Abstract
Cerebral malaria is the most severe complication of human infection with Plasmodium falciparum. It was shown that Plasmodium berghei ANKA-induced cerebral malaria was prevented in 100% of mice depleted of CD8+ T cells 1 day prior to the development of neurological signs. However, the importance of parasites in the brains of these mice was never clearly investigated. Moreover, the relevance of this model to human cerebral malaria has been questioned many times, especially concerning the relative importance of leukocytes versus parasitized erythrocytes sequestered in the brain. Here, we show that mice protected from cerebral malaria by CD8+ T-cell depletion have significantly fewer parasites in the brain. Treatment of infected mice with an antimalarial drug 15 to 20 h prior to the estimated time of death also protected mice from cerebral malaria without altering the number of CD8+ T cells in the brain. These mice subsequently developed cerebral malaria with parasitized red blood cells in the brain. Our results clearly demonstrated that sequestration of CD8+ T cells in the brain is not sufficient for the development of cerebral malaria in C57BL/6 mice but that the concomitant presence of parasitized red blood cells is crucial for the onset of pathology. Importantly, these results also demonstrated that the experimental cerebral malaria model shares many features with human pathology and might be a relevant model to study its pathogenesis.
Malaria causes 1 million deaths per year, most of them due to cerebral malaria (CM) induced by infection with Plasmodium falciparum. The precise mechanism responsible for this neuropathology remains far from being fully understood. Cytoadherence of parasitized red blood cells (pRBC) to endothelial cells of the cerebral microvasculature in P. falciparum-infected people has been implicated as the major process responsible for development of human CM (HCM) (5, 20, 30). However, the observation of this phenomenon in non-CM cases (29) suggested that other factors are involved. The first evidence of leukocyte involvement in HCM pathogenesis came from the observation that individuals suffering from thymic abnormality were protected from severe malaria (11). Moreover, leukocytes were detected in the brains of patients that had died of CM (25, 31). Also, histological analysis of brains from Malawian children that had died of CM revealed an important accumulation of intravascular leukocytes and platelets compared with brains of children dying of other complications of malaria (9, 15).
Infection of the malaria-susceptible C57BL/6 mouse strain with Plasmodium berghei ANKA provides an experimental model of CM that shares some characteristics with HCM and has been extensively used to dissect the mechanisms involved in CM (reviewed in reference 28). While pRBC sequestration has been demonstrated for P. falciparum, it remains to be clearly shown in experimental CM (ECM). Nevertheless, recent studies have shown that P. berghei ANKA can be sequestered in vivo in different organs, including the brain (13, 16). On the other hand, several reports have unequivocally demonstrated that the development of ECM in P. berghei ANKA-infected mice was dependent on the presence of T cells (3, 4, 6, 36). In particular, recent studies demonstrated a role for CD8+ T cells in the effector phase. By comparing rodent models of CM and non-CM it was possible to establish a correlation between the number of CD8+ T cells sequestered in the brain and the development of pathology (3, 4, 23). These cells were sequestered just before the mice started to show neurological signs (27). More importantly, it was shown that depletion of CD8+ T cells 1 day prior to the development of neurological signs protects 100% of the mice (4). Altogether, these results clearly demonstrated that CD8+ T cells were crucial effectors of ECM pathogenesis in mice. Yet, the relevance of P. berghei ANKA infection in C57BL/6 to HCM has been frequently questioned (35), especially regarding the relative importance of leukocytes versus pRBC sequestration in the brain. Thus, we sought to establish whether the presence of pRBCs is necessary for the onset of ECM in P. berghei ANKA-infected C57BL/6 mice.
Here, we clearly demonstrated that brain sequestration of CD8+ T cells is not sufficient for the development of ECM in C57BL/6 mice. The concomitant presence of both CD8+ T cells and pRBC in the brain seemed to play a crucial role in the onset of neuropathology. Importantly, these results demonstrated that ECM could be a relevant model for studying HCM.
MATERIALS AND METHODS
Animals.
Studies were carried out in C57BL/6 male mice (from Jackson Laboratories), aged 6 to 10 weeks, bred and housed in the specific-pathogen-free animal facilities of the Instituto Gulbenkian the Ciência (IGC) and of the Instituto de Medicina Molecular, according to the guidelines of the Animal Care Committee of the IGC. In one group of experiments BALB/c male mice from the same origin were also used.
Parasite and pathology.
Parasite blood stages of P. berghei ANKA clone 1.49L, a P. berghei ANKA line expressing luciferase (clone 676m1cl1) (17), a P. berghei ANKA line expressing green fluorescent protein (GFP) (12), or P. berghei NK65 were stored as a stabilate in liquid nitrogen. Mice were infected intraperitoneally (i.p.) with 106 (P. berghei ANKA) or 3.5 × 106 (P. berghei NK65) parasitized red blood cells (pRBC).
Between 90 and 100% of P. berghei ANKA-infected mice succumb to CM between days 6 and 8 postinfection (p.i.) with moderate parasitemia (6 to 11%). Mice were considered to have CM if they displayed neurological symptoms such as paralysis, deviation of the head, ataxia, convulsions, and coma (10). P. berghei NK65-infected mice have moderate parasitemia at day 7 p.i. but do not develop ECM, dying around day 21 p.i. with high parasitemia (34). The percentage of parasitemia was determined on tail blood smears stained with Giemsa and calculated as the number of pRBC per 100 RBC.
CD8+ T-cell depletion.
Mice were injected i.p. with 250 μg of a rat anti-mouse CD8 monoclonal antibody (MAb) (YTS-169; produced in the laboratory) at day 6 p.i., 1 day before the onset of CM. Depletion was confirmed for each mouse by flow cytometry 5 h after antibody treatment. Briefly, blood lymphocytes, collected from the tail, were stained with conjugated anti-CD3 (145-2C11) and anti-CD8 (53-6.7; BD Biosciences) MAbs. RBCs were then eliminated using BD lysis buffer (Becton Dickinson), and lymphocytes were analyzed on a FACSCalibur instrument (Becton Dickinson).
Antimalarial treatment.
Plasmodium berghei ANKA-infected mice were treated with pyrimethamine, 15 to 20 h before the estimated time of death by ECM. The drug was prepared at 1.25 mg/ml as described previously (24) and injected i.p. in a single injection (100 μl/mouse). Briefly, drug was prepared in 20 ml phosphate-buffered saline (PBS) to which 3 drops of dimethyl sulfoxide (DMSO) and 8 drops of HCl were added. Alternatively, the drug was prepared in DMSO at 7 mg/ml and then diluted 1/100 in drinking water supplied to the mice (pH 3.5 to 5.0) for overnight treatment. Control infected mice were either injected with the vehicle or supplied with drinking water containing 1% DMSO.
Parasite and CD8+ T-cell quantification by quantitative real-time PCR (qRT-PCR).
Mice were killed and perfused intracardially with PBS to remove circulating RBC and leukocytes from the brain. Brains were crushed in a denaturing solution (4 M guanidine thiocyanate, 25 mM sodium citrate, pH 7.0, 0.5% sarcosyl, and 0.7% β-mercaptoethanol in diethyl pyrocarbonate [DEPC]-treated water), and RNA was further extracted using a Qiagen RNeasy minikit according to the manufacturer's instructions. The synthesis of the first-strand cDNA from the RNA templates was carried out using the AMV reverse trancriptase kit (Roche). The relative expression of parasite 18S or host CD8β genes was performed using the SYBR green PCR master mix (Applied Biosystems) on an ABI Prism 7900HT system (Applied Biosystems) as previously described (7) and using the housekeeping gene hprt as an endogenous reference. P. berghei 18S (7)- and mouse hprt and CD8β (14)-specific primer sequences were 5′-AAGCATTAAATAAAGCGAATACATCCTTAC-3′ and 5′-GGAGATTGGTTTTGACGTTTATGTG-3′; 5′-TGCTCGAGATGTGATGAAGG-3′ and 5′-TCCCCTGTTGACTGGTCATT-3′; and 5′-TGCTCGAGATGTGATGAAGG-3′ and 5′-TCCCCTGTTGACTGGTCATT-3′, respectively. Standard curves were performed using plasmids encoding cDNA fragments cloned into TOPO TA (Invitrogen) corresponding to the amplified regions. The relative changes in gene expression between experimental and control groups were calculated by the 2−ΔΔCT method (19) using hprt as an internal control gene.
Ex vivo bioluminescence imaging.
Mice were injected with a P. berghei ANKA line expressing luciferase (17). On day 7 p.i., when control mice (nondepleted C57BL/6-infected mice) were showing signs of ECM, mice were injected i.p. with luciferin (Caliper Life Sciences) and sacrificed after 10 min. Mice were then perfused intracardially with PBS, and their brains were removed immediately to quantify pRBC. Luciferase-expressing pRBC were visualized using an intensified-charge-coupled device (I-CCD) photon-counting video camera from the in vivo Imaging System IVIS Lumina (Xenogen) (13), with a 15-cm field of view (FOV), a small binning factor, and an exposure time of 300 s. The bioluminescence was quantified using the software Living Image (Xenogen), applying a region of interest adjusted to the organs' shape and using average radiance (p/s/cm2/sr) as the unit.
Flow cytometry of brain lymphocytes.
Plasmodium berghei ANKA- or P. berghei NK65-infected mice were killed at different time points p.i. and perfused intracardially with PBS. The brains were removed, and lymphocytes were isolated (33). Briefly, brains were homogenized in PBS supplemented with 2% fetal calf serum (FCS) and 2 U/ml DNase (Sigma). Tissue extracts were then centrifuged, and the pellet was further purified by centrifugation at 1,100 × g for 20 min in 30% (vol/vol) Percoll (Pharmacia) at room temperature. Cell suspensions were incubated with FcBlock CD16/CD32 (clone 2.4G2) followed by incubation with conjugated anti-CD8 antibody (clone YTS169), both produced at the IGC. Fluorescence-activated cell sorter (FACS) analysis was performed on a FACS-Calibur instrument run with the CellQuest program (Becton Dickinson). Data analysis was performed with FlowJo (Tree Star) software.
Assessment of brain vascular permeability.
Blood-brain barrier (BBB) permeability during infection was assessed using the Evans blue assay as described previously (18). Briefly, mice were injected i.v. with 200 μl of PBS-2% Evans blue, sacrificed 1 h later, and perfused intracardially with PBS. Brains were surgically removed, weighed, and placed in 2 ml 100% formamide (Merck) for 48 h at 37°C to extract the Evans blue dye from the tissue. Absorbance was then measured on the formamide at 620 nm (Bio-Rad). Evans blue concentrations per g of brain tissue were calculated from a standard curve prepared with a known concentration of Evans blue in formamide.
Statistical analysis.
Differences between mean values of two groups, at a given time point, were analyzed for statistical significance with SPSS software (version 11.5) using the nonparametric Mann-Whitney test. P < 0.05 was considered significant. Values of parasitemia indicated in the text represent the means ± standard deviations (SD) of the data.
RESULTS
CD8+ T-cell depletion reduces the accumulation of pRBC in the brains of infected mice.
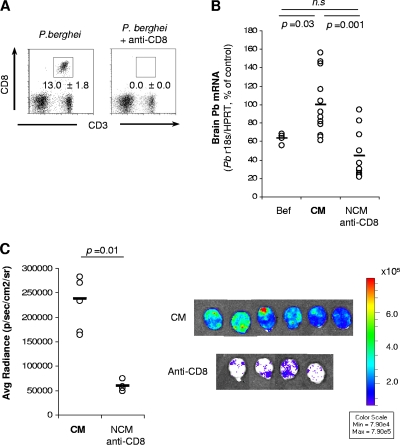
To determine the relevance of pRBC in the onset of ECM in infected mice, we first had to establish whether depletion of CD8+ T cells, which is known to protect 100% of mice from developing ECM (4), has any effect on the accumulation of pRBC in the brain. To that end, P. berghei ANKA-infected mice were treated with anti-CD8 antibodies on the day before the estimated onset of ECM (day 6 p.i.). The presence of pRBC in the brain was assessed on the following day by quantifying parasite load by qRT-PCR after intracardiac perfusion. Five hours after depletion, all injected mice showed almost no circulating CD8+ T cells (Fig. 1A). As previously reported, CD8-depleted mice did not develop ECM (4). At day 7 p.i., pRBC accumulation in the brain was significantly lower in the CD8-depleted mice than in nondepleted animals showing signs of ECM (Fig. 1B), despite a significant increase (P = 0.02) in the amount of circulating pRBC (11.88 ± 2.99 versus 8.99 ± 3.48, respectively). This finding may be explained by the absence of pRBC accumulation in the brain in the CD8-depleted mice. No differences were observed between CD8-depleted mice at day 7 p.i. and mice sacrificed before depletion (Fig. 1B).
FIG. 1.
Accumulation of parasites is reduced in the brains of CD8+ cell-depleted mice. Mice were infected with P. berghei ANKA (A and B) or a P. berghei ANKA line expressing luciferase (C). Groups of mice were treated with anti-CD8 antibodies 20 to 24 h before CM onset. (A) Depletion was checked by flow cytometry of blood lymphocytes 5 h after antibody injection. Data represent means ± SD (n = 5 mice per group). (B) When control mice developed CM, brains were harvested after intracardiac perfusion, and parasite mRNA expression was quantified by qRT-PCR. A group of mice was also analyzed before being treated with anti-CD8 antibodies (Bef). Each symbol represents one individual mouse from a pool of two independent experiments. (C) Mice were infected with luciferase-expressing P. berghei ANKA. When control mice developed CM, brain-accumulated pRBC were visualized after luciferin injection followed by intracardiac perfusion. n.s., not significantly different (Mann-Whitney test).
Mice were infected with luciferase-expressing P. berghei ANKA. A group was also treated with anti-CD8 using the same schedule as before. When nondepleted mice were showing signs of ECM, luciferin was injected, mice were sacrificed and perfused intracardially, and pRBC accumulation in the brain was quantified by bioluminescence. Confirming the above results, pRBC accumulation in the brain was significantly lower in CD8-depleted mice than in nondepleted ones showing signs of ECM (Fig. 1C).
These data demonstrate that the amount of pRBC in the brain is significantly decreased in mice protected from ECM by CD8 depletion. This suggests that in addition to brain-recruited CD8+ T cells, the presence of pRBC in this organ seems to be crucial for the onset of ECM.
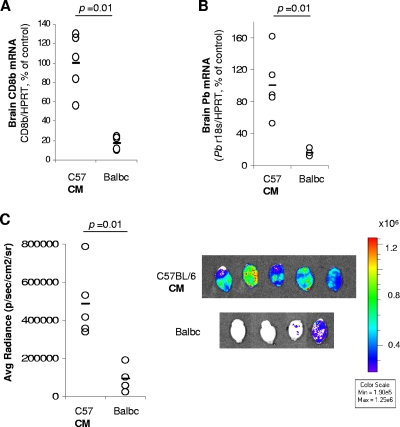
Infected BALB/c mice have reduced accumulation of pRBC in the brain.
It was previously shown that P. berghei ANKA-infected BALB/c mice, a mouse strain resistant to ECM, do not sequester CD8+ T cells in the brain at day 6 to 7 p.i. (4). To determine whether these mice also do not accumulate pRBC in the brain, we quantified the parasite load in the brain by qRT-PCR after intracardiac perfusion. C57BL/6 and BALB/c mice were infected with P. berghei ANKA. At day 6 p.i., C57BL/6 mice that presented symptoms of ECM were sacrificed, whereas BALB/c mice were sacrificed on day 7 p.i. On the day of sacrifice, BALB/c and C57BL/6 mice had similar values of parasitemias (6.43 ± 1.09 versus 6.88 ± 1.17, respectively). As expected, infected BALB/c mice had significantly lower expression of CD8β mRNA in the brain than infected C57BL/6 mice (Fig. 2A). Also, pRBC accumulation in the brain, as measured by qRT-PCR, was significantly lower in BALB/c mice than in C57BL/6 mice (Fig. 2B). C57BL/6 and BALB/c mice were also injected with luciferase-expressing P. berghei ANKA to quantify pRBC accumulation in the brain by bioluminescence. pRBC accumulation was significantly lower in BALB/c than in C57BL/6 mice showing signs of ECM (Fig. 2C), supporting the previous results.
FIG. 2.
Accumulation of parasites is reduced in the brains of BALB/c mice. C57BL/6 (C57) or BALB/c mice were infected with P. berghei ANKA (A and B) or luciferase-expressing P. berghei ANKA (C). C57BL/6 mice were sacrificed when showing signs of CM (day 6 p.i.), and BALB/c mice were sacrificed on day 7 p.i., and brains were harvested after intracardiac perfusion. CD8β mRNA expression (A) and parasite mRNA expression (B) were quantified by qRT-PCR. (C) Brain-accumulated pRBC were visualized after luciferin injection followed by intracardiac perfusion. Each symbol represents one individual mouse. Data are representative of two independent experiments.
These data demonstrate that BALB/c mice infected with P. berghei ANKA, a mouse model of CM resistance, do not sequester CD8+ T cells nor do they accumulate pRBC in the brain.
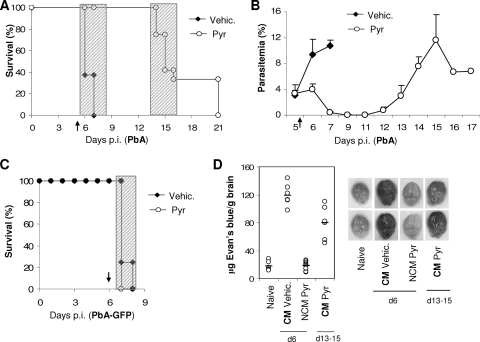
Treatment with antimalarial drugs delays development of ECM in P. berghei ANKA-infected mice.
To establish the importance of pRBC accumulation in the brain in ECM, we next determined the effect of decreasing parasite load in P. berghei-infected mice some hours before death. To that end, P. berghei ANKA-infected mice were treated with pyrimethamine 15 to 20 h before the approximate time of death. Treatment protected 100% of the mice from death for at least 6 days (Fig. 3A). Less than 20 h after treatment, parasitemia was already significantly lower in pyrimethamine-treated mice than in controls and was reduced to less than 1% on the following day (Fig. 3B, day 6 and 7 p.i., respectively). Parasitemia began to increase again between days 11 and 12 p.i., and 70 to 100% of the mice treated with pyrimethamine developed neurological signs characteristic of ECM at days 13 to 15 p.i. and died in less than 6 h (Fig. 3A). These mice developed the neurological syndrome only when their parasitemia was similar to that of the nontreated P. berghei ANKA-infected mice with ECM at day 6 to 7 p.i. (Fig. 3A and B).
FIG. 3.
P. berghei ANKA-infected mice treated with antimalarial drugs do not develop ECM in the following days. P. berghei ANKA (A, B, and D)- or P. berghei ANKA-GFP (C)-infected mice were treated with pyrimethamine (Pyr) or vehicle (Vehic.) 15 to 20 h before the onset of CM (arrow): at the end of day 5 p.i. for P. berghei ANKA- and at day 6 p.i. for P. berghei ANKA-GFP-infected mice. The effects of vehicle or pyrimethamine treatment on survival (A and C) and parasitemia (B) are shown. Shaded areas indicate the times when mice displayed and died with CM symptoms. Data are representative of two independent experiments (n = 5 mice per group). (D) Qualitative (right) and quantitative (left) brain capillary permeability was assessed after intracardiac perfusion of Evans blue-injected mice. Naive (N), CM (vehicle-treated), and NCM (Pyr-treated) mice at day 6 p.i. and CM (Pyr-treated) mice at days 13 to 15 of infection were analyzed. Data represent the pooled results of two independent experiments. Each symbol represents one individual mouse.
When mice were injected with P. berghei ANKA-GFP (a transgenic parasite line resistant to pyrimethamine) (12) and treated with pyrimethamine, they were not protected from ECM (Fig. 3C). This clearly demonstrates that the protection from ECM is due to the decrease in parasitemia and not due to a secondary effect of the drug on the host.
Breakdown of the blood-brain barrier (BBB) has been associated with the development of CM both in mice and in humans (1). Thus, to confirm that pyrimethamine-treated mice were dying later with ECM, breakdown of the BBB was evaluated by measuring Evans blue accumulation in brain tissue. As expected, BBB permeability became significantly enhanced in vehicle-treated P. berghei ANKA-infected mice with ECM at day 6 to 7 p.i. compared to that in the uninfected controls (Fig. 3D). However, no extravasation of Evans blue was observed in pyrimethamine-treated mice at days 6 to 7 p.i. (Fig. 3D). In contrast, increased BBB permeability was observed in pyrimethamine-treated mice dying of ECM between days 13 and 15 p.i.
Altogether, these results strongly suggest that in P. berghei ANKA-infected mice parasite load is critical, in the last few hours before death, for the BBB breakdown and consequent development of the pathology.
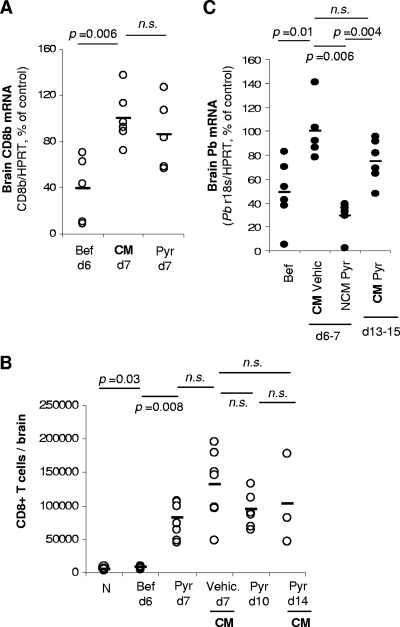
CD8+ T cells still accumulate in the brains of infected mice protected from ECM by pyrimethamine treatment.
We and others had reported a direct requirement of brain-sequestered CD8+ T cells for the onset of ECM (3, 4, 23). CD8+ T cells were quantified in the brains of pyrimethamine-treated P. berghei ANKA-infected mice to rule out that protection against ECM conferred by pyrimethamine treatment was associated with a decrease in CD8+ T cells migration/sequestration to the brain. Compared to noninfected control mice, both groups of mice showed a similar increase of CD8β mRNA expression at day 7 p.i., in spite of the absence of neurological signs in pyrimethamine-treated animals (Fig. 4A). These results were confirmed by quantifying brain-sequestered CD8+ T cells by flow cytometry (Fig. 4B). Furthermore, these cells were significantly increased in the brain until mice developed ECM signs around day 14 p.i. (Fig. 4B).
FIG. 4.
Mice protected from ECM still accumulate CD8+ T cells in the brain but not pRBC. P. berghei ANKA-infected mice were treated with pyrimethamine (Pyr) or vehicle (Vehic.) 20 h before the onset of CM. Brains were harvested after intracardiac perfusion at different days p.i., before (Bef) or after pyrimethamine treatment (A to C). Sequestration of CD8+ T cells was assessed either by qRT-PCR (A) or by flow cytometry (B) in independent experiments. (C) Brain sequestration of parasites was assessed by qRT-PCR. Data are representative of three independent experiments. Each symbol represents one individual mouse. n.s., not significantly different (Mann-Whitney test).
These results clearly show that protection from ECM in pyrimethamine-treated mice was not due to an absence of CD8+ T cell recruitment to the brain. Furthermore, they demonstrate that brain sequestration of CD8+ T cells, although necessary, is not sufficient to induce the neurological signs of ECM.
Accumulation of pRBCs in the brain is concomitant with ECM.
To ascertain that brain accumulation of pRBCs is necessary for the later development of ECM in pyrimethamine-treated mice, qRT-PCR was performed on material extracted from the brains of these animals after intracardiac perfusion. The amount of pRBC accumulation in the brains of vehicle-treated mice increased significantly between days 6 and 7 p.i., when mice showed clear signs of ECM. As expected, brain parasite load was not increased in pyrimethamine-treated mice at day 7 p.i. (Fig. 4C). However, between days 13 and 15 p.i., when pyrimethamine-treated mice clearly showed signs of ECM, pRBC accumulation was significantly increased. These results clearly demonstrate that accumulation of pRBCs in the brain is concomitant with the onset of ECM.
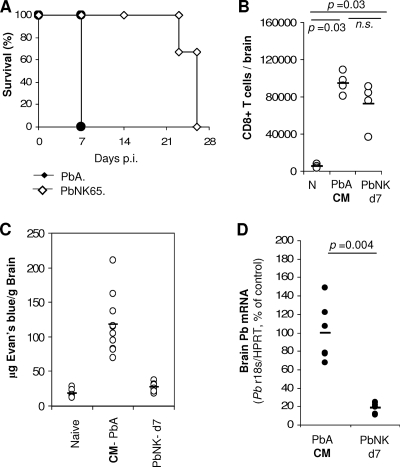
The importance of both CD8+ T-cell and pRBC recruitment to the onset of ECM was further assessed in P. berghei NK65-infected C57BL/6 mice which do not develop ECM (Fig. 5A). Interestingly, the numbers of CD8+ T cells accumulating in the brains of non-ECM P. berghei NK65-infected mice (day 7 p.i.) and P. berghei ANKA-infected mice showing neurological signs (days 6 to 7 p.i.) were similar and in both cases significantly higher than in noninfected C57BL/6 mice (Fig. 5B). No BBB breakdown was detected in P. berghei NK65-infected mice in spite of CD8+ T-cell sequestration in the brain (Fig. 5C). However, P. berghei ANKA-infected mice with ECM showed a significantly higher parasite load in the brain than P. berghei NK65-infected mice on the same day p.i. (Fig. 5D). These results clearly demonstrate that CD8+ T-cell sequestration in the brain can occur in the absence of pRBC accumulation, but they also indicate that both play an important role in BBB breakdown and the onset of ECM.
FIG. 5.
CD8+ T cells but not P. berghei NK65 parasites accumulate in the brain in a non-CM model. Mice were infected i.p. with P. berghei ANKA or P. berghei NK65. (A) Survival of P. berghei NK65-infected mice is shown. (B to D) At day 7 p.i., when P. berghei ANKA-infected mice developed CM, brains were harvested after intracardiac perfusion. In independent experiments, sequestration of CD8+ T cells was assessed by flow cytometry (B), quantitative brain capillary permeability was assessed in Evans blue-injected mice (C), and parasite mRNA expression was quantified by qRT-PCR (D). Data are representative of two independent experiments. Each symbol represents one individual mouse. n.s., not significantly different (Mann-Whitney test).
DISCUSSION
The rapid administration of antimalarial drugs at the onset of neurological symptoms is responsible for the rescue of most children (∼80%) while the remaining 20% still succumb to CM (21). In the latter cases, it is important to find an additional therapy targeting other contributors to pathogenesis. To achieve that, it is fundamental to understand the late cerebral events implicated in pathogenesis and, for that purpose, murine models of CM can be of crucial importance. Nevertheless, the relevance of these models to HCM has been frequently questioned in regard to the relative importance of leukocytes versus pRBC sequestration in the brain (35). This study provides evidence that ECM in C57BL/6 mice occurs only when there is an accumulation of both pRBC and CD8+ T cells in the brain, supporting the idea that C57BL/6/P. berghei ANKA might be a good experimental model to study HCM.
There is a consensus that the presence of CD8+ T cells in the brain is required for the development of ECM. It was shown that depletion of these cells the day before death by ECM can protect 100% of mice (4). Here, we showed that the protection given by CD8+ T-cell depletion was associated with a significant decrease of pRBC accumulation in the brain. Interestingly, BALB/c mice, an ECM-resistant strain that does not sequester CD8+ T cells in the brain, also do not accumulate pRBC in the brain. The absence of CD8+ T cells in the brains of BALB/c mice was reported to be associated with a lower upregulation of the chemokine receptor CXCR3 in splenic T cells (32). In ECM-susceptible strains such as C57BL/6, CD8+ T cells are recruited to the brain by chemokines, namely, CXCR3 ligands, and then adhere to activated endothelial cells (8, 27). Our results demonstrated that the presence of CD8+ T cells at the late stage of infection is required for pRBC accumulation in the brain. The local presence of CD8+ T cells could be crucial for modifying the brain microenvironment, such as the activated status of endothelial cells, by inducing the upregulation of adhesion molecules that could be further required for pRBC accumulation. Whether this accumulation is mediated by direct interaction with the endothelial cells or by interaction with other cells sequestered in the endothelium of the brain is not known. Nevertheless, it might be possible that pRBC accumulation, and not CD8+ T cells, is the ultimate event that leads to BBB breakdown. Alternatively, both could be acting together where pRBC accumulation in the brain might be important for in loco reactivation or function of CD8+ T cells. It has been proposed that activated brain endothelial cells could phagocytize the parasite and process and present antigen to CD8+ T cells. This would induce the killing of the endothelial cell by CD8+ T cells, through the perforin pathway, which could culminate in BBB breakdown (23, 26, 27). For that, pRBC recruitment to the brain would be an essential step.
Recent studies have shown, by in vivo imaging, lower brain accumulation of pRBC in mice protected from ECM by different ways (2, 22). In these studies, and in agreement with our results, mice protected either by depletion of CD25+ cells (2) or by IP-10 deficiency (22) also showed a decrease in sequestration of CD8+ T cells in the brain. Nevertheless, mice injected with anti-IP10 antibodies, which protected 50 to 80% of mice from developing CM, also had lower numbers of CD8+ T cells in the brain, but no significant differences were reported for the accumulation of pRBC at day 6 p.i. (22). However, an enormous variability in bioluminescence levels was detected in that specific experiment (22). We know that pRBC accumulate mainly in the last 20 h before death by ECM. The quantification of pRBC accumulated in the brain by bioluminescence or qRT-PCR is dependent on the clinical score of the mice at the time of sacrifice and analysis. Thus, this could explain the apparent contradiction of our results.
By using different experimental models it was possible to find a correlation between the development of ECM and the sequestration of CD8+ T cells in the brains of infected mice (3, 4, 23). Importantly, we report here for the first time that CD8+ T cells are sequestered in the brains of mice infected with a non-ECM-causing parasite line (P. berghei NK65). However, accumulation of pRBC was not observed in the brains of these mice at day 7 p.i., which is in agreement with the requirement for pRBC accumulation in the brain for the onset of ECM. The most probable explanation for these observations could be the inability of the P. berghei NK65 parasite to adhere to activated endothelial cells in the brain, namely, by not expressing the appropriate ligands. Alternatively, the profile of cytokines or other immune modulators released during the immune response to this parasite could induce the upregulation of adhesion molecules in the brain endothelium essential for CD8+ T-cell sequestration but not for subsequent pRBC accumulation. A third possibility is that the subset of CD8+ T cells generated in response to P. berghei NK65 infection, and then sequestered in the brain, might be different from those in P. berghei ANKA-infected mice. In that case, once sequestered in the brain, these cells could not generate the local microenvironment required for the accumulation of pRBC.
The importance of pRBC accumulation in the brain for the onset of ECM in mouse models was previously studied by different groups and has been very controversial. Histological evidence of concomitant pRBC and leukocyte sequestration was previously shown in the CM model of (BALB/c × C57BL/6)F1 mice (16), but the relative importance of each was not further dissected. More recently, using P. berghei ANKA parasites expressing luciferase, it was proposed that ECM could occur in the absence of pRBC brain sequestration (13). However, the analysis was made on all organs at the same time and the amount of blood accumulated in each organ analyzed was quite different and much higher than in the brain. This fact may have masked the accumulation of pRBC in the brain. In fact, it is now possible to detect significant accumulation of pRBC in the same model of CM by analyzing only the brains (7, 22).
In agreement with a role for brain recruitment of pRBC in the onset of ECM, we clearly showed that P. berghei ANKA-infected mice were completely protected from developing ECM on the day following a single treatment with the antimalarial drug pyrimethamine. Critically, this protection was not associated with the absence of CD8+ T-cell sequestration, since there was still accumulation of these cells in the brain. Moreover, these mice developed ECM later, when the parasitemia reach a certain level and parasites were detected in the brain. Brain endothelial cells, as well as CD8+ T cells, became activated during the immune response to the parasite (reviewed in reference 27). Both events appear necessary for subsequent brain sequestration of CD8+ T cells (27). Our data demonstrated that CD8+ T cells sequestered in the brain even when parasites were eliminated by pyrimethamine treatment in the late stage of infection. This was probably due to the activation of CD8+ T cells and brain-endothelial cells prior to parasite elimination by pyrimethamine treatment. Thus, elimination of parasites by pyrimethamine treatment does not prevent CD8+ T-cell sequestration but protects mice from ECM, reinforcing the importance of pRBC accumulation in the brain just prior to the onset of ECM and their ability to induce the pathology. It is possible that, as mentioned above, pRBC accumulation in the brain is necessary for in loco reactivation or function of the CD8+ T cells that have been accumulated, modifying their phenotype and rending them “pathogenic.” Interestingly, the number of sequestered CD8+ T cells in the brain during this time did not decrease significantly, but whether they correspond to the same sequestered cell population or to successive sequestrations of different subsets of CD8+ T cells remains to be established. It will be interesting to know how long CD8+ T cells might remain in the brain if the antimalarial treatment is maintained for a longer period.
Altogether, this study reveals that brain sequestration of CD8+ T cells, although required, is not sufficient for CM onset in C57BL/6 mice. Concomitant recruitment of pRBC, which is in part dependent on the sequestration of CD8+ T cells, seems to play a critical role in the onset of ECM. These findings provide new important insights into the role(s) and interaction(s) between these two major players in ECM pathogenesis. Moreover, our data provide evidence that the C57BL/6-P. berghei ANKA mouse model of ECM might have much stronger resemblance to HCM than had been asserted.
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia (FCT) with the coparticipation of FEDER, Portugal (POCTI/MGI/46719/2002). F. G. Baptista was supported by IEFP, Portugal, Ana C. Pena by grant IMM/BI/47-2008, and Ana Pamplona by grant SFRH/BPD/26633/2006, both from FCT, Portugal.
We thank Ligia Deus, Ana Rita França, and Silvia Portugal for help with qRT-PCR, Rosa Maria Cabrita for antibody production, Dolores Bonaparte and Alina Costa for animal production and care, and Teresa Pais for useful discussions.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 6 July 2010.
REFERENCES
- 1.Adams, S., H. Brown, and G. Turner. 2002. Breaking down the blood-brain barrier: signaling a path to cerebral malaria? Trends Parasitol. 8:360-366. [DOI] [PubMed] [Google Scholar]
- 2.Amante, F. H., A. C. Stanley, L. M. Randall, Y. Zhou, A. Haque, K. McSweeney, A. P. Waters, C. J. Janse, M. F. Good, G. R. Hill, and C. R. Engwerda. 2007. A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am. J. Pathol. 171:548-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagot, S., F. Nogueira, A. Collette, V. do Rosário, F. Lemonier, P. A. Cazenave, and S. Pied. 2004. Comparative study of brain CD8+ T cells induced by sporozoites and those induced by blood-stage Plasmodium berghei ANKA involved in the development of cerebral malaria. Infect. Immun. 72:2817-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belnoue, E., M. Kayibanda, A. M. Vigário, J. C. Deschemin, N. van Rooijen, M. Viguier, G. Snounou, and L. Rénia. 2002. On the pathogenic role of brain-sequestered αβCD8+ T cells in experimental cerebral malaria. J. Immunol. 169:6369-6375. [DOI] [PubMed] [Google Scholar]
- 5.Berendt, A. R., G. D. Tumer, and C. I. Newbold. 1994. Cerebral malaria: the sequestration hypothesis. Parasitol. Today 10:412-414. [DOI] [PubMed] [Google Scholar]
- 6.Boubou, M. I., A. Collette, D. Voegtlé, D. Mazier, P. A. Cazenave, and S. Pied. 1999. T cell response in malaria pathogenesis: selective increase in T cells carrying the TCR Vβ8 during experimental cerebral malaria. Int. Immunol. 11:1553-1562. [DOI] [PubMed] [Google Scholar]
- 7.Bruna-Romero, O., J. C. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499-1502. [DOI] [PubMed] [Google Scholar]
- 8.Campanella, G. S., A. M. Tager, J. K. El Khoury, S. Y. Thomas, T. A. Abrazinski, L. A. Manice, R. A. Colvin, and A. D. Luster. 2008. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc. Natl. Acad. Sci. U. S. A. 105:4814-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, I. A., M. M. Awburn, R. O. Whitten, C. G. Harper, N. G. Liomba, M. E. Molyneux, and T. E. Taylor. 2003. Tissue distribution of migration inhibitory factor and inducible nitric oxide synthase in falciparum malaria and sepsis in African children. Malar. J. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Souza, J. B., and E. M. Riley. 2002. Cerebral malaria: the contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect. 4:291-300. [DOI] [PubMed] [Google Scholar]
- 11.Edington, G. M. 1967. Pathology of malaria in West Africa. Br. Med. J. i:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franke-Fayard, B., H. Trueman, J. Ramesar, J. Mendoza, M. van der Keur, R. van der Linden, R. E. Sinden, A. P. Waters, and C. J. Janse. 2004. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137:23-33. [DOI] [PubMed] [Google Scholar]
- 13.Franke-Fayard, B., C. J. Janse, M. Cunha-Rodrigues, J. Ramesar, P. Büscher, I. Que, C. Löwik, P. J. Voshol, M. A. den Boer, S. G. van Duinen, M. Febbraio, M. M. Mota, and A. P. Waters. 2005. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc. Natl. Acad. Sci. U. S. A. 102:11468-11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii, Y., K. Kitaura, K. Nakamichi, T. Takasaki, R. Suzuki, and I. Kurane. 2008. Accumulation of T-cells with selected T-cell receptors in the brains of Japanese encephalitis virus-infected mice. Jpn. J. Infect. Dis. 61:40-48. [PubMed] [Google Scholar]
- 15.Grau, G. E., C. D. Mackenzie, R. A. Carr, M. Redard, G. Pizzolato, C. Allasia, C. Cataldo, T. E. Taylor, and M. E. Molyneux. 2003. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J. Infect. Dis. 187:461-466. [DOI] [PubMed] [Google Scholar]
- 16.Hearn, J., N. Rayment, D. N. Landon, D. R. Katz, and J. B. de Souza. 2000. Immunopathology of cerebral malaria: morphological evidence of parasite sequestration in murine brain microvasculature. Infect. Immun. 68:5364-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janse, C. J., J. Ramesar, and A. P. Waters. 2006. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 1:346-356. [DOI] [PubMed] [Google Scholar]
- 18.Kuschnaroff, L. M., L. Overbergh, H. Sefriouni, H. Sobis, M. Vandeputte, and M. Waer. 1999. Effect of staphylococcal enterotoxin B injection on the development of experimental autoimmune encephalomyelitis: influence of cytokine and inducible nitric oxide synthase production. J. Neuroimmunol. 99:157-168. [DOI] [PubMed] [Google Scholar]
- 19.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 20.MacPherson, G. G., M. J. Warrell, N. J. White, S. Looareesuwan, and D. A. Warrell. 1985. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol. 119:385-401. [PMC free article] [PubMed] [Google Scholar]
- 21.Newton, C. R., and S. Krishna. 1998. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol. Ther. 79:1-53. [DOI] [PubMed] [Google Scholar]
- 22.Nie, C. Q., N. J. Bernard, M. U. Norman, F. H. Amante, R. J. Lundie, B. S. Crabb, W. R. Heath, C. R. Engwerda, M. J. Hickey, L. Schofield, and D. S. Hansen. 2009. IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog. 5:e1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nitcheu, J., O. Bonduelle, C. Combadiere, M. Tefit, D. Seilhean, D. Mazier, and B. Combadiere. 2003. Perforin-dependent brain infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J. Immunol. 170:2221-2228. [DOI] [PubMed] [Google Scholar]
- 24.Ono, T., T. Tadakuma, and A. Rodriguez. 2007. Plasmodium yoelii yoelii 17XNL constitutively expressing GFP throughout the life cycle. Exp. Parasitol. 115:310-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patnaik, J. K., B. S. Das, S. K. Mishra, S. Mohanty, S. K. Satpathy, and D. Mohanty. 1994. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am. J. Trop. Med. Hyg. 5:642-647. [PubMed] [Google Scholar]
- 26.Potter, S., T. Chan-Ling, H. J. Ball, H. Mansour, A. Mitchell, L. Maluish, and N. H. Hunt. 2006. Perforin mediated apoptosis of cerebral microvascular endothelial cells during experimental cerebral malaria. Int. J. Parasitol. 36:485-496. [DOI] [PubMed] [Google Scholar]
- 27.Rénia, L., S. M. Potter, M. Mauduit, D. S. Rosa, M. Kayibanda, J. C. Deschemin, G. Snounou, and A. C. Grüner. 2006. Pathogenic T cells in cerebral malaria. Int. J. Parasitol. 36:547-554. [DOI] [PubMed] [Google Scholar]
- 28.Schofield, L., and G. E. Grau. 2005. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 5:722-735. [DOI] [PubMed] [Google Scholar]
- 29.Silamut, K., N. H. Phu, C. Whitty, G. D. Turner, K. Louwrier, N. T. Mai, J. A. Simpson, T. T. Hien, and N. J. White. 1999. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am. J. Pathol. 155:395-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor, T. E., W. J. Fu, R. A. Carr, R. O. Whitten, J. S. Mueller, N. G. Fosiko, S. Lewallen, N. G. Liomba, and M. E. Molyneux. 2004. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 10:143-145. [DOI] [PubMed] [Google Scholar]
- 31.Toro, G., and G. Román. 1978. Cerebral malaria. A disseminated vasculomyelinopathy. Arch. Neurol. 35:271-275. [DOI] [PubMed] [Google Scholar]
- 32.Van den Steen, P. E., K. Deroost, I. Van Aelst, N. Geurts, E. Martens, S. Struyf, C. Q. Nie, D. S. Hansen, P. Matthys, J. Van Damme, and G. Opdenakker. 2008. CXCR3 determines strain susceptibility to murine cerebral malaria by mediating T lymphocyte migration toward IFN-gamma-induced chemokines. Eur. J. Immunol. 38:1082-1095. [DOI] [PubMed] [Google Scholar]
- 33.Vigário, A. M., O. Gorgette, H. C. Dujardin, T. Cruz, P. A. Cazenave, A. Six, A. Bandeira, and S. Pied. 2007. Regulatory CD4+ CD25+ Foxp3+ T cells expand during experimental Plasmodium infection but do not prevent cerebral malaria. Int. J. Parasitol. 37:963-973. [DOI] [PubMed] [Google Scholar]
- 34.Waki, S., J. Tamura, M. Imanaka, S. Ishikawa, and M. Suzuki. 1982. Plasmodium berghei: isolation and maintenance of an irradiation attenuated strain in the nude mouse. Exp. Parasitol. 53:335-340. [DOI] [PubMed] [Google Scholar]
- 35.White, N. J., G. D. Turner, I. M. Medana, A. M. Dondorp, and N. P. Day. 2010. The murine cerebral malaria phenomenon. Trends Parasitol. 1:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yañez, D. M., D. D. Manning, A. J. Cooley, W. P. Weidanz, and H. C. van der Heyde. 1996. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J. Immunol. 157:1620-1624. [PubMed] [Google Scholar]