Abstract
The nontoxic cholera toxin B subunit (CTB) was evaluated as a potential delivery molecule for the Plasmodium vivax ookinete surface protein, Pvs25. Recombinant Pvs25 was expressed as a secreted protein in the yeast Pichia pastoris, as a mixture of isoforms including multimers and the A and B monomers. The A isoform with the presumed native protein fold was the most abundant, accounting for more than 40% of all expressed protein. The molecularly uniform A isoform was chemically conjugated to CTB via its primary amines, and the fusion protein, retaining GM1-ganglioside affinity, was administered to BALB/c mice by the subcutaneous (s.c.) or intranasal (i.n.) route. Immunization of mice with conjugated Pvs25 without supplemental adjuvant induced antisera that specifically recognized P. vivax ookinetes in vitro. Furthermore, the antisera, when mixed with parasitized blood isolated from P. vivax patients from Thailand, was found to reduce parasite transmission to mosquitoes, conferring a 93 to 98% (s.c.) or a 73 to 88% (i.n.) decrease in oocyst number. Unconjugated Pvs25 alone conferred only a 23 to 60% (s.c.) or a 0 to 6% (i.n.) decrease in oocyst number. Coadministration of extraneous adjuvants, however, further enhanced the vaccine efficacy up to complete blockade. Taken together, we conclude that a weakly immunogenic Pvs25 by itself, when linked to CTB, transforms into a potent transmission-blocking antigen in both i.n. and s.c. routes. In addition, the present study is, to the best of our knowledge, the first demonstration of the immune potentiating function of CTB for a vaccine antigen delivered by the s.c. route.
Malaria is one of the most serious infectious diseases, with high mortality and morbidity, especially in tropical regions of the world. The disease causes 350 to 500 million clinical cases every year, and the estimated annual mortality exceeds 1.1 million (28). Implementation of many malaria control measures, including chemotherapy and insecticide-treated bed nets, have made a significant contribution to the reduction of malaria cases worldwide; however, these control measures are suboptimal, and hence new tools, particularly vaccines, should be used for local elimination and the ultimate eradication of malaria from the globe (9, 10, 24). The development of effective and affordable malaria vaccines is therefore likely to benefit global public health (Malaria Vaccine Technology Roadmap [MVTR], 2006 [http://www.malariavaccineroadmap.net/pdfs/Malaria_Vaccine_TRM_Final.pdf]).
Although Plasmodium falciparum causes the highest mortality rates among the four Plasmodium species known to infect humans (18), P. vivax malaria has the highest morbidity and is an important cause of recurrent malaria. This species is therefore an important target of malaria control efforts (4-6; MVTR). Furthermore, because global malaria eradiation is the ultimate goal, the value of developing vaccines against P. vivax cannot be underestimated (4-6; MVTR). Several promising vaccine candidates have been intensively investigated, such as those targeting the asexual stages, i.e., the sporozoite, hepatic and erythrocytic stages, which are designed to prevent infection and to reduce disease severity. On the other hand, transmission-blocking vaccines (TBVs) that target the sexual stage, in which the parasite undergoes sporogonic development in anopheline mosquitoes, prevent parasite transmission from mosquitoes to humans (7, 14, 25). TBVs induce antibodies that react with the ookinete surface proteins (OSPs) of malaria parasites within the mosquito midgut, and as such they do not directly protect vaccinated individuals from infection. They could, however, contribute to elimination of the disease by lowering the parasite transmission frequency below the threshold at which the parasite can maintain its life cycle (4, 6). In addition, TBVs, when combined with vaccines targeting other stages of the infection, could prevent transmission of parasites that have escaped the immune response. Furthermore, TBVs could also prevent transmission of drug-resistant parasites, which will likely to emerge when mass administration of primaquine is initiated. Therefore, TBVs might function as a “safety net” for pre-erythrocytic and erythrocytic vaccines, as well as other nonvaccine interventions.
We have recently tested whether a mucosal vaccination regime can be applied to TBVs, on the premise that noninvasive and easy-to-administer mucosal vaccines are advantageous in a mass vaccination campaign in a region where malaria is endemic (1-3). In these animal studies, we have demonstrated the potential of mucosal vaccines to block parasite transmission, but enhancement of the mucosal immunogenicity of recombinant antigens was found to be critically dependent upon the use of cholera toxin (CT) adjuvant. CT is well known for its high immune potentiating function for admixed antigens administered through the mucosal, particularly the intranasal (i.n.), route (13). However, its clinical application is hampered by its severe toxicity (26). Thus, alternative vaccine formulations not using the CT holotoxin are highly desirable.
Here, we extended our previous studies to test our hypothesis that the immunogenicity of a Plasmodium vivax malaria OSP, Pvs25, becomes substantially augmented when physically linked to the nontoxic B subunit of CT (CTB), even without supplementation with extraneous adjuvants. This should, in theory, effectively reduce parasite transmission to mosquitoes. Furthermore, we tested the TBV efficacy of the engineered fusion complex in a subcutaneous (s.c.) immunization regimen to test the immune potentiating function of CTB with this particular immunization route.
MATERIALS AND METHODS
Expression of Pvs25H protein from the methylotrophic yeast Pichia pastoris.
The Pvs25 coding region (Ala23 to Leu195) was amplified by PCR from plasmid Pvs25#26_SalI_pEU3, which harbors the coding region for the extracellular domain of the Pvs25 protein (12), with a sense primer (5′-GCCGTCACGGTAGACACC-3′) and an antisense primer containing an EcoRI site, a hexahistidine-coding sequence and a termination codon (5′-GGGAATTCTTAATGATGGTGATGGTGATGTGGTCCAAGGCATACATTTTTCTCTTTGTC-3′). The DNA fragment was amplified by using Vent DNA polymerase (New England Biolabs, Beverly, MA), was purified by using a PCR Purification Kit (Qiagen, Inc., Valencia, CA), and then digested with EcoRI. The fragment was subcloned into the SnaBI and EcoRI sites of the P. pastoris expression vector pPIC9K (Life Technologies, Carlsbad, CA) to construct the plasmid pPvs25H, which was designed to express an α-factor signal-Pvs25-hexahistidine fusion protein (see Fig. 1a).
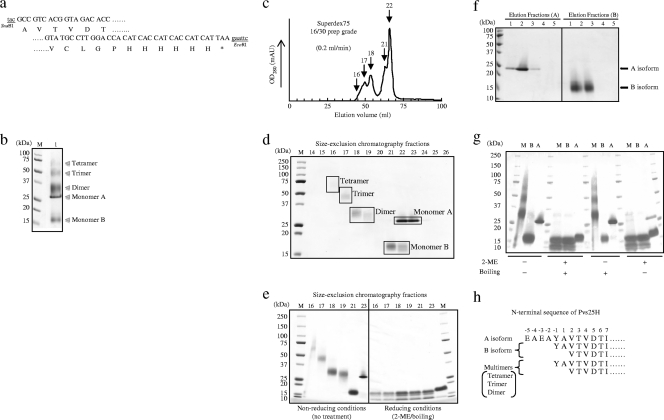
FIG. 1.
Construction and expression analysis of Pvs25H in Pichia pastoris. (a) The 5′- and 3′-coding regions of the Pvs25 gene sequence with its predicted amino acid sequence (Ala23 to Leu195). The Pvs25 gene fused to a hexahistidine tag at its C terminus was inserted into the SnaBI and EcoRI sites of pPIC9K, and the gene was integrated into the chromosomal DNA of P. pastoris strain GS115 by homologous recombination. (b) After selection of a high-producer clone that secreted the recombinant protein, nickel-affinity chromatography-purified Pvs25H was analyzed by SDS-PAGE (lane 1). M, molecular marker. (c) The affinity-purified Pvs25H was fractionated by size exclusion chromatography from which at least five chromatographic peaks were observed. (d) Size exclusion chromatography fractions 14-26 were subjected to SDS-PAGE (15% acrylamide). Based on the apparent molecular mass of each protein band, the fractions 16, 17, 18-19, 21-22, and 22-23 were defined as the tetramer, trimer, dimer, monomer B, and monomer A, respectively. (e) Selected chromatography peaks were subjected to 5 to 20% acrylamide gradient SDS-PAGE under nonreducing or reducing (10% 2-ME and boiling) conditions. (f) Hydrophobic interaction chromatography of the Pvs25H-A and Pvs25H-B monomeric isoforms. Elution fractions (A) are eluates of 2 M NH4SO4 (Pvs25H-A) and elution fractions (B) are eluates of PBS (Pvs25H-B). (g) Each isoform (M, a mixture of dimer, trimer and tetramer; B, B isoform; A, A isoform) was subjected to 15% acrylamide SDS-PAGE under various conditions, as indicated. (h) The N-terminal protein sequence of each isoform. Positively numbered amino acid residues (Ala1 and the following) are residues of the Pvs25 protein; negatively numbered amino acids are derivatives of the pPIC9K α-factor secretion signal. The A monomer revealed a single, unique sequence, but the B monomer and the multimers showed a mixture of different N termini.
P. pastoris recombination was performed according to the manufacturer's instructions (Life Technologies). Approximately 10 μg of linearized pPvs25H plasmid digested with SalI was electroporated into P. pastoris strain GS115 by using a Gene Pulser (1.5 kV, 200 Ω, 25 μF; Bio-Rad Laboratories, Inc., Redmond, WA). Immediately after electroporation, the cells were plated on minimal dextrose medium and incubated for 72 h at 29°C. Colonies were transferred to yeast extract-peptone-dextrose (YPD) medium containing increasing concentrations of Geneticin (G418; 1 to 5 mg/ml; Nacalai Tesque, Inc., Kyoto, Japan) for the selection of clones containing multiple copies of the desired gene. Clones that acquired a phenotype resistant to the highest level of G418 (5 mg/ml) were analyzed for production of the Pvs25H protein. Selected clones were cultured in BMGY medium with vigorous shaking in a baffled flask at 30°C until the optical density at 600 nm (OD600) reached 2.0; the cultured cells were then transferred to BMMY medium containing 0.5% methanol to induce gene expression. Cells were cultured for an additional 72 h, with supplementation with 0.5% methanol for every 24 h of continued induction. The culture supernatant was collected by two rounds of centrifugation (9,600 × g) for 10 min, followed by filtration (FastCap filter with a 0.2-μm pore size; Nalgene Nunc International, Inc., Rochester, NY) to remove residual cells completely. Proteins secreted into the supernatant were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie brilliant blue or subjected to immunoblot analysis with anti-Pvs25 antiserum or anti-histidine tag monoclonal antibody (Roche, Basel, Switzerland). The supernatant was supplemented with 20 mM imidazole and applied to an immobilized metal ion affinity chromatography column (HisTrap FF Ni Sepharose 6 Fast Flow; GE Healthcare, Little Chalfont, United Kingdom). After a washing step with a buffer containing 20 mM imidazole, the Pvs25H protein was eluted with phosphate-buffered saline (PBS) containing 500 mM imidazole. The affinity-purified protein was used for size exclusion chromatography with a flow rate of 0.2 ml/min (HiLoad 16/60 Superdex 75-pg column; GE Healthcare) to separate the monomeric from the multimeric isoforms. The monomeric isoforms that were a mixture of A and B isoforms were then subjected to hydrophobic interaction chromatography (HIC; HiTrap Phenyl Sepharose HP; GE Healthcare), in which both the A and the B isoforms bound to the hydrophobic ligand by adjusting the concentration of ammonium sulfate in the solution to 2 M. Then, a two-step elution process was performed, using 1 M ammonium sulfate for elution of the A isoform, followed by PBS elution of the B isoform.
This highly purified A isoform (Pvs25H-A) was used for all immunization experiments. The endotoxin content of the Pvs25H-A protein was measured by the Limulus amebocyte lysate test (Pyrogent Single Test Vials; Cambrex, East Rutherford, NJ), and the endotoxin content was found to be <0.05 endotoxin unit (EU)/μg of protein.
Immobilized tris[2-carboxyethyl]phosphine hydrochloride on a beaded agarose support (TCEP; Pierce, Rockford, IL) was used to generate a reduced form of the sulfhydryl groups for recombinant protein samples. In addition, 5,5′-dithio-bis-(2-nitrobenzoic acid) (Ellman's reagent; Pierce) and a cysteine hydrochloride monohydrate standard were used to estimate the amounts of free sulfhydryls.
The N-terminal protein sequences were analyzed by the Edman degradation method as described elsewhere, using a protein sequencer (Shimadzu, Kyoto, Japan).
All recombinant DNA experiments were conducted according to the Safety Guidelines for Gene Manipulation Experiments of the University of the Ryukyus.
Chemical conjugation between Pvs25H-A and CTB.
Recombinant CTB was expressed and purified as described previously (11), and purified Pvs25H-A was chemically conjugated to CTB by using the heterobifunctional cross-linker N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP; Thermo Scientific, Inc., Rockford, IL). One milligram of Pvs25H-A (2 mg/ml in PBS-EDTA) was incubated with SPDP (0.6 mM, final concentration) for 1 h at room temperature to add pyridyl disulfide groups to the primary amines of the protein. The reaction mixture was then desalted and buffer exchanged to PBS by a size exclusion membrane filter (Amicon Ultra-15, MWCO 10,000; Millipore, Billerica, MA) to remove excess reagent and by-products (pyridine 2-thione). Similarly, 1 mg of CTB (2 mg/ml in PBS-EDTA) was incubated with SPDP (0.6 mM, final concentration) for 1 h at room temperature and then desalted and buffer exchanged to PBS. Pyridyldithiol-activated CTB was then incubated with dithiothreitol (DTT; 50 mM, final) for 30 min at room temperature to expose the sulfhydryl groups and then desalted and buffer exchanged to PBS as before. Finally, equal amounts of pyridyldithiol-activated Pvs25H-A and sulfhydryl-activated CTB were mixed, followed by incubation at room temperature overnight for conjugation. The conjugated sample was desalted as before, and the endotoxin content was measured to confirm that there had been no significant contamination during the conjugation process.
To evaluate the conjugation efficiency, untreated Pvs25H-A was separately incubated either with untreated CTB or pyridyldithiol-activated CTB (CTBSPDP). Similarly, pyridyldithiol-activated Pvs25H-A (Pvs25H-ASPDP) was separately incubated with either untreated or DTT-treated CTB (CTBDTT or CTBSPDP/DTT, respectively) (see Results and Fig. 2a for details). Each conjugation sample was analyzed by GM1-enzyme-linked immunosorbent assay (GM1-ELISA) as described previously (11). Briefly, 5 μg of monosialoganglioside GM1 (Sigma-Aldrich, St. Louis, MO)/ml, a receptor for CT, diluted with bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.6]; 50 μl/well) was coated onto a 96-well microtiter plate (Sumitomo Bakelite Co., Ltd., Tokyo, Japan), and the plate was incubated at 4°C overnight. After washing the plate twice with PBS containing 0.05% Tween 20 (PBS-T) and once with PBS, the plate was blocked with PBS containing 10% skim milk for 2 h at 37°C. Each conjugation sample (2 μg of total protein/well) was then applied to the wells, and the plate was incubated for 2 h at 37°C, followed by incubation with rabbit anti-CT antiserum (1/4,000; Sigma-Aldrich) or mouse anti-Pvs25 antiserum (1/500) for 2 h at 37°C. This was followed by the addition of anti-rabbit or anti-mouse IgG conjugated to alkaline phosphatase (1/4,000; Sigma-Aldrich). Finally, p-nitrophenylphosphate (Bio-Rad) was added, and the plate was incubated for 20 min at 37°C. The OD415 was measured by using a microplate reader (Bio-Rad).
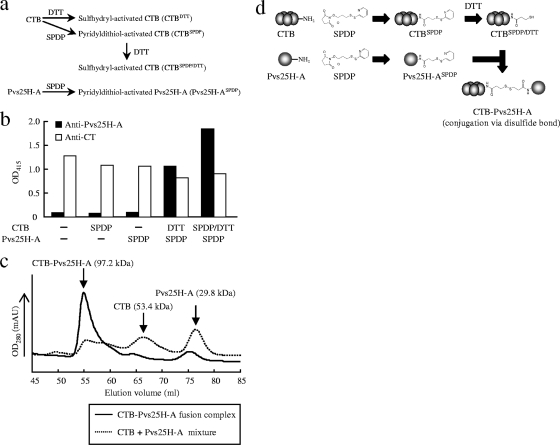
FIG. 2.
Chemical conjugation of Pvs25H-A to cholera toxin B subunit (CTB). Various conjugation methods were evaluated for the generation of the CTB-Pvs25H-A fusion complex. The heterobifunctional cross-linker N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP) was used to link Pvs25H-A to CTB via the primary amines. (a) Either CTB or SPDP-modified CTB (CTBSPDP) was first treated with DTT to expose free sulfhydryls (designated as CTBDTT and CTBSPDP/DTT, respectively), and then SPDP-modified Pvs25H-A (Pvs25H-ASPDP) was separately mixed with each of them to generate the fusion complex. (b) The CTB-Pvs25H-A fusion complex was analyzed by GM1-ELISA using anti-cholera toxin (CT) (□) or anti-Pvs25H-A (▪) antiserum. (c) The CTB-Pvs25H-A fusion protein (solid line) or a mixture of CTB and Pvs25H-A (dotted line) was subjected to size exclusion chromatography. For the fusion protein, the peaks for CTB and Pvs25H-A disappeared, and a new peak emerged, indicating generation of the fusion complex. (d) The conjugation scheme chosen for production of the CTB-Pvs25H-A fusion complex. See Materials and Methods for the detailed conjugation method.
To evaluate the conjugation state of the fusion complex, 1 mg of conjugation sample was subjected to size exclusion chromatography (HiLoad 16/60 Superdex 75-pg column; GE Healthcare). Molecular weight standards (Gel Filtration Calibration kits LMW; GE Healthcare) were used to estimate the molecular weights of CTB, Pvs25H-A, and their fusion complex by calculating the partition coefficient (Kav) values for each protein standard and sample protein.
Immunization with CTB-Pvs25H-A fusion protein and analysis of induced antibodies.
Eight-week-old female BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). Mice (four or eight mice per group) were immunized via the s.c. or the i.n. route with 30 μg of Pvs25H-A, a mixture of CTB and Pvs25H-A (30 μg each) or 60 μg of CTB-Pvs25H-A fusion protein. Where indicated, incomplete Freund's adjuvant (IFA; Difco Laboratories, Detroit, MI) or 0.1 to 1.0 μg of CT (List Biological Laboratories, Campbell, CA) was used for s.c. or i.n. adjuvants, respectively. The mice were immunized three times, at weeks 0, 2, and 3.
Mice were anesthetized 1 week after the third immunization (week 4) by intraperitoneal injection of pentobarbital sodium salt (Nacalai Tesque, Inc.) and were sacrificed by exsanguination to collect serum. For specific serum antibody analysis, ELISA plates (Sumilon; Sumitomo Bakelite Co.) were coated with Pvs25H-A (5 μg/ml) in bicarbonate buffer by incubating the plate at 4°C overnight. The plate was washed twice with PBS-T and once with PBS. The plate was blocked with 1% bovine serum albumin (BSA) in PBS for 2 h at 37°C. Twofold serial dilutions of the antisera starting with 50-fold dilution with 0.5% BSA in PBS were applied to the wells in duplicate, which were then incubated for 2 h at 37°C. The plate was then incubated with anti-mouse IgG conjugated to alkaline phosphatase (1/4,000; Sigma-Aldrich) for 2 h at 37°C. p-Nitrophenylphosphate (Bio-Rad) was added to the plate for color development, and the absorbance at OD415 was measured after 20 min of incubation at 37°C, using a microplate reader (Bio-Rad). The antibody titer was defined as the serum dilution that gave an OD415 value equal to 0.1 or as the serum dilution where a one magnitude higher dilution gave an OD415 value of <0.1.
All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of the Ryukyus, and the experiments were conducted according to the Ethical Guidelines for Animal Experiments of the University of the Ryukyus.
Mosquito membrane feeding assay.
Heparinized syringes were used for peripheral blood collection, with written informed consent, from P. vivax patients who came to a malaria clinic in the Mae Sod district in the Tak province of northwestern Thailand. Single species infection with P. vivax was confirmed by Giemsa stain of thick and thin blood smears. The levels of parasitemias and the gametocytemias were 0.22 and 0.02%, respectively, for the volunteer P. vivax patient 1, and 0.23 and 0.01%, respectively, for the volunteer P. vivax patient 2. Collected blood was aliquoted into tubes (300 μl/tube), and the plasma was removed after brief centrifugation. Pooled mouse antisera were mixed with an equal volume of heat-inactivated normal human AB serum prepared from malaria-naive donors. The test antiserum sample was mixed and incubated with P. vivax-infected blood cells (1:1 [vol/vol] ratio) for 15 min at room temperature. The mixture was then applied to a membrane feeding apparatus kept at 37°C to allow starved Anopheles dirus A mosquitoes (Bangkok colony, Armed Forces Research Institute of Medical Sciences) to feed on the blood meals for 30 min. Fully engorged mosquitoes were separated from unfed mosquitoes, maintained for a week in an insectary kept at 26°C, and provided with 10% sucrose water. For each blood sample-serum mixture, 20 mosquitoes were dissected, and the number of oocysts in the midgut was counted under a microscope after 0.5% mercurochrome staining.
All human subject research conducted in the present study was reviewed and approved by the Ethics Committee of the Thai Ministry of Public Health and the Institutional Review Board of the Walter Reed Army Institute of Research.
Detection of native Pvs25 in antisera from immunized mice.
Peripheral blood from P. vivax-infected patients was collected as described above. The gametocytemic patient blood was used to grow zygotes and ookinetes in vitro, as described previously (23). They were spotted onto slides and fixed with acetone as described previously (1-3). The slides were blocked with 5% nonfat milk in PBS and incubated with mouse antisera derived from immunization with CTB-Pvs25H-A fusion protein emulsified with IFA or with the fusion protein supplemented with CT (1 μg), after dilution of the antisera 100-fold with 5% nonfat milk in PBS. The samples were washed with ice-cold PBS and incubated with Alexa 488-conjugated anti-mouse antibody (Invitrogen, Carlsbad, CA). After a wash with ice-cold PBS, the slides were viewed by confocal scanning laser microscopy (LSM5 Pascal; Carl Zeiss MicroImaging, Thornwood, NY).
Statistical analysis.
The Wilcoxon-Mann-Whitney test was used to compare antibody titers or the number of oocysts per mosquito between the nonimmune and an indicated immunization group or between indicated two immunization groups. The Kruskal-Wallis test was used to compare antibody titers or the number of oocysts per mosquito among three groups (i.e., S, M, and L). The chi-square test was used to analyze the difference in the proportion of parasite-free mosquitoes out of the total number of mosquitoes examined between the nonimmune and an indicated immunization group or between indicated two immunization groups. All statistical analysis were conducted by using JMP software version 8.0 (SAS Institute, Inc., Cary, NC).
RESULTS
Expression and purification of recombinant Pvs25H-A isoform from P. pastoris.
Ookinete surface proteins (OSPs) contain several intramolecular disulfide bonds, e.g., the 11 disulfide bonds in Pvs25, which are important for overall structural integrity and native antigenicity. For this reason, E. coli expression systems are unsuitable, and therefore yeast Saccharomyces cerevisiae expression systems are used (12, 15, 19, 22). We explored other, more efficient recombinant protein expression systems for Pvs25 and found a higher production efficiency in the yeast P. pastoris. We constructed a plasmid for secretory expression of Pvs25 as a C-terminal hexahistidine-tagged protein (Pvs25H) (Fig. 1a). The culture supernatant of recombinant P. pastoris was found to contain several protein species that were not present in the culture supernatant of vector-transformed clones (Fig. 1b), and these protein bands specifically reacted with anti-Pvs25 antiserum, as well as with an anti-hexahistidine tag monoclonal antibody (data not shown). Secreted Pvs25H was affinity purified on a nickel Sepharose column and further separated into large (fractions 16 to 19) and small (fractions 21 to 23) protein species by size exclusion chromatography (Fig. 1c and d). At least five protein bands were identified on SDS-PAGE (Fig. 1b) and their corresponding peaks by size exclusion chromatography (fractions 16, 17, 18-19, 21-22, and 22-23 in Fig. 1c and d). The apparent molecular masses of these protein species based on gel mobility on SDS-PAGE were estimated to be 63.2, 47.3, 33.2, 16.1, and 28.3 kDa, respectively. The calculated molecular mass of Pvs25H (based on its deduced amino acid sequence) is 20.2 kDa, to which the fraction 21 and 22 protein species corresponded most closely. We therefore concluded that this was monomeric Pvs25H. Furthermore, the apparent molecular masses of protein species found in fractions 18 and 19, 17, and 16 corresponded very well to multiples of the molecular mass of the apparent monomer, so we concluded that they represented the dimer, trimer and tetramer of Pvs25H, respectively. The protein species that appeared in fractions 22 and 23 exhibited markedly different gel mobility (28.3 kDa) from that of the monomeric protein (16.1 kDa) (Fig. 1d), although these two showed extensively overlapping chromatographic peaks on size exclusion chromatography (Fig. 1c). We concluded that the protein species in fractions 22 and 23 was a monomeric protein with a distinct hydrophobic character, and thus decided to further separate these two monomers based on hydrophobicity profile by HIC. HIC clearly separated the two monomeric isoforms (Fig. 1f), and we named the less hydrophobic isoform and the more hydrophobic isoform the A and B monomeric isoforms, respectively (19). The observed lower gel mobility for the A isoform than the B isoform on SDS-PAGE under nonreducing conditions (Fig. 1d) was consistent with the results of HIC: the more hydrophilic the molecule, the fewer SDS molecules bind, and the protein becomes less negatively charged, resulting in reduced gel mobility on SDS-PAGE.
Molecular characterization of Pvs25H isoforms.
The A isoform appeared as a single sharp band, but the other isoforms, including the multimers and the B monomer, appeared as diffuse bands on SDS-PAGE (Fig. 1b and d to g), indicating that the A isoform is constrained to a more uniform molecular configuration than the other isoforms. In addition, all of the isoforms, except the A isoform, generated at least three identical protein bands when samples were heat treated in the presence of SDS and β-mercaptoethanol (2-ME) prior to SDS-PAGE. The A isoform appeared as a single protein band with a slightly higher molecular mass than that for the largest protein band among the three bands observed for the other isoforms (Fig. 1e).
To characterize the molecular configuration of each isoform furthermore, mixtures of the multimers, including dimers, trimers, and tetramers and the A and B monomers, were subjected to SDS-PAGE after various treatments, as indicated in Fig. 1g. The gel mobility of all of the isoforms did not show any noticeable changes after boiling (100°C, 2 min), but 2-ME treatment (10% [vol/vol]) resulted in a banding pattern very similar to the pattern observed for complete denaturation (2-ME and boiling), except that the A isoform seemed to be slightly more resistant to the reducing agent than the other isoforms. This became particularly notable when lower concentrations of the reducing agent were used (data not shown). These results suggest that the multimers comprised B monomers self-cross-linked by intermolecular disulfide bonds and that the A isoform has a higher molecular rigidity than the other isoforms. Because all of the isoforms exhibited a strong resistance to the boiling and SDS, but not to the 2-ME and SDS treatment, it is plausible that the physical integrity of the protein is critically, if not completely, maintained by disulfide bonds.
Next, to evaluate the status of the covalent disulfide bonds in the Pvs25H protein, Ellman's test was conducted for each isoform. No isoform reacted with the Ellman's reagent, suggesting either that no reduced sulfhydryls were present or that the molecules were inaccessible to the reagent. However, treatment with TCEP immobilized on agarose prior to the Ellman's test resulted in the detection of 4 to 6 molecules of reduced sulfhydryls per molecule of B monomer or multimeric isoforms, but fewer than 0.3 molecules of reduced sulfhydryls were detected per molecule of A monomer. These results indicated that the B and multimeric isoforms have disulfide bonds that are more accessible to surface-immobilized TCEP than those of the A isoform, indicating that the A isoform has more deeply buried disulfide bonds than the other isoforms. It is indicative of the higher molecular flexibility of the B isoform and the multimers than of the A isoform. Interestingly, however, denaturation of proteins with 2% SDS or 6 M guanidine hydrochloride, or TCEP agarose treatment in the presence of these denaturants prior to the Ellman's test, did not further increase the level of free sulfhydryls. These results strongly support the notion that all of the isoforms are tightly packed molecules and that their rigidity is maintained by intramolecular disulfide bonds and other noncovalent interactions.
