Abstract
Streptococcus intermedius is an opportunistic pathogen of humans that causes purulent infections, including brain and liver abscesses. This pathogen secretes a human-specific cytolysin, intermedilysin, which has been recognized as a major virulence factor. However, most of the expressional control mechanisms of ily are still unknown. To determine these mechanisms, we analyzed the nucleotide sequence of the ily promoter region. We found a highly homologous region to the catabolite-repressible element (cre) in the ily promoter region and observed a considerable decrease in the amount of secreted intermedilysin when cells were grown in a culture medium containing high concentrations of glucose/utilizable carbohydrates. Disruption of the ccpA gene, which encodes catabolite control protein A, did not induce catabolite repression of ily by glucose/utilizable carbohydrates. In cre mutants, catabolite repression of ily was partially restored, and purified catabolite control protein A bound to an oligonucleotide containing the cre consensus sequence in the ily promoter region. In addition, a prolonged lag phase and slower doubling time of the ccpA mutant cells were observed. Our data show that S. intermedius can modulate ily expression and growth rate through catabolite control protein A-mediated monitoring of the extracellular glucose/utilizable carbohydrate concentration.
Streptococcus intermedius is a member of the Anginosus group of streptococci, constituting a part of the normal flora in the human oral cavity and the upper respiratory, gastrointestinal, and female urogenital tracts. The Anginosus group includes opportunistic pathogens that cause purulent infections and abscesses (4, 15, 16, 31, 40, 41). Streptococcus intermedius is an important human pathogen and a leading cause of deep-seated infections, including brain and liver abscesses (40, 41). This pathogen secretes a human-specific cytolysin, intermedilysin (ILY), originally identified in investigations using S. intermedius strain UNS46 isolated from a human liver abscess (26). ILY is a member of the cholesterol-dependent cytolysins (CDCs), which also include pneumolysin from Streptococcus pneumoniae, streptolysin O from Streptococcus pyogenes, perfringolysin O from Clostridium perfringens, and listeriolysin O from Listeria monocytogenes (3, 29). Cytolysins are major virulence factors of the bacteria producing them, and by using toxin gene-deficient or knockout mutants these have been determined to be key factors in infection events (5, 8, 14, 35, 36). ILY is considered to be the major virulence factor of S. intermedius, essential for invasion of and cytotoxicity to human cells, due to the following: (i) the production level of ILY from isolates found in deep-seated abscesses is 6.2- to 10.2-fold higher than that from the strains found in normal habitats, such as dental plaque, in contrast to the expression levels of other potential virulence factors, such as hyaluronidase and sialidase, where no significant difference in levels has been found (27); (ii) an ily knockout strain showed greatly decreased adherence, invasion, and cytotoxicity of human liver (HepG2) cells, and incubating ILY+ strain UNS38 with antibody to ILY caused drastic reductions in adherence and invasion of the HepG2 cells (36).
Many bacterial species monitor metabolic enzyme activity in order to control the hierarchical utilization of available sugars, a process known as carbon catabolite repression (CCR). The mechanism of CCR in low-GC-content Gram-positive bacteria has been clarified by studies on Bacillus subtilis (7, 9, 11, 38). CCR is controlled by the histidine-containing protein (HPr), phosphoenolpyruvate:sugar phosphotransferase system protein, and catabolite control protein A (CcpA), which is a LacI/GalR-type repressor. The uptake of a preferred carbohydrate such as glucose, fructose, or mannose leads to an increase in cellular levels of fructose-1,6-bisphosphate, which in turn triggers ATP-dependent HPr kinase/phosphatase-catalyzed phosphorylation of HPr at Ser-46. Only seryl-phosphorylated forms of HPr can bind to CcpA, and the P-Ser-HPr/CcpA complexes bind in turn to the catabolite-repressible element (cre). Several in vivo and in vitro studies have shown that the cre consensus sequence is WTGNAANCGNWNNCW (N is any base and W is A or T), where the underlined bases are involved in CcpA binding (18, 25, 32, 39). If the cre site is located within the promoter region or open reading frame, CcpA binding inhibits the RNA polymerase interaction with the promoter or its progression through to DNA, thereby repressing transcription (19). The regulation of virulence genes by CcpA has been highlighted, and many streptococcal virulence factors (e.g., those for streptolysin S and the multiple virulence gene regulator of group A streptococci [GAS] and fructan hydrolase of Streptococcus mutans) have been found to be controlled by CCR (1, 2, 17, 20, 33, 34).
Although only Autoinducer-2 (a LuxS product used by several bacteria in quorum-sensing signaling) has so far been reported to be an activation factor for ily expression (30), most of the expressional control mechanisms of ily remain unknown. To reveal these mechanisms, we analyzed the nucleotide sequence of the ily promoter region for putative control elements. Here, we show that CcpA can control ily expression by direct interaction with the cre site located 3 nucleotides behind the Pribnow box.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Streptococcus intermedius was cultured at 37°C under anaerobic conditions (CO2:H2:N2, 5:10:85). Brain heart infusion (BHI) broth (Becton-Dickinson, Palo Alto, CA), BHI broth without dextrose (United States Biological, Swampscott, MA) supplemented with glucose at the concentrations indicated, or 3-(N-morpholino)propanesulfonic acid (MOPS)-buffered BHI (MOPS-BHI) medium was used for culture. The MOPS-BHI medium contained 100 mM MOPS buffer (pH 7.4) and 18.5 g/liter BHI broth or 17.5 g/liter BHI broth without dextrose, and it was supplemented with glucose or other sugars at the specified concentrations. An enzyme immunoassay (EIA) and quantitative reverse transcription-PCR (qRT-PCR) were performed using the modified MOPS-BHI medium in which the amount of BHI broth was reduced to 50% of the amount present in the MOPS-BHI medium. Escherichia coli was grown in Luria-Bertani (LB) medium at 37°C under aerobic conditions. Antibiotics were added at the following concentrations: ampicillin at 50 μg/ml for E. coli culture, chloramphenicol at 20 μg/ml for E. coli and 2 μg/ml for S. intermedius, and erythromycin at 1 μg/ml for S. intermedius.
