain AB307-0294 and the complemented epsA mutant AB307.45/pNLAC1::epsA were similar (P = 0.09).
Ascites fluid is known to contain active complement; therefore, we studied the effect of human serum on AB307-0294 compared to that on the Tn mutants AB307.30 and AB307.45 and their complemented derivatives. AB307-0294 and AB307.30/pNLAC1::ptk were resistant to the bactericidal activity of 90% human serum (Fig. 6 A). In contrast, AB307.30, AB307.30/pNLAC1, AB307.45, and AB307.45/pNLAC1 were dramatically sensitive to 90% human serum, undergoing a rapid (<1 h), nearly 5-log kill (P < 0.0001, compared to AB307-0294 and AB307.30/pNLAC1::ptk) (Fig. 6A and B). Interestingly, complementation of AB307.45 with pNLAC1::espA did not restore resistance to 90% human serum but did restore partial resistance to 20% human serum (Fig. 6C). These data further confirm the importance of the capsule as an A. baumannii virulence factor and of PTK and EpsA as essential proteins for serum survival.
FIG. 6.
AB307.30 (ptk mutant, capsule minus) and AB307.45 (epsA mutant, capsule minus) were significantly killed in human serum compared to their wild-type parent, AB307-0294 (capsule positive). (A) The survival in 90% human serum of AB307-0294 (n = 6), AB307.30 (n = 8), AB307.30/pNLAC1 (n = 4), and AB307.30/pNLAC1::ptk (n = 4) was assessed at 0, 1, 2, and 3 h. AB307.30 and AB307.30/pNLAC1 were significantly killed in serum compared to AB307-0294 (*, P < 0.0001; two-tailed unpaired t test). The rates of survival of wild-type strain AB307-0294 and the complemented ptk mutant AB307.30/pNLAC1::ptk were similar (P = 0.1). (B) The survival in 90% human serum of AB307-0294 (n = 6), AB307.45 (n = 4), AB307.45/pNLAC1 (n = 4), and AB307.45/pNLAC1::epsA (n = 4) was assessed at 0, 1, 2, and 3 h. AB307.45, AB307.45/pNLAC1, and AB307.45/pNLAC1::epsA were significantly killed in serum compared to AB307-0294 (*, P < 0.0001; two-tailed unpaired t test). (C) The survival in 20% human serum of AB307-0294 (n = 4) AB307.45 (n = 4), AB307.45/pNLAC1 (n = 4), and AB307.45/pNLAC1::epsA (n = 4) was assessed at 0, 1, 2, and 3 h. AB307.45 and AB307.45/pNLAC1 were significantly killed in serum compared to AB307-0294 (*, P < 0.0001; two-tailed unpaired t test). The survival of the complemented epsA mutant AB307.45/pNLAC1::epsA was significantly greater than that of the noncomplemented mutants AB307.45 and AB307.45/pNLAC1 (P = 0.0007 and 0.0002, respectively) but still significantly less than that of the wild-type strain, AB307-0294 (P = 0.0002). All strains were also assessed in the presence of 90% heat-inactivated (56°C for 30 min) normal human serum, and their levels of growth were similar (data are not shown).
Capsule is a critical protectin in vivo.
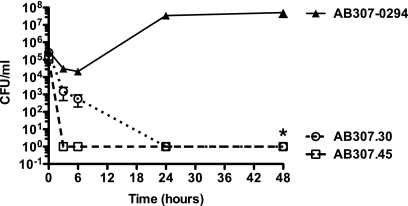
A validation of our in vitro findings was needed to confirm that capsule, and therefore PTK and EpsA, were essential for survival in vivo. This was accomplished by comparing the levels of growth and survival of AB307-0294, AB307.30, and AB307.45 in an established rat soft tissue infection model. This model is particularly clinically relevant since A. baumannii has been increasingly recognized as a cause of a variety of soft tissue infections (7, 12). Further, it has been validated for studying A. baumannii by our group (37). Compared to AB307-0294, AB307.30 and AB307.45 demonstrated a highly significant decrease in survival in this model (P < 0.0001) (Fig. 7). These data clearly demonstrated that capsule is critical for the survival of AB307-0294 in vivo and therefore that PTK and EpsA are essential in this setting.