Finally, the N-terminal protein sequences determined by the Edman degradation method for each isoform supported the results of SDS-PAGE, in that the multimers and the B isoform contain a mixture of polypeptide species with multiple N termini; however, the A isoform comprises a single polypeptide with a longer, unique N terminus (Fig. 1h). These results suggested that the multimers and the B isoform contain the same set of polypeptide species, with multiple primary structures and presumably various folding configurations. By using different combinations of structurally heterologous polypeptides, different isoforms might be generated.
Taken together, we concluded that the A isoform, which has a more uniform protein configuration, is less hydrophobic and has higher molecular rigidity than the B isoform, and perhaps it most closely resembles the native Pvs25 protein at the structural level. The proportions of each isoform expression were estimated to be 42% (A isoform), 27% (B isoform), 16% (dimer), 10% (trimer), and 5% (tetramer). Thus, the A isoform was produced most abundantly among all of the isoforms. The final protein yields of the total Pvs25H and the A isoform using our expression and purification method were 30 to 50 mg and 12 to 20 mg/liter of culture medium, respectively. We confirmed that the purified Pvs25H-A contained endotoxin at levels less than 0.05 EU/μg of protein. Based on these observations, we decided to use the A isoform (Pvs25H-A) as a TBV antigen to be linked to CTB.
Chemical conjugation of Pvs25H-A to CTB and its molecular evaluation.
Recombinant CTB was expressed by P. pastoris strain GS115 and purified as previously reported (11). Pvs25H-A was chemically conjugated to CTB by using the heterobifunctional cross-linker SPDP (Fig. 2). Because CTB contains two cysteine residues per monomeric subunit, which, in the native form, are involved in an intramolecular disulfide bond, the existence of reduced sulfhydryls in our recombinant CTB was determined by using Ellman's reagent, and none was detected (data not shown).
Various conjugation schemes were evaluated for efficiency of linking Pvs25H-A to CTB via SPDP (Fig. 2a and b). Consistent with the results of Ellman's test for Pvs25H-A and CTB, SPDP modification of only one protein failed to generate the CTB-Pvs25H-A fusion complex (Fig. 2b). Thus, at least one partner protein had to be treated with the reducing agent to expose free sulfhydryls and make it reactive toward the pyridyldithiol groups added to the partner protein. Because intact disulfide bonds might be important for the overall structural integrity and native antigenicity of Pvs25 (12, 15, 19, 22), we avoided treating it with reducing agents. Therefore, the CTB or SPDP-modified CTB (CTBSPDP) was treated instead with DTT (designated as CTBDTT or CTBSPDP/DTT in Fig. 2a). Although both CTBDTT and CTBSPDP/DTT, when reacted with SPDP-modified Pvs25H-A (Pvs25H-ASPDP), generated substantial levels of fusion complex with retained affinity for GM1-ganglioside, sequential treatment of CTB with SPDP and then with DTT resulted in an even higher specific reactivity toward Pvs25 antiserum (Fig. 2b).
Second, to evaluate the homogeneity of the fusion complex and the stoichiometry of each component within the complex, proteins before and after the conjugation process were analyzed by size exclusion chromatography (Fig. 2c). The two chromatographic peaks for Pvs25H-A and CTB in a mixed sample disappeared, and a new single peak emerged with an apparent molecular mass of 97.2 kDa. Because the molecular masses of Pvs25H-A and CTB are 29.8 and 53.4 kDa, respectively, based on the Kav values of chromatography standard proteins, the average stoichiometric ratio for CTB and Pvs25H-A was calculated to be 1:1.5, indicating that one CTB pentamer molecule carries one to two molecules of Pvs25H-A on its surface. Alternatively, if it is assumed that the fusion complex is highly homogeneous and its stoichiometric ratio is 1:1, the 14-kDa discrepancy between the observed and calculated fusion complex mass may be explained by irregularities in the molecular shape, resulting in a higher apparent molecular mass.
Taking all of the results together, we decided to use the Pvs25H-ASPDP + CTBSPDP/DTT conjugation method (Fig. 2d) to generate the fusion complex for all immunization experiments.
Immunogenicity in mice of Pvs25H-A and its fusion protein with CTB when administered by the s.c. or the i.n. route.
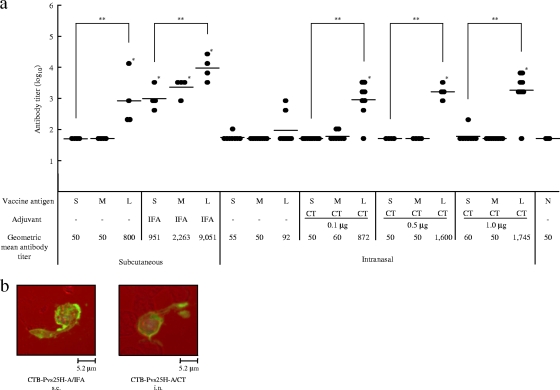
BALB/c mice were immunized with Pvs25H-A (designated as “S” in Fig. 3 and 4), a mixture of Pvs25H-A and CTB (designated as “M” in Fig. 3 and 4), or CTB-Pvs25H-A fusion protein (designated as “L” in Fig. 3 and 4), by the s.c. or the i.n. route, with or without the indicated adjuvants, at weeks 0, 2, and 3. Antisera were collected at week 4, and the Pvs25H-A-specific IgG titers were determined (Fig. 3a). We demonstrated that: (i) s.c. immunization tended to induce a higher response than i.n. immunization in both the absence and the presence of adjuvants; (ii) the fusion protein (L) consistently induced a higher response than antigen alone (S) or the mixture of proteins (M), regardless of adjuvant supplementation; (iii) supplementation with adjuvants was required for substantial augmentation of the IgG response for both immunization routes; (iv) IFA significantly augmented the response elicited by unfused or CTB-mixed antigen, but CT only marginally affected the response elicited by these antigens; and (v) CT did not exhibit a dose-dependent augmentation effect on the IgG response in the dose range used in the present study (0.1 to 1.0 μg). Finally, we confirmed that the antisera specifically recognized the P. vivax ookinete surface by immunofluorescence (Fig. 3b).
FIG. 3.
Immunogenicity of the CTB-Pvs25H-A fusion protein for induction of a Pvs25-specific serum IgG response. (a) Female BALB/c mice (four or eight mice per group) were immunized with Pvs25H-A alone (30 μg) (S), a mixture of cholera toxin B subunit (CTB; 30 μg) and Pvs25H-A (30 μg) (M), or the CTB-Pvs25H-A fusion protein (60 μg), by the subcutaneous (s.c.) or the intranasal (i.n.) route (L), three times, at weeks 0, 2, and 3. Serum samples were collected a week after the third immunization and were evaluated for Pvs25-specific IgG titers. All mice received the same amount of Pvs25H-A antigen, i.e., 30 μg per injection. IFA and CT at various doses (0.1 to 1.0 μg) were used as s.c. and i.n. vaccine adjuvants, respectively. Nonimmune serum (N) was used as a negative control. Antibody titers were defined as the serum dilution that gave an OD415 of 0.1 or the serum dilution for which a one-point-higher dilution (2-fold) gave an OD415 of <0.1. *, Significantly different from nonimmune serum as determined by the Wilcoxon-Mann-Whitney test (P < 0.05); **, significantly different among the three groups (S, M, and L) as determined by the Kruskal-Wallis test (P < 0.001). (b) Ookinete-specific reactivity of induced antisera analyzed by immunofluorescence. The antisera derived from s.c. immunization with the CTB-Pvs25H-A fusion protein emulsified with IFA (CTB-Pvs25H-A/IFA), or the fusion protein administered i.n. with CT (1 μg) (CTB-Pvs25H-A/CT) specifically recognized native Pvs25 protein expressed on the surface of Plasmodium vivax ookinetes. Scale bar, 5.2 μm.
FIG. 4.
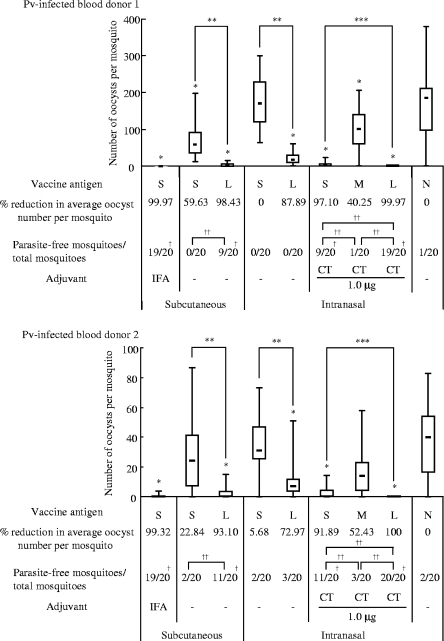
Transmission-blocking vaccine (TBV) effects of the induced mouse antisera on Plasmodium vivax oocyst development in the Anopheles dirus A mosquito midgut. TBV effects on oocyst numbers induced by antisera (1/2 dilution) obtained from mice immunized with each antigen formulation (S, M, and L) as described in Fig. 3. N, nonimmune serum. Either IFA or CT was used as an adjuvant, as indicated. The data are expressed as the median values of oocyst number found per mosquito (bar within the box) with the 25 and 75% quartiles (the box) and ranges (whiskers above and below the box). The percent reduction was calculated as the reduction in the average oocyst number for each immunization group compared to the average oocyst number for the unimmunized control group (N). The number of parasite-free mosquitoes per total number of mosquitoes examined (20 mosquitoes) is provided. The analysis was performed twice, using different blood samples, as indicated in the upper panel (P. vivax [Pv]-infected blood donor 1) and the lower panel (Pv-infected blood donor 2). M groups without adjuvant supplementation, M and L groups with IFA supplementation, and all groups with 0.1- and 0.5-μg CT supplementation were excluded from membrane feeding analysis. *, P < 0.001 versus the nonimmune (N) group as determined by the Wilcoxon-Mann-Whitney test; **, P < 0.001 between the S and L groups as determined by the Wilcoxon-Mann-Whitney test; ***, P < 0.001 among the three groups (S, M, and L) as determined by the Kruskal-Wallis test; †, P < 0.005 versus the nonimmune (N) group as determined by the chi-square test; ††, P < 0.005 between the indicated two groups as determined by the chi-square test.
Transmission-blocking effect of the induced mouse antisera against field strains of P. vivax parasites.
The TBV efficacy of the induced mouse antisera against P. vivax parasites in infected blood samples from patients was evaluated by the membrane feeding assay. The same experiments were performed twice, using blood samples from two volunteer donors (Fig. 4). The average number of oocysts observed per mosquito fed on patient blood mixed with antisera induced by s.c. immunization of mice with Pvs25H-A/IFA was reduced by >99.9% compared to the naive control serum (N). Omission of the adjuvant significantly abated the effect down to 20 to 60% reduction; however, conjugating the antigen to CTB resulted in a dramatic restoration of the vaccine efficacy back to >90%. A similar tendency, albeit with significantly lower efficacy, was observed for i.n. immunization, in that antisera induced by i.n. immunization with the fusion protein decreased the oocyst number by 70 to 90%, whereas unfused antigen conferred only a 0 to 6% blocking effect. As expected, CT supplementation augmented the effect for i.n.-administered antigens, in that both unfused and CTB-fused antigens conferred a blocking effect of >90%. Interestingly, however, addition of CTB to the mixture of antigen and CT significantly abated the vaccine efficacy down to 40 to 50%. The reason for this is unknown, and it could not have been predicted from the antibody titers (Fig. 3a). Taken together, we concluded that chemical coupling of Pvs25 to CTB is a potentially promising strategy to enhance the transmission-blocking efficacy in i.n. and s.c immunization regimes.
DISCUSSION
Pvs25 is one of the top-priority P. vivax TBV candidates, and the production of stable and functional forms of the antigen in the most appropriate formulation is crucial (4; MVTR). In the present study, we investigated the methylotrophic yeast P. pastoris as a production host for Pvs25. The yield of Pvs25H was comparable to that reported previously for its expression in S. cerevisiae (19). When expressed in S. cerevisiae, this protein was also produced as a mixture of various isoforms (19). Although we observed a similar protein expression pattern, i.e., multimers and the A and B monomers, higher proportions of the molecularly homogeneous A isoform than the heterogeneous B and multimeric isoforms were produced when Pvs25H was expressed in P. pastoris and not in S. cerevisiae. This might present an advantage of using P. pastoris expression system for Pvs25 vaccine production rather than S. cerevisiae system. The P. pastoris-derived A isoform could be as conveniently and efficiently purified from the culture supernatant as reported for S. cerevisiae-derived A isoform, by a combination of affinity, size exclusion, and hydrophobic interaction chromatographies.
The next critical step in vaccine generation is the optimization of vaccine antigen formulations; a search for the optimal antigen formulation is often considered to be as important as choosing the best antigen among many vaccine candidate antigens. Pvs25H antigen adsorbed onto Alhydrogel (Brentag Biosector, Frederilssund, Denmark) has recently been shown to induce antibody effectively in human volunteers in a phase 1 clinical trial, and the antigen was found to be efficacious, as evidenced by significant transmission-blocking activity observed in the membrane feeding assay (17). That study confirmed that Pvs25 is a very promising TBV candidate; however, it is highly desirable to induce higher levels of transmission-blocking immunity for practical vaccine development (17). Another phase 1 clinical trial using Montanide ISA 51, a water-in-oil emulsion, has recently been completed; however, due to an unexpected frequent local reactogenicity, the vaccine efficacy has not been verified (29). Therefore, there seems to be an increasing demand for the development of a new immune-enhancing vaccine platform technology for malaria OSPs, because they are low-molecular-weight proteins that are by themselves not sufficiently immunogenic.
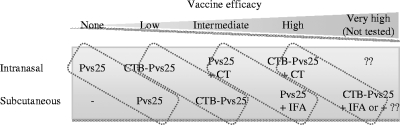
There have been several reports showing examples of chemical conjugation of Plasmodium falciparum OSPs with potential antigen carrier molecules such as the outer membrane protein of Neisseria meningitidis (30), exoprotein A of Pseudomonas aeruginosa (16), ovalbumin (16), and a P. falciparum OSP itself by chemical crosslinking (16). All of these were demonstrated to increase TBV efficacy, but no attempts have yet been made to enhance the immunogenicity of P. vivax OSPs by coupling them to other proteins. In the present study we evaluated CTB as a potential carrier for Pvs25. First, to extend our previous study where CT was used as adjuvant (1-3), we tested our hypothesis that the mucosal immunogenicity of Pvs25 would increase when the protein was coupled to the nontoxic CTB subunit, even in the absence of CT supplementation. Second, to explore CTB's less-characterized immune potentiating properties for s.c.-delivered antigens, we immunized mice with the CTB-Pvs25H-A fusion protein by an s.c. route, in the presence or absence of IFA. Our principal finding was that the coupling of the antigen to CTB profoundly enhanced its immunogenicity in i.n. as well as in s.c. immunization regimes, without supplementation with extraneous adjuvants. However, the membrane feeding assay revealed that there was still much room for improvement (Fig. 4). For instance, although i.n. administration of the fusion protein alone conferred a relatively high transmission-blocking immunity (88 and 73% decreases in oocyst number for blood samples from donors 1 and 2, respectively) compared to unfused antigen alone or the unimmunized control group, only a few mosquitoes (0/20 to 3/20) were free of parasites. Supplementation of CT to the fusion protein increased the efficacy close to complete blockade (>99.9%), significantly increasing the number of mosquitoes free of parasites (19/20 to 20/20). Because supplementation of CT to unfused antigen resulted in an intermediate level of protection (97% [9/20] and 92% [11/20] decrease in oocyst number for blood samples from donors 1 and 2, respectively), we concluded that both the CT supplementation and the CTB-coupling strategies contributed to the increased vaccine efficacy, although the former was more efficient than the latter. A similar tendency was observed for the s.c. immunization regime: s.c. administration of the fusion protein alone conferred a more than 90% decrease in oocyst number, in which approximately half of the mosquitoes were free of parasites (9/20 to 11/20); however, the use of IFA with unfused antigen contributed more than the fusion method. We observed that the efficacy of the fusion protein administered alone by the s.c. route was almost equal to that attained by the unfused antigen administered i.n. with CT supplementation, in terms of the average numbers of oocyst per mosquito (>90%) as well as the number of mosquitoes free of parasites (9/20 to 11/20). Similarly, the vaccine efficacy for unfused antigen administered s.c. with IFA was almost equal to the level attained by the fusion protein administered i.n. with CT in terms of the average number of oocysts per mosquito (>99%) as well as the number of mosquitoes free of parasites (19/20 to 20/20). Although we did not assess the vaccine efficacy of the fusion protein emulsified in an oil adjuvant such as IFA, IFA was very effective in that almost complete blockade was observed for unfused antigen. It is likely that a combination of oil adjuvant and the CTB-coupling strategy would further enhance the efficacy. Taken together, we conclude that (i) s.c. immunization is more efficacious than i.n. immunization, inducing “one-level-higher” immunity than i.n. immunization, and (ii) a CTB-coupling strategy is substantially effective in enhancing transmission-blocking immunity, but supplementation with extraneous adjuvants is expected to induce an even higher immunity (Fig. 5).
FIG. 5.
Schematic summary of the observed or expected (but not tested) transmission-blocking vaccine (TBV) efficacy induced by each immunization regime. s.c. immunization tended to induce “one-level-higher” immunity than i.n. immunization in our experimental model. Identical or similar vaccine formulations are encircled by a dotted line. CTB, cholera toxin B subunit; CT, cholera toxin; IFA, incomplete Freund's adjuvant.
The clinical use of CT, particularly as a nasal adjuvant, is hampered by its toxicity (26). Furthermore, the nontoxic CTB has yet to be proven a safe nasal vaccine delivery molecule. Therefore, an alternative approach for using CTB as a vaccine antigen carrier is highly desirable. Although there have been numerous reports demonstrating enhanced mucosal immunogenicity of various antigens by coupling to CTB (13), very few systematic studies have been conducted to assess CTB's antigen carrier capacity for s.c.-delivered antigens. Our present study clearly demonstrated the potential of CTB in s.c. vaccine platform design. Furthermore, it is notable that, unlike antigens emulsified with oil adjuvant such as IFA or antigens administered with an aluminum hydroxide adjuvant, the protein-only CTB-coupled antigens are likely to be much less reactogenic. It is believed that recent innovations in effective but much less locally reactogenic and safer oil adjuvants such as MF59 (Chiron Corp., Emeryville, CA) (20, 21), the Montanide ISA series (Seppic, Inc., Fairfield, NJ) (20, 29), and the GlaxoSmithKline adjuvant systems (GlaxoSmithKline, Brentford, United Kingdom) (8, 27), will expedite malaria vaccine development. It is also possible that protein delivery molecules will ultimately be combined with effective oil or other adjuvants, including aluminum hydroxide. This is supported by our recent unpublished study in which a recombinant malaria antigen administered with an alum adjuvant only marginally enhanced its immunogenicity, whereas the same antigen loaded onto carrier molecules became highly immunogenic when applied together with the alum.
In the present study, we did not assess the molecular mechanisms of the immune potentiating function of CTB. However, our observation that simple mixing of Pvs25H-A with CTB did not produce a profound immune enhancement implies that its immunogenicity results from the antigen delivery, rather than a physiological cell activation, as occurs for CT. Further experiments are ongoing to characterize the immune potentiating function of CTB using the C-terminal 19-kDa fragment of merozoite surface protein 1 from Plasmodium yoelii. The results of these studies will help us judge whether CTB could contribute to a new platform technology for the design of s.c.-delivered subunit vaccines against infectious diseases such as malaria.
Acknowledgments
We thank the staff of the Department of Entomology, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand, for their technical assistance.
This study was supported by the following grants: the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT); Grants-in-Aid for Scientific Research (19406009 and 20590425) and Scientific Research on Priority Areas (21022034) from MEXT; a grant from The Okinawa Industry Promotion Public Corp. (Naha, Okinawa, Japan); and a Cooperative Research Grant from NEKKEN, 2010.
Editor: J. H. Adams
Footnotes
Published ahead of print on 28 June 2010.
REFERENCES
- 1.Arakawa, T., A. Komesu, H. Otsuki, J. Sattabongkot, R. Udomsangpetch, Y. Matsumoto, N. Tsuji, Y. Wu, M. Torii, and T. Tsuboi. 2005. Nasal immunization with a malaria transmission-blocking vaccine candidate, Pfs25, induces complete protective immunity in mice against field isolates of Plasmodium falciparum. Infect. Immun. 73:7375-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa, T., M. Tachibana, T. Miyata, T. Harakuni, H. Kohama, Y. Matsumoto, N. Tsuji, H. Hisaeda, A. Stowers, M. Torii, and T. Tsuboi. 2009. Malaria ookinete surface protein-based vaccination via the intranasal route completely blocks parasite transmission in both passive and active vaccination regimens in a rodent model of malaria infection. Infect. Immun. 77:5496-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa, T., T. Tsuboi, A. Kishimoto, J. Sattabongkot, N. Suwanabun, T. Rungruang, Y. Matsumoto, N. Tsuji, H. Hisaeda, A. Stowers, I. Shimabukuro, Y. Sato, and M. Torii. 2003. Serum antibodies induced by intranasal immunization of mice with Plasmodium vivax Pvs25 co-administered with cholera toxin completely block parasite transmission to mosquitoes. Vaccine 21:3143-3148. [DOI] [PubMed] [Google Scholar]
- 4.Arevalo-Herrera, M., C. Chitnis, and S. Herrera. 2010. Current status of Plasmodium vivax vaccine. Hum. Vaccin. 6:124-132. [DOI] [PubMed] [Google Scholar]
- 5.Bill and Melinda Gates Foundation. 2009. Global health program. Bill and Melinda Gates Foundation, Seattle, WA. http://www.gatesfoundation.org/global-health/Documents/malaria-strategy.pdf.
- 6.Birkett, A. J. 2010. PATH Malaria Vaccine Initiative (MVI): perspectives on the status of malaria vaccine development. Hum. Vaccin. 6:139-145. [DOI] [PubMed] [Google Scholar]
- 7.Carter, R. 2001. Transmission blocking malaria vaccines. Vaccine 19:2309-2314. [DOI] [PubMed] [Google Scholar]
- 8.Garcon, N., P. Chomez, and M. Van Mechelen. 2007. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev. Vaccines 6:723-739. [DOI] [PubMed] [Google Scholar]
- 9.Genton, B. 2008. Malaria vaccines: a toy for travelers or a tool for eradication? Expert Rev. Vaccines 7:597-611. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood, B. M., D. A. Fidock, D. E. Kyle, S. H. Kappe, P. L. Alonso, F. H. Collins, and P. E. Duffy. 2008. Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118:1266-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harakuni, T., H. Sugawa, A. Komesu, M. Tadano, and T. Arakawa. 2005. Heteropentameric cholera toxin B subunit chimeric molecules genetically fused to a vaccine antigen induce systemic and mucosal immune responses: a potential new strategy to target recombinant vaccine antigens to mucosal immune systems. Infect. Immun. 73:5654-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hisaeda, H., A. W. Stowers, T. Tsuboi, W. E. Collins, J. S. Sattabongkot, N. Suwanabun, M. Torii, and D. C. Kaslow. 2000. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 68:6618-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmgren, J., J. Adamsson, F. Anjuere, J. Clemens, C. Czerkinsky, K. Eriksson, C. F. Flach, A. George-Chandy, A. M. Harandi, M. Lebens, T. Lehner, M. Lindblad, E. Nygren, S. Raghavan, J. Sanchez, M. Stanford, J. B. Sun, A. M. Svennerholm, and S. Tengvall. 2005. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol. Lett. 97:181-188. [DOI] [PubMed] [Google Scholar]
- 14.Kaslow, D. C. 1997. Transmission-blocking vaccines: uses and current status of development. Int. J. Parasitol. 27:183-189. [DOI] [PubMed] [Google Scholar]
- 15.Kaslow, D. C., I. C. Bathurst, T. Lensen, T. Ponnudurai, P. J. Barr, and D. B. Keister. 1994. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect. Immun. 62:5576-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubler-Kielb, J., F. Majadly, Y. Wu, D. L. Narum, C. Guo, L. H. Miller, J. Shiloach, J. B. Robbins, and R. Schneerson. 2007. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc. Natl. Acad. Sci. U. S. A. 104:293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malkin, E. M., A. P. Durbin, D. J. Diemert, J. Sattabongkot, Y. Wu, K. Miura, C. A. Long, L. Lambert, A. P. Miles, J. Wang, A. Stowers, L. H. Miller, and A. Saul. 2005. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23:3131-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendis, K., B. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97-106. [DOI] [PubMed] [Google Scholar]
- 19.Miles, A. P., Y. Zhang, A. Saul, and A. W. Stowers. 2002. Large-scale purification and characterization of malaria vaccine candidate antigen Pvs25H for use in clinical trials. Protein Expr. Purif. 25:87-96. [DOI] [PubMed] [Google Scholar]
- 20.Peek, L. J., C. R. Middaugh, and C. Berkland. 2008. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 60:915-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachdeva, S., A. Mohmmed, P. V. Dasaradhi, B. S. Crabb, A. Katyal, P. Malhotra, and V. S. Chauhan. 2006. Immunogenicity and protective efficacy of Escherichia coli expressed Plasmodium falciparum merozoite surface protein-142 using human compatible adjuvants. Vaccine 24:2007-2016. [DOI] [PubMed] [Google Scholar]
- 22.Saxena, A. K., K. Singh, H. P. Su, M. M. Klein, A. W. Stowers, A. J. Saul, C. A. Long, and D. N. Garboczi. 2006. The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms. Nat. Struct. Mol. Biol. 13:90-91. [DOI] [PubMed] [Google Scholar]
- 23.Suwanabun, N., J. Sattabongkot, T. Tsuboi, M. Torii, N. Maneechai, N. Rachapaew, N. Yim-amnuaychok, V. Punkitchar, and R. E. Coleman. 2001. Development of a method for the in vitro production of Plasmodium vivax ookinetes. J. Parasitol. 87:928-930. [DOI] [PubMed] [Google Scholar]
- 24.Targett, G. A., and B. M. Greenwood. 2008. Malaria vaccines and their potential role in the elimination of malaria. Malar. J. 7(Suppl. 1):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuboi, T., M. Tachibana, O. Kaneko, and M. Torii. 2003. Transmission-blocking vaccine of vivax malaria. Parasitol. Int. 52:1-11. [DOI] [PubMed] [Google Scholar]
- 26.van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165:4778-4782. [DOI] [PubMed] [Google Scholar]
- 27.Waitumbi, J. N., S. B. Anyona, C. W. Hunja, C. M. Kifude, M. E. Polhemus, D. S. Walsh, C. F. Ockenhouse, D. G. Heppner, A. Leach, M. Lievens, W. R. Ballou, J. D. Cohen, and C. J. Sutherland. 2009. Impact of RTS,S/AS02(A) and RTS,S/AS01(B) on genotypes of P. falciparum in adults participating in a malaria vaccine clinical trial. PLoS One 4:e7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. 2005. World health report. World Health Organization, Geneva Switzerland.
- 29.Wu, Y., R. D. Ellis, D. Shaffer, E. Fontes, E. M. Malkin, S. Mahanty, M. P. Fay, D. Narum, K. Rausch, A. P. Miles, J. Aebig, A. Orcutt, O. Muratova, G. Song, L. Lambert, D. Zhu, K. Miura, C. Long, A. Saul, L. H. Miller, and A. P. Durbin. 2008. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 3:e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu, Y., C. Przysiecki, E. Flanagan, S. N. Bello-Irizarry, R. Ionescu, O. Muratova, G. Dobrescu, L. Lambert, D. Keister, Y. Rippeon, C. A. Long, L. Shi, M. Caulfield, A. Shaw, A. Saul, J. Shiver, and L. H. Miller. 2006. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. U. S. A. 103:18243-18248. [DOI] [PMC free article] [PubMed] [Google Scholar]