TABLE 1.
Bacterial strains and plasmids used in the study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| S. intermedius strains | ||
| UNS38 | ILY high-producing strain from human brain abscess | 36 |
| UNS38 B3 | ily knockout strain derived from UNS38 | 36 |
| UNS38 ΔccpA | ccpA knockout strain derived from strain UNS38 | Present study |
| UNS38 EMr | Insertion of EM cassette in front of ily promoter region of UNS38 | Present study |
| UNS38 creE | Same as UNS38 but with three point mutations in cre site | Present study |
| UNS38 creS | Same as UNS38 but with two nucleotide deletions and one point mutation in cre site | Present study |
| E. coli strains | ||
| C600 | thr-1 leuB6 thi-1 lacY supE44 rfbD1 fhuA21 | Lab collection |
| DH5αZ1 | F−endA1 hsdR17(rK− mK+) supE44 thi-1 recA1 gyrA(Nalr) relA1 Δ(lacZYA-argF)U169 deoR φ80dlacZΔM15 | 23 |
| Plasmids | ||
| pSET1 | S. suis replicating plasmid with Cm resistance | 37 |
| pSET1 Δlac | Deletion of lac promoter in pSET1 | Present study |
| pSET1 Δlac p15A | pSET1 Δlac carrying p15A replication origin | Present study |
| pccpA | pSET1 Δlac p15A carrying ccpA and putative native promoter | Present study |
| pUHE212-1 | IPTG-inducible expression vector with N-terminal six-His tag | 10 |
| pN-his ccpA | pUHE212-1 carrying ccpA | Present study |
Databases and multiple sequence alignment.
Nucleotide and protein sequences were obtained from GenBank or the Microbes genomic BLAST databases by an Entrez cross-database search at the National Center for Biotechnology Information (National Institutes of Health). A multiple sequence alignment was constructed with the Parallel PRRN program (Kyoto University Bioinformatics Center, Japan) (12).
Generation of the ccpA knockout mutant and cre mutants.
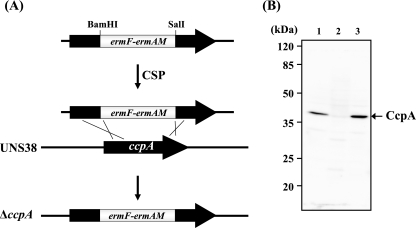
A ccpA (DDBJ accession no. AB566422) knockout mutant (ΔccpA) was produced by homologous recombination (Fig. 1 A). Briefly, a 1,125-bp DNA fragment including the putative native promoter and coding region of ccpA (GenBank accession no. AB543256) was amplified by PCR from S. intermedius type strain NCDO2227 genomic DNA with primers ccpA EcoRI F and ccpA PstI R (Table 2). The 5′ region of the ccpA DNA fragment (437 bp) was amplified by using ccpA EcoRI F and internal primer ccpA BamHI R (Table 2) and then digested with BamHI. The 3′ region of the latter (525-bp) DNA fragment was amplified using internal primer ccpA SalI F and ccpA PstI R (Table 2) and then digested with SalI. The erythromycin resistance cassette was amplified from ily knockout mutant UNS38 B3 (36) genomic DNA using primers erm (BamHI) F and erm (SalI) R (Table 2). The BamHI- and SalI-digested erythromycin cassette was ligated to the BamHI-digested 5′ region and SalI-digested 3′ region of the 1,125-bp DNA fragment. This ligated fragment was amplified by nested PCR with the primers nested ccpA F and nested ccpA R (Table 2). UNS38 ΔccpA was produced by transformation of competence-stimulating peptide (CSP; DSRIRMGFDFSKLFGK)-treated UNS38 with the PCR amplicon. Colonies were selected and isolated on a BHI agar plate containing 1 μg/ml erythromycin. Disruption of ccpA was confirmed by PCR and immunoblotting using anti-CcpA mouse antiserum (Fig. 1B and data not shown).
FIG. 1.
(A) Schematic of the strategy for producing the ΔccpA strain by allelic exchange mutagenesis; ermF-ermAM, erythromycin resistance genes for the erythromycin cassette. (B) CcpA immunoblotting analysis for confirmation of the ΔccpA and ΔccpA/pccpA strain. UNS38, ΔccpA, and ΔccpA/pccpA cells were cultured in BHI medium. Whole-cell extracts (10 μg) were separated by 12.0% SDS-PAGE. Immunodetection was carried out with anti-CcpA mouse serum. Lane 1, UNS38; lane 2, ΔccpA; lane 3, ΔccpA/pccpA.
TABLE 2.
Oligonucleotides used in the study
| Target or purpose | Primer or probe | Sequence (5′-3′) |
|---|---|---|
| PCR primers | ||
| pSET1 | pSET delta lac F | CTTGCATGCCTGCAGGTCGACTCTAGAG |
| pSET delta lac R | CTCGCGCATGCGAAAGCCCCTAGAAGACG | |
| p15A | p15A PstI | AGACTGCAGGGATATATTCCGCTTCCTCGC |
| p15A SphI | ATCGCATGCGCTTGGACTCCTGTTGATAG | |
| ccpA | ccpA EcoRI F | AAGAATTCAAAATGCTTGAAAGTGTTTCCAATAAGTG |
| ccpA PstI R | GACTGCAGGCCTAACGGCCTCTTCTTTATTTCC | |
| his ccpA BamHI F | GAGGATCCATGAACACAGACGATACTG | |
| his ccpA PstI R | GCCCTGCAGTTATTTCCTTGTTGAACAACG | |
| Disruption of ccpA | ccpA SalI F | CTATGGATGCGGTCGACTGTCTTGCAAAAGGCAACCG |
| ccpA BamHI R | CTTTGGATCCAGCGTATTAACAACAGAGAC | |
| nested ccpA F | GTGATAAAATGATTTTGGTAGGAATGTGAAAACG | |
| nested ccpA R | GTTGAACAACGCTCAATCAGACCATGTGCC | |
| Erythromycin cassette | erm (BamHI) F | AATGGATCCCCCGATAGCTTCCGCTATTG |
| erm (SalI) R | CAGTAGTCGACCTAATAATTTATCTAC | |
| Mutagenesis of cre | ily up F | AATACCAAGCCCAATGCAA CTCCTG |
| ily up R | CTTTATAAGTCGACAGAAGCCCATTTTTCC | |
| ily promo. F | CTGTTTCTAACTAGATCTACTTCCCC | |
| ily no. 3 R | GTAGTCTGTGTTGTTTTGGATAGTTGC | |
| nested PCR F | CGACACAATGACTAAAGTGTATCACTCATC | |
| nested PCR R | GAGATTGGTACAGCTGGACTTTGAGCAC | |
| mt-cre EcoRI F | GAAAGAATTCGCAATTTAGCAAAAGGAGGC | |
| mt-cre EcoRI R | GCGAATTCTTTCATTTATATATTAACACTATGATGAGC | |
| mt-cre SphI F | GAAAGCATGCAATTTAGCAAAAGGAGGC | |
| mt-cre SphI R | ATTGCATGCTTTCATTTATATATTAACACTATGATGAGC | |
| qRT-PCR for ily | qRT-ily F | CTATTAGTGTAAACTTACCGGGATTG |
| qRT-ily R | GGACTATTTGGAGAGTCTACGCTAGC | |
| qRT-PCR for gyrB | qRT-gyrB F | GATGAGGCACTAGCAGGTTTTGC |
| qRT-gyrB R | GTGAACAGTTGTCCCTGTTCG | |
| Probes for EMSA | cre sense | ATAAATGAAAGCGTTAGCAATTTAGCAAA |
| cre antisense | TTTGCTAAATTGCTAACGCTTTCATTTAT | |
| cre EcoRI sense | ATAAATGAAAGAATTCGCAATTTAGCAAA | |
| cre EcoRI antisense | TTTGCTAAATTGCGAATTCTTTCATTTAT | |
| cre SphI sense | ATAAATGAAAGCATGCAATTTAGCAAA | |
| cre SphI antisense | TTTGCTAAATTGCATGCTTTCATTTAT | |
| non-cre sense | ATCGGTCTGTTATTTGTGTGTTTTTTATA | |
| non-cre antisense | TATAAAAAACACACAAATAACAGACCGAT |
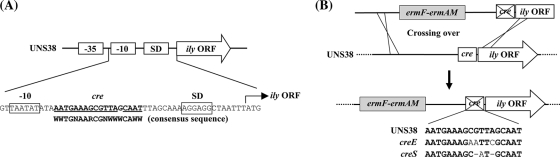
For mutagenesis of the putative cre site located 3 bp behind the putative ily promoter −10 region (see Fig. 5A), an erythromycin cassette was inserted 391 bp upstream from the ily translation start site (ATG). The procedure was performed as follows: the 1,408-bp DNA fragment within the region from bp 1799 to 391 upstream of the ily translation start site was amplified from UNS38 genomic DNA using primers ily up F and ily up R (Table 2). The amplified fragment (ily UP) was digested with SalI. A 1,555-bp DNA fragment including the putative native promoter and a portion of the ily coding region was amplified from UNS38 genomic DNA using primers ily promo. F and ily No. 3 R (Table 2). The latter amplified fragment (ily no. 3) was digested with BglII. The BamHI- and SalI-digested erythromycin cassette was ligated with SalI-digested ily UP and BglII-digested 3′ ily no. 3. The ligated fragment was then amplified by using primers nested PCR F and nested PCR R (Table 2). UNS38 erythromycin resistance (EMr) was produced by transformation of CSP-treated UNS38 with the PCR amplicon. Colonies were selected and isolated on a BHI agar plate containing 1 μg/ml erythromycin. Insertion of the erythromycin cassette was confirmed by PCR and sequencing (data not shown).
FIG. 5.
(A) Schematic of a partial sequence of the UNS38 promoter region. The cre-like sequence of the ily promoter region and its consensus sequence are shown with bold letters, and the nucleotide sequences corresponding to the consensus sequence are underlined. ily ORF, ily open reading frame coding region; −10 and −35, ily promoter regions; SD, Shine-Dalgarno sequence. (B) Strategy for site-directed mutagenesis of cre. The EcoRI or SphI site was introduced by PCR in the cre consensus sequence using UNS38 EMr (38 EM) genomic DNA (for details, see Materials and Methods). UNS38 creE (creE) and UNS38 creS (creS) were produced by transformation of CSP-treated UNS38, with each PCR amplicon carrying mutations in cre. In creE, the EcoRI site was introduced in the cre consensus sequence with three point mutations (plain text); in creS, the SphI site was introduced with two nucleotide deletions (-) and one point mutation (plain text).
Mutations were introduced by PCR in the cre putative site (Fig. 5B). Primers mt-cre EcoRI F and mt-cre EcoRI R (Table 2) were designed to introduce mutagenesis into the putative cre site to create an EcoRI restriction site in the cre consensus sequence. A 3,918-bp DNA fragment of the cre upstream region including an erythromycin cassette was amplified from UNS38 EMr genomic DNA using primers ily up F and mt-cre EcoRI R (Table 2). A 1,119-bp DNA fragment of the cre downstream region was amplified from UNS38 EMr genomic DNA using primers mt-cre EcoRI F and ily no. 3 R (Table 2). The amplified fragments were digested with EcoRI and ligated, and the resulting fragment was amplified using primers nested PCR F and nested PCR R (Table 2). UNS38 creE was produced by transformation of CSP-treated UNS38 with the PCR amplicon; UNS38 creS was produced similarly by using primers mt-cre SphI F and mt-cre SphI R (Table 2). The cre mutants were selected and isolated on a BHI agar plate containing 1 μg/ml erythromycin. Introduction of mutations was confirmed by nucleotide sequencing (data not shown).
Complementation of the S. intermedius UNS38 ΔccpA strain.
For complementation of the UNS38 ΔccpA mutant, Streptococcus suis-E. coli shuttle vector pSET1 (37) was modified as follows. pSET1 was kindly supplied by T. Sekizaki (Research Center for Food Safety, The University of Tokyo). Primers pSET1 delta lac F and pSET1 delta lac R (Table 2) were designed to conduct the PCR for removal of the lac promoter and lacZα for α-complementation in pSET1, creating SphI restriction sites in the resulting PCR product. The amplified fragment was digested with SphI, self-ligated, and transformed in E. coli C600. The resultant plasmid (pSET1 Δlac) was digested with PstI and SphI. Primers p15A PstI F and p15A SphI R (Table 2) were designed to amplify a p15A replication origin of E. coli from pZA43 by PCR (23), creating PstI and SphI restriction sites in the resulting PCR product. This amplified fragment was digested with PstI and SphI, ligated to digested pSET1 Δlac, and transformed into E. coli DH5αZ1 (23). The resultant plasmid (pSET1 Δlac p15A) was used for construction of the plasmid to complement UNS38 ΔccpA. A ccpA fragment containing the putative native promoter was amplified by PCR using primers ccpA EcoRI F and ccpA PstI R (Table 2) from S. intermedius type strain NCDO2227 genomic DNA. The amplified fragment was digested with EcoRI and PstI and cloned into the corresponding sites in pSET1 Δlac p15A. The resultant plasmid (pccpA) was transformed into a CSP-treated UNS38 ΔccpA mutant. Transformants were selected and isolated on a BHI agar plate containing 2 μg/ml chloramphenicol. Complementation of ΔccpA was checked by PCR (data not shown) and Western blotting using anti-CcpA mouse antiserum (Fig. 1B).
Human erythrocyte agar plating.
Hemolysis induced by the bacterial cells was examined on human erythrocyte agar plates at 37°C for 1 day under anaerobic conditions. Human blood was obtained from healthy Japanese volunteers and stored in sterilized Alsever solution at 4°C. Human blood cells (5 ml) in Alsever solution (5 ml) were washed three times with phosphate-buffered saline (PBS) by centrifugation (1,000 × g) and resuspended in 5 ml of PBS. PBS-suspended human erythrocytes were added to MOPS-BHI medium containing 1% agar at a final concentration of 10% (vol/vol).
Preparation of His-tagged recombinant CcpA.
The ccpA gene was amplified from the chromosomal DNA of S. intermedius type strain NCDO2227, using primers his ccpA BamHI F and his ccpA PstI R (Table 2). The amplified fragment was digested with BamHI and PstI and cloned into pUHE212-1 (10). The resultant plasmid (pN-his ccpA) was transformed into E. coli DH5αZ1. Hyperexpression of the recombinant protein was induced by adding 1 mM isopropyl-β-d-thiogalactopyranoside to E. coli cells in mid-log phase, and incubation continued at 37°C for 3 h. The cells were then harvested by centrifugation (5,000 × g) and resuspended in 20 mM Tris-HCl buffer (pH 8.0) containing 1 mM EDTA, 20% sucrose, and 1 mg/ml lysozyme. The suspension was sonicated with an Astrason XL2020 ultrasonic processor and diluted 5-fold with 20 mM Tris-HCl buffer (pH 8.0) containing 10 mM MgCl2. The resultant cell extract was centrifuged at 10,000 × g for 30 min to remove unbroken cells. The supernatant was loaded onto a Ni-nitrilotriacetic acid agarose column (Qiagen GmbH, Hilden, Germany) equilibrated with buffer A (20 mM Tris-HCl [pH 8.0], 300 mM NaCl, and 20 mM imidazole). Recombinant CcpA (N-his CcpA) was eluted with a linear gradient of 20 to 250 mM imidazole in buffer A. Peak fractions were diluted 10-fold with buffer B (20 mM Tris-HCl [pH 8.0], 1 mM EDTA, and 10% glycerol) and loaded onto an Econo-Pac high-Q cartridge (Bio-Rad Laboratories, Richmond, CA). N-his CcpA was eluted with a linear gradient of 0 to 1.0 M NaCl in buffer B, and the fractions were frozen at −80°C until use.