FIG. 7.
AB307.30 and AN307.45 were significantly killed in the rat soft tissue infection model compared to their wild-type parent, AB307-0294. Rats were challenged with AB307-0294 (4 to 6 rats per time point), AB307.30 (4 rats), and AB307.45 (7 rats). Bacterial titers were determined at 0, 3, 6, 24, and 48 h. Data are means ± SEM. The survival of AB307.30 and AB307.45 was significantly decreased compared to that of AB307-0294 (*, P < 0.0001; two-tailed unpaired t test).
DISCUSSION
Although recent reports have described the production of two surface polysaccharides produced by A. baumannii, the extracellular polysaccharide poly-beta-(1-6)-N-acetylglucosamine (9) and LPS (27), remarkably, almost nothing is known about any aspect of the capsular polysaccharides produced by this bacterial pathogen. The data presented in this report begin to fill in this critical knowledge gap. First, it was clearly demonstrated that the K1 capsule from the A. baumannii strain AB307-0294 was necessary for optimal growth in human ascites fluid (Fig. 5) and survival in human serum (Fig. 6), both of which contain complement, a critical host defense system that is instrumental in protecting against extracellular bacterial pathogens such as A. baumannii. Further, capsule was requisite for survival in a rat soft tissue infection model (Fig. 7). Most importantly, the magnitude and durability of the clearance of the capsule-minus mutants AB307.30 and AB307.45 was remarkable (Fig. 7). These data clearly established capsule as a critical protectin for A. baumannii.
Our strategy to identify genes important for the growth and survival of A. baumannii by screening Tn mutants on human ascites fluid plates is an innovative modification of previously established methods. Ascites fluid plates represent an ex vivo screening tool reflective of inflammatory extracellular fluid and therefore mimic the host environment that occurs with infection. This simple innovation was critical for the efficiency of our approach. By selecting for Tn mutants on laboratory medium and then screening for decreased or absent growth on ascites fluid plates, we were able to identify genes that are both requisite and expressed in vivo in one step. This approach is advantageous in that it is unbiased and highly efficient. We do not select the genes but allow the genetic screen, designed to identify the phenotype of in vivo essentiality, to dictate the choices. This genetic approach also avoids a potential problem that may occur when using in silico, genomic, or proteomic approaches. Genes/proteins predicted to possess biologic significance by these methods might subsequently prove not to have a phenotype of interest, often after the cumbersome and time-consuming process of construct generation and testing.
Although the mechanism by which capsule confers this survival advantage has not yet been established in vivo, given the increased serum sensitivity of AB307.30 and AB307.45 in vitro, it is logical to infer that resistance to the bactericidal activity of complement is one means. Whether capsule also serves to protect against the effects of professional phagocytes (e.g., neutrophils and macrophages) or antimicrobial peptides remains to be tested. To date, we have not performed any studies on the mechanisms by which PTK and EpsA facilitate capsule expression. However, a substantial body of work has been done on their E. coli orthologs, Wcz and Wza, respectively. These proteins are part of a multiprotein complex that spans the cell membrane. Although the precise roles of the participating proteins in this process are still being defined, Wcz is an inner membrane tyrosine kinase that regulates capsule assembly and transport and Wza is an outer membrane protein that, in a complex with Wza, enables the transport of capsule across the periplasm and outer membrane (10, 11, 14). In Acinetobacter lwoffiiand Acinetobacter johnsonii, the ptk ortholog wzc has also been shown to possess autophosphorylating tyrosine kinase activity (15, 16, 30), and in A. lwoffii, it has been demonstrated that it is required for optimal expression of the extracellular galactosamine-containing lipoheteropolysaccharide bioemulsifier (30).
One of the promises of the field of microbial pathogenesis was to identify potential drug targets. A strategy to inactivate such targets is supported by recent results (31, 33), thereby validating the potential of virulence factors or their regulators as drug targets. PTK and EpsA possess many of the characteristics requisite for an anti-virulence drug target. The loss of PTK or EpsA resulted in a complete and durable ≥5-log kill of AB307-0294 in vivo, fulfilling an important criterion for a potential antivirulence drug target. Next, and importantly, PTK and EpsA do not have any significant homology to human proteins (data not shown). PTK and EpsA were 100% prevalent in the 8 A. baumannii strains for which sequence is available: 6 A. baumannii genomes in the public domain (A. baumannii strains ATCC 17978, ATCC 0057, AYE, ACICU, SDF, and ATCC 19606) and 2 A. baumannii strains sequenced by our group in addition to AB307-0294, namely, AB853 (blood isolate from Iraq) and AB979 (environmental isolate from Iraq) (unpublished data). The putative protein sequences of PTK of AB307-0294 and EpsA of AB307-0294 were compared to the sequences in these A. baumannii strains and were found to have 91 to 100% identities and 96 to 100% similarities. These data suggest that PTK and EpsA are potential antimicrobial targets.
In summary, we have demonstrated the importance of the K1 capsule from the A. baumannii strain AB307-0294 as a protectin in vitro and in vivo. These results have important biologic and translational implications.
Acknowledgments
This work was supported by a VA Merit Review grant from the Department of Veterans Affairs (T.A.R.), a U.S. Army Medical Research Acquisition Activity grant under contract W81XWH-05-1-0627 (T.A.R., A.A.C), and an Interdisciplinary Grant from the University at Buffalo (T.A.R., T.C.U., L.W. S.).
Editor: J. N. Weiser
Footnotes
Published ahead of print on 19 July 2010.
REFERENCES
- 1.Adams, M. D., K. Goglin, N. Molyneaux, K. M. Hujer, H. Lavender, J. J. Jamison, I. J. MacDonald, K. M. Martin, T. Russo, A. A. Campagnari, A. M. Hujer, R. A. Bonomo, and S. R. Gill. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 190:8053-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anstey, N. M., B. J. Currie, M. Hassell, D. Palmer, B. Dwyer, and H. Seifert. 2002. Community-acquired bacteremic Acinetobacter pneumonia in tropical Australia is caused by diverse strains of Acinetobacter baumannii, with carriage in the throat in at-risk groups. J. Clin. Microbiol. 40:685-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anstey, N. M., B. J. Currie, and K. M. Withnall. 1992. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin. Infect. Dis. 14:83-91. [DOI] [PubMed] [Google Scholar]
- 4.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 6.Campagnari, A. A., T. F. Ducey, and C. A. Rebmann. 1996. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect. Immun. 64:3920-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2004. Acinetobacter baumannii infections among patients at military medical facilities treating injuried U.S. service members, 2002-2004. MMWR Morb. Mortal. Wkly. Rep. 53:1063-1066. [PubMed] [Google Scholar]
- 8.Chen, M. Z., P. R. Hsueh, L. N. Lee, C. J. Yu, P. C. Yang, and K. T. Luh. 2001. Severe community-acquired pneumonia due to Acinetobacter baumannii. Chest 120:1072-1077. [DOI] [PubMed] [Google Scholar]
- 9.Choi, A. H., L. Slamti, F. Y. Avci, G. B. Pier, and T. Maira-Litran. 2009. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 191:5953-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, R. F., K. Beis, C. Dong, C. H. Botting, C. McDonnell, R. C. Ford, B. R. Clarke, C. Whitfield, and J. H. Naismith. 2007. The 3D structure of a periplasm-spanning platform required for assembly of group 1 capsular polysaccharides in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 104:2390-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuthbertson, L., I. L. Mainprize, J. H. Naismith, and C. Whitfield. 2009. Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram-negative bacteria. Microbiol. Mol. Biol. Rev. 73:155-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, K. A., K. A. Moran, C. K. McAllister, and P. J. Gray. 2005. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg. Infect. Dis. 11:1218-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Department of Veterans Affairs, U.S. Veterans Health Administration. 2004. Update of the Colleagues' Letter sent April 23, 2004 concerning Acinetobacter baumanii, p. 539/111. Department of Veterans Affairs, Veterans Health Administration, Washington, DC.
- 14.Dong, C., K. Beis, J. Nesper, A. L. Brunkan-Lamontagne, B. R. Clarke, C. Whitfield, and J. H. Naismith. 2006. Wza, the translocon for E. coli capsular polysaccharides, defines a new class of membrane protein. Nature 444:226-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doublet, P., C. Vincent, C. Grangeasse, A. J. Cozzone, and B. Duclos. 1999. On the binding of ATP to the autophosphorylating protein, Ptk, of the bacterium Acinetobacter johnsonii. FEBS Lett. 445:137-143. [DOI] [PubMed] [Google Scholar]
- 16.Duclos, B., C. Grangeasse, E. Vaganay, M. Riberty, and A. J. Cozzone. 1996. Autophosphorylation of a bacterial protein at tyrosine. J. Mol. Biol. 259:891-895. [DOI] [PubMed] [Google Scholar]
- 17.Falagas, M. E., and E. A. Karveli. 2007. The changing global epidemiology of Acinetobacter baumannii infections: a development with major public health implications. Clin. Microbiol. Infect. 13:117-119. [DOI] [PubMed] [Google Scholar]
- 18.Fournier, P. E., and H. Richet. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 42:692-699. [DOI] [PubMed] [Google Scholar]
- 19.Garzoni, C., S. Emonet, L. Legout, R. Benedict, P. Hoffmeyer, L. Bernard, and J. Garbino. 2005. Atypical infections in tsunami survivors. Emerg. Infect. Dis. 11:1591-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaynes, R., and J. R. Edwards. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848-854. [DOI] [PubMed] [Google Scholar]
- 21.Gkrania-Klotsas, E., and R. C. Hershow. 2006. Colonization or infection with multidrug-resistant Acinetobacter baumannii may be an independent risk factor for increased mortality. Clin. Infect. Dis. 43:1224-1225. [DOI] [PubMed] [Google Scholar]
- 22.Jain, R., and L. H. Danziger. 2004. Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann. Pharmacother. 38:1449-1459. [DOI] [PubMed] [Google Scholar]
- 23.Jerassy, Z., A. M. Yinnon, S. Mazouz-Cohen, S. Benenson, Y. Schlesinger, B. Rudensky, and D. Raveh. 2006. Prospective hospital-wide studies of 505 patients with nosocomial bacteraemia in 1997 and 2002. J. Hosp. Infect. 62:230-236. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, E. N., T. C. Burns, R. A. Hayda, D. R. Hospenthal, and C. K. Murray. 2007. Infectious complications of open type III tibial fractures among combat casualties. Clin. Infect. Dis. 45:409-415. [DOI] [PubMed] [Google Scholar]
- 25.Joly-Guillou, M. L. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 11:868-873. [DOI] [PubMed] [Google Scholar]
- 26.Loehfelm, T. W., N. R. Luke, and A. A. Campagnari. 2008. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J. Bacteriol. 190:1036-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luke, N. R., S. L. Sauberan, T. A. Russo, J. M. Beanan, R. Olson, T. W. Loehfelm, A. D. Cox, F. St. Michael, E. V. Vinogradov, and A. A. Campagnari. 2010. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect. Immun. 78:2017-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maegele, M., S. Gregor, E. Steinhausen, B. Bouillon, M. M. Heiss, W. Perbix, F. Wappler, D. Rixen, J. Geisen, B. Berger-Schreck, and R. Schwarz. 2005. The long-distance tertiary air transfer and care of tsunami victims: injury pattern and microbiological and psychological aspects. Crit. Care Med. 33:1136-1140. [DOI] [PubMed] [Google Scholar]
- 29.Michaelsen, T. E., A. Aase, J. Kolberg, E. Wedge, and E. Rosenqvist. 2001. PorB3 outer membrane protein on Neisseria meningitidis is poorly accessible for antibody binding on live bacteria. Vaccine 19:1526-1533. [DOI] [PubMed] [Google Scholar]
- 30.Nakar, D., and D. L. Gutnick. 2003. Involvement of a protein tyrosine kinase in production of the polymeric bioemulsifier emulsan from the oil-degrading strain Acinetobacter lwoffii RAG-1. J. Bacteriol. 185:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payne, D. J. 2008. Microbiology. Desperately seeking new antibiotics. Science 321:1644-1645. [DOI] [PubMed] [Google Scholar]
- 32.Peleg, A. Y., H. Seifert, and D. L. Paterson. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasko, D. A., C. G. Moreira, R. de Li, N. C. Reading, J. M. Ritchie, M. K. Waldor, N. Williams, R. Taussig, S. Wei, M. Roth, D. T. Hughes, J. F. Huntley, M. W. Fina, J. R. Falck, and V. Sperandio. 2008. Targeting QseC signaling and virulence for antibiotic development. Science 321:1078-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richet, H., and P. E. Fournier. 2006. Nosocomial infections caused by Acinetobacter baumannii: a major threat worldwide. Infect. Control Hosp. Epidemiol. 27:645-646. [DOI] [PubMed] [Google Scholar]
- 35.Robenshtok, E., M. Paul, L. Leibovici, A. Fraser, S. Pitlik, I. Ostfeld, Z. Samra, S. Perez, B. Lev, and M. Weinberger. 2006. The significance of Acinetobacter baumannii bacteraemia compared with Klebsiella pneumoniae bacteraemia: risk factors and outcomes. J. Hosp. Infect. 64:282-287. [DOI] [PubMed] [Google Scholar]
- 36.Russo, T. A., J. M. Beanan, R. Olson, S. A. Genagon, U. Macdonald, J. J. Cope, B. A. Davidson, B. Johnston, and J. R. Johnson. 2007. A killed, genetically engineered derivative of a wild-type extraintestinal pathogenic E. coli strain is a vaccine candidate. Vaccine 25:3859-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo, T. A., J. M. Beanan, R. Olson, U. MacDonald, N. R. Luke, S. R. Gill, and A. A. Campagnari. 2008. Rat pneumonia and soft-tissue infection models for the study of Acinetobacter baumannii biology. Infect. Immun. 76:3577-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo, T. A., U. MacDonald, J. M. Beanan, R. Olson, I. J. MacDonald, S. L. Sauberan, N. R. Luke, L. W. Schultz, and T. C. Umland. 2009. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J. Infect. Dis. 199:513-521. [DOI] [PubMed] [Google Scholar]
- 39.Scott, P., G. Deye, A. Srinivasan, C. Murray, K. Moran, E. Hulten, J. Fishbain, D. Craft, S. Riddell, L. Lindler, J. Mancuso, E. Milstrey, C. T. Bautista, J. Patel, A. Ewell, T. Hamilton, C. Gaddy, M. Tenney, G. Christopher, K. Petersen, T. Endy, and B. Petruccelli. 2007. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 44:1577-1584. [DOI] [PubMed] [Google Scholar]
- 40.Tognim, M. C., S. S. Andrade, S. Silbert, A. C. Gales, R. N. Jones, and H. S. Sader. 2004. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multidrug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. Int. J. Infect. Dis. 8:284-291. [DOI] [PubMed] [Google Scholar]
- 41.van der Ley, P., O. Kuipers, J. Tommassen, and B. Lugtenberg. 1986. O-antigenic chains of lipopolysaccharide prevent binding of antibody molecules to an outer membrane pore protein in Enterobacteriaceae. Microb. Pathog. 1:43-49. [DOI] [PubMed] [Google Scholar]
- 42.van Dessel, H., T. E. Kamp-Hopmans, A. C. Fluit, S. Brisse, A. M. de Smet, L. Dijkshoorn, A. Troelstra, J. Verhoef, and E. M. Mascini. 2002. Outbreak of a susceptible strain of Acinetobacter species 13 (sensu Tjernberg and Ursing) in an adult neurosurgical intensive care unit. J. Hosp. Infect. 51:89-95. [DOI] [PubMed] [Google Scholar]
- 43.Van Looveren, M., and H. Goossens. 2004. Antimicrobial resistance of Acinetobacter spp. in Europe. Clin. Microbiol. Infect. 10:684-704. [DOI] [PubMed] [Google Scholar]
- 44.Villegas, M. V., and A. I. Hartstein. 2003. Acinetobacter outbreaks, 1977-2000. Infect. Control Hosp. Epidemiol. 24:284-295. [DOI] [PubMed] [Google Scholar]