Anti-CcpA mouse antiserum and anti-ILY mouse monoclonal antibody.
To obtain anti-CcpA mouse antiserum, 50 μg purified N-his CcpA in 500 μl PBS was emulsified with an equal volume of Freund's complete adjuvant and administered into mice (intraperitoneal injection [i.p.]). Two weeks later, a booster shot of 50 μg of antigen was administered using Freund's incomplete adjuvant i.p. Mice were sacrificed at 1 week after the booster, and antisera were collected for immunoblotting. To obtain anti-ILY mouse monoclonal antibodies, 50 μg purified native ILY in 500 μl PBS was emulsified with an equal volume of Freund's complete adjuvant and administered into BALB/c mice i.p. Two weeks later, the same volume of the first booster shot of 50 μg of the antigen was administered using Freund's incomplete adjuvant i.p. After a further 2 weeks a final booster with 25 μg antigen in 100 μl PBS was administered to each mouse (intravenous injection [i.v.]). Three days after the final booster, mice were sacrificed and the spleen cells of each mouse were collected and used in hybridoma preparations using SP2/0-Ag14 myeloma as a hybridization partner according to the established PEG protocol (27). Anti-ILY antibody-secreting hybridomas were screened by EIA in 1 μg ILY (fixed)/well in 96-well microtiter plates, and each hybridoma clone was established by the limiting dilution method as described previously (27). Highly reactive monoclonal antibodies were selected and used in ILY immunoblotting and the EIA.
EIA to determine ILY in culture supernatants.
S. intermedius cells were grown in the modified MOPS-BHI medium containing 0.1% or 1% glucose at 37°C for 24 h under anaerobic conditions, and then the culture supernatant was separated by centrifugation (5,000 × g). To estimate the amount of ILY in the culture supernatant of individual strains an EIA was carried out as follows: 50 μl of each culture supernatant diluted with Tris-buffered saline (TBS; 0.9% NaCl and 10 mM TrisHCl [pH 7.4]) was dispensed into the wells of black-masked 96-well EIA plastic plates (ASAHI Glass Co., Ltd., Japan) and dried at 37°C for 24 h. Wells were then blocked with 300 μl/well blocking solution (1% bovine serum albumin in TBS) at room temperature for 1 h. After washing twice with 300 μl/well TBS, 50 μl/well of hybridoma culture supernatant cocktail containing five anti-ILY mouse monoclonal antibodies recognizing different immunodeterminants was dispensed into the wells and reacted with the ILY present at room temperature for 1 h. Each well was washed five times with 300 μl/well TBS. Subsequently, 50 μl/well of 2,500-fold-diluted alkaline phosphatase-labeled anti-mouse IgG(H+L) goat IgG (Promega, Madison, WI) with the blocking solution was dispensed into each well and reacted with any immunocomplex present on the wells at room temperature for 1 h. Each well was then washed five times with 300 μl/well of TBS and 50 μl/well of substrate solution containing 100 mM NaCl, 5 mM MgCl2, and 2 mM 4-methylumbelliferyl phosphate disodium salt (Sigma Chemical Co., St. Louis, MO), and 100 mM Tris-HCl (pH 9.5) was dispensed into each well. After 60 min at 25°C,the fluorescence in each well was measured using an EIA plate reader (TECAN Infinite M200; excitation at 380 nm and emission at 450 nm). Purified ILY prepared as described previously (26) was used to construct a standard curve.
Quantitative RT-PCR analysis.
S. intermedius cells were grown in the modified MOPS-BHI medium at 37°C for 14 h or 24 h under anaerobic conditions, and then cells were separated by centrifugation (5,000 × g). Total RNA was isolated from cells using the FastRNA Pro Blue kit (Qbiogene Inc., Carlsbad, CA) and a FastPrep cell disruptor (Savant Instruments, Holbrook, NY) according to the manufacturer's recommendations. Contaminated chromosomal DNA in the RNA sample was degraded using an RNase-free DNase set (Qiagen GmbH, Hilden, Germany). Degraded DNA and DNase were removed from the RNA sample by using the RNeasy minikit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's recommendations. The RNA concentration was measured using a BioSpec minispectrophotometer (Shimadzu, Kyoto, Japan). cDNA was synthesized using high-capacity cDNA reverse transcription kits (Applied Biosystems, Warrington, United Kingdom), and cDNA corresponding to 100 ng of input RNA was used as template in each real-time PCR mixture. Real-time PCR was carried out in 96-well plates on an ABI Prism 7000 apparatus with Power SYBR Green PCR master mix (Applied Biosystems, Warrington, United Kingdom) and the primer set of qRT-ily F and qRT-ily R (Table 2), which are specific for ily. The amount of qRT-PCR product of gyrB (DDBJ accession no. AB566421) of strain UNS38, which was amplified with the specific primer set of qRT-gyrB F and qRT-gyrB R, was used as an internal control to normalize the amount of total RNA in each sample. To prepare calibration curves for the primer set, we used cDNA from UNS38 creE cultured under high glucose conditions as the template in a five-step dilution (corresponding to 100, 50, 25, 12.5, and 6.25 ng of input RNA). Thermal cycling conditions were as follows: initial denaturation at 50°C for 2 min and then 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The amounts of target RNAs were calculated from the calibration curves.
Electrophoretic mobility shift assay.
Biotin labeling of the 3′ end of single-strand oligonucleotides was performed by using a biotin 3′ end DNA labeling kit according to the manufacturer's instructions (Thermo Fisher Scientific Inc., Rockford, IL). Biotin-labeled single-strand oligonucleotides (Table 2) were mixed and annealed with equal amounts of labeled complementary oligonucleotide (cre sense-cre antisense, cre EcoRI sense-cre EcoRI antisense, and cre SphI sense-cre SphI antisense). The mixture was incubated for 1 h at room temperature. Purified 100 nM N-his CcpA and a 5 nM biotin-labeled double-stranded oligonucleotide probe were mixed in a solution containing 2 μM nonlabeled double-stranded oligonucleotide (non-cre sense and non-cre antisense), 1 mg/ml bovine serum albumin, 7.5% glycerol, 5 mM MgCl2, 1.5 mM EDTA, 1.5 mM dithiothreitol, 75 mM NaCl, 0.3% NP-40, and 15 mM Tris-HCl (pH 7.5) and then incubated at room temperature for 20 min. The DNA-protein complexes were separated from unbound probe on native 4% polyacrylamide gels which had been prerun in 0.25× Tris-borate-EDTA buffer (22.5 mM Tris-borate [pH 8.3], 0.5 mM EDTA), followed by electroblotting to a positively charged nylon membrane. The blotted membrane was treated with a chemiluminescent nucleic acid detection module according to the manufacturer's instructions (Thermo Fisher Scientific Inc.) and exposed to a lumino-image analyzer LAS-4000 miniEPUV (Fuji Film, Tokyo, Japan).
Gel electrophoresis and immunoblotting.
S. intermedius cells were grown in BHI broth at 37°C under anaerobic conditions. The culture supernatant and cells were separated by centrifugation (5,000 × g). The cells were washed three times with PBS and resuspended in 1 ml or 0.5 ml of 20 mM HEPES-KOH, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, and 25% glycerol. Samples were then added to tubes containing lysing matrix B (Qbiogene Inc., Carlsbad, CA) and lysed in a FastPrep cell disruptor (Savant Instruments, Holbrook, NY). To obtain the soluble protein fraction, samples were centrifuged at 17,400 × g for 30 min and the supernatant retained. Total protein (10 μg) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the established Laemmli method (21). For immunoblotting analysis, the gel-resolved proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Blots were incubated with anti-CcpA mouse serum or anti-ILY monoclonal antibody and developed with an Immobilon Western chemiluminescent horseradish peroxidase substrate (Millipore) or 5-bromo-4-chloro-3′-indolylphosphate (BCIP)/nitroblue tetrazolium chloride (NBT) using horseradish peroxidase or alkaline phosphatase-conjugated anti-mouse immunoglobulin G as the secondary antibody.
Statistics.
Data are presented as means ± standard deviations (SD). Statistical comparisons were made using an unpaired two-tailed Student's t test.
RESULTS
Production of ΔccpA and its complementation strain.
The nucleotide sequence of the coding region of ily and its promoter and the potential regulatory region have been reported for strains UNS38 (GenBank accession no. AB212797) and UNS46 (GenBank accession no. AB029317), isolated from brain and liver abscesses, respectively. To investigate the regulation of ily expression, the possible regulatory elements around the ily promoter region were searched using an 18-bp cre consensus sequence, WWTGNAARCGNWWWCAWW (where W is A or T, N is any base, and R is G or A), with alignment of 22 cre genes functional in B. subtilis (25). Interestingly, a highly homologous region to the cre consensus sequence was found 3 nucleotides behind the Pribnow box in the promoter region of both strains UNS38 and UNS46, with 17 bp of the 18-bp potential cre sequence of the ily promoter corresponding to the cre consensus sequence (see Fig. 5A). This result strongly suggested that CcpA could regulate ily expression by CCR. The ccpA gene was identified from the whole-genome sequence of S. intermedius type strain NCDO2227 (K. Kikuchi et al., unpublished data, and encodes a 36.7-kDa protein. A multiple sequence alignment search revealed that NCDO2227 CcpA shares 53% identity with CcpA from B. subtilis subsp. subtilis strain 168, whereas the degree of homology to CcpA with S. pneumoniae ATCC 700669 is 89%. We introduced a ccpA knockout mutation in the UNS38 genome by insertion of an erythromycin cassette (Fig. 1A) in order to investigate whether CCR-controlled ily expression was observable in this mutant. To exclude the possibility that the mutant phenotypes result from other mutations in the chromosome, ΔccpA was complemented in trans with a recombinant plasmid carrying ccpA, including its putative native promoter (pccpA). Immunoblotting analysis using anti-CcpA mouse antiserum was conducted to confirm the deletion of ccpA and complementation by pccpA (Fig. 1B). The immunoblotting result showed a band corresponding to the molecular weight of CcpA from the UNS38 cell extract that was not present in the ΔccpA cell extract. Recovery of CcpA was observed in the cell extract of the ΔccpA complementation strain. The level of CcpA in the ΔccpA complementation strain cells was much higher than that in the wild-type cells, possibly resulting from the increased ccpA copy number due to plasmid complementation.
Effect of ccpA knockout on ILY secretion.
When S. intermedius was cultured in a medium containing a high glucose concentration (>0.5%), the culture pH fell to <5.0 due to lactate accumulation and was accompanied by a loss of ILY activity and a reduction in the level of ILY in the culture supernatant. Therefore, the MOPS-BHI medium was used, maintaining a neutral pH in the culture medium for at least 24 h under the culture conditions.
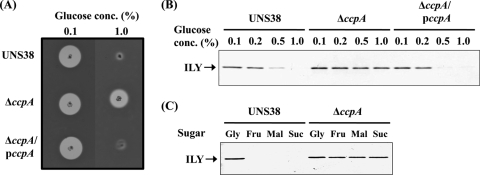
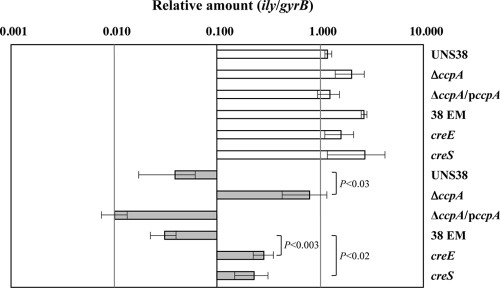
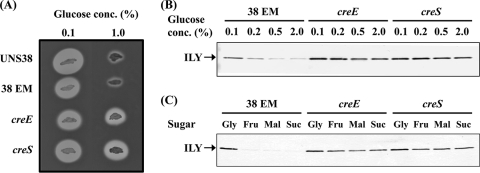
UNS38, ΔccpA, and the ΔccpA complementation strain (ΔccpA/pccpA) were inoculated onto human erythrocyte agar plates containing 0.1% or 1.0% glucose to confirm whether CcpA contributes to ily repression by CCR (Fig. 2 A). When a human erythrocyte agar plate containing 0.1% glucose was used for the hemolysis assay, the sizes of the beta-hemolysis zone surrounding the UNS38, ΔccpA, and ΔccpA/pccpA cells were similar. However, in the presence of high levels of glucose (1.0%), ILY production was repressed in both UNS38 and ΔccpA/pccpA cells with a much reduced zone of beta-hemolysis observed around the UNS38 cells. Conversely, a zone of hemolysis was observed around ΔccpA cells even under high glucose culture conditions. We further examined the correlation between the amount of ILY secreted and the glucose concentration in the culture medium by using immunoblotting analysis (Fig. 2B). UNS38, ΔccpA, and ΔccpA/pccpA cells were cultured in MOPS-BHI medium containing the concentrations of glucose indicated. The amount of ILY secreted into the culture supernatant reduced with increasing glucose concentration in both UNS38 and ΔccpA/pccpA cells. On addition of more than 0.5% glucose to the culture medium, only a weak ILY signal was detected. Moreover, as shown in Fig. 2C, addition of other preferred carbohydrates such as maltose and sucrose instead of glucose showed a similar repressive effect on ILY production. However, for ΔccpA, an apparent ILY signal was detected even under high concentrations of glucose or other preferred and utilizable carbohydrates. Addition of a nonpreferred carbohydrate such as glycerol did not repress ILY production in either UNS38 or ΔccpA cells (Fig. 2C). These data strongly suggested that CcpA could repress ily transcription by CCR. To confirm this possibility, we examined the level of ily mRNA in UNS38, ΔccpA, and ΔccpA/pccpA cells under the low glucose condition (0.1%) and the high glucose condition (1.0%) by using qRT-PCR (Fig. 3). To avoid exhaustion of glucose by overgrowing the cells, we used a modified MOPS-BHI medium, in which the amount of BHI broth was reduced to 50% of the amount present in the MOPS-BHI medium. The relative amounts of ily (ily/gyrB) were measured in UNS38, ΔccpA, and ΔccpA/pccpA cells under the low and high glucose conditions. A striking reduction in ily expression to 4% of that observed under the low glucose condition was observed in UNS38 and ΔccpA/pccpA under the high glucose condition. However, the level of ily expressed by ΔccpA was the same under both high and low glucose conditions; at 24 h the level of ily expressed by ΔccpA was 20-fold greater than that expressed by UNS38 (P < 0.03) under the high glucose condition. At the 24-h time point examined these strains were in different growth phases (Fig. 4). Therefore, we also compared the amount of ily transcript in log-phase cultures (optical density at 600 nm [OD600] of approximately 0.4) under the high glucose condition and observed the amount of ily mRNA transcribed in ΔccpA to be 4.4-fold higher than that in UNS38 (P ≤ 0.002) and 9.3-fold higher in ΔccpA/pccpA (P < 0.001). We also analyzed the amount of secreted ILY by performing EIA on culture supernatants that were identical to those used for the qRT-PCR experiment (Table 3). A significant amount of ILY was detected in culture supernatants under the low glucose condition in UNS38, ΔccpA, and ΔccpA/pccpA cells, although ILY could not be detected in UNS38 and ΔccpA/pccpA under the high glucose condition. However, ΔccpA secreted the same levels of ILY in the culture medium even under the high glucose condition. These data clearly showed that ily expression was regulated by CcpA monitoring of extracellular glucose/utilizable carbohydrate in the culture environment.
FIG. 2.
Effects of glucose/utilizable carbohydrates on ILY secretion by ΔccpA and ΔccpA/pccpA strains. (A) UNS38, ΔccpA, and ΔccpA/pccpA cells were inoculated onto human erythrocyte agar plates containing 0.1% or 1.0% glucose and then incubated at 37°C for 1 day. (B) Cells were grown for 24 h at 37°C in MOPS-BHI medium containing different concentrations of glucose (0.1 to 1.0%). (C) UNS38, ΔccpA, and ΔccpA/pccpA cells were grown for 18 h at 37°C in MOPS-BHI medium containing 0.1% glucose and 1.0% glycerol (Gly), fructose (Fru), maltose (Mal), or sucrose (Suc), and the OD600 of the cultures was measured. Standardized amounts of the culture supernatants were analyzed by 12.0% SDS-PAGE. Anti-ILY monoclonal antibody was used as a probe for immunodetection of ILY.
FIG. 3.
Relative expression levels of the ily gene under low and high glucose conditions. Strains were grown for 24 h at 37°C in modified MOPS-BHI medium with 0.1% or 1.0% glucose. The ily expression levels in UNS38, ΔccpA, ΔccpA/pccpA, UNS38 EMr (38 EM), UNS38 creE (creE), and UNS38 creS (creS) under the low glucose condition (0.1%; open bars) and the high glucose condition (1.0%; gray bars) are indicated relative to the gyrB expression level. The results are plotted on a logarithmic scale. The data are plotted as mean values ± standard deviations of three replicates. Statistically significant values (P < 0.05) are shown (Student's t test).
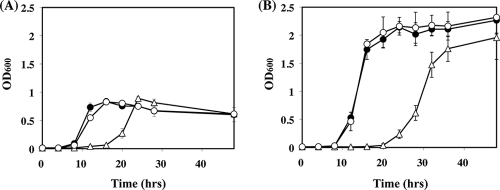
FIG. 4.
Growth curves of UNS38, ΔccpA, and ΔccpA/pccpA strains. Strains were cultured in BHI medium with 0.1% (A) or 1.0% (B) glucose, and the OD600 was measured at the indicated time points. The graphed data are the mean values ± standard deviations of at least four replicates of independent experiments. Symbols: closed circles, UNS38; open triangles, ΔccpA; open circles, ΔccpA/pccpA.
TABLE 3.
ILY secretion into the culture medium
| Glucose concn (%) | Amt of ILY secreteda |
|||||
|---|---|---|---|---|---|---|
| UNS38 | ΔccpA | ΔccpA/pccpA | 38 EM | creE | creS | |
| 0.1 | 8.08 ± 0.04 | 5.36 ± 0.38 | 2.50 ± 0.50 | 4.66 ± 0.46 | 7.94 ± 0.18 | 8.90 ± 0.64 |
| 1.0 | ND | 6.44 ± 0.16 | ND | ND | 1.54 ± 0.10 | 1.28 ± 0.06 |
The values shown are means ± standard deviations (n = 3) of the amount of ILY (μg/ml/OD600 units) in the culture supernatant of individual strains. ND, not detected under the assay condition.
During these experiments, we observed that ΔccpA exhibited a slower growth rate than UNS38 and ΔccpA/pccpA. Therefore, the growth curve of these strains was examined by using BHI medium containing 0.1% or 1.0% glucose (Fig. 4A and B). UNS38 and ΔccpA/pccpA cells had an 8-h lag phase and then grew logarithmically until 12 h in 0.1% glucose or 16 h in 1.0% glucose, following which they entered the stationary phase. However, the ΔccpA cells (Fig. 4A and B) had a prolonged lag phase (16 h in 0.1% glucose or 20 h in 1.0% glucose) and then grew logarithmically (until 24 h in 0.1% glucose or 32 h in 1.0% glucose) before entering stationary phase. Both the UNS38 and ΔccpA/pccpA cells grew with the same doubling time: around 70 min in 0.1% glucose and 60 min in 1.0% glucose. However, the doubling time of the ΔccpA cells was relatively much longer: 115 min in 0.1% glucose and 85 min in 1.0% glucose.
Isolation and characterization of the cre mutants.
To confirm that the putative cre site serves as a CcpA recognition element (Fig. 5 A), two cre-mutated strains were constructed to introduce point and deletion mutations into the cre site of UNS38 EMr (Fig. 5B). UNS38 creE was mutated at three nucleotide positions and UNS38 creS had two nucleotide deletions and one point mutation in the cre site. These mutations did not cause the growth defect observed in the case of ΔccpA. To estimate the level of ILY secretion, UNS38, UNS38 EMr, UNS38 creE, and UNS38 creS were inoculated onto human erythrocyte agar plates containing 0.1% or 1.0% glucose. When human erythrocyte agar containing 0.1% glucose was used for the hemolysis assay, the sizes of the surrounding beta-hemolysis zones were similar in all cases. In contrast, high levels of glucose (1.0%) repressed ILY production in both UNS38 and UNS38 EMr with only a small zone of beta-hemolysis observed (Fig. 6 A). However, a zone of hemolysis was found around the cre mutant cells even in the presence of 1.0% glucose.
FIG. 6.
Effects of glucose/utilizable carbohydrates on ILY secretion by the cre mutant strains. (A) UNS38, 38 EM, creE, and creS cells were inoculated onto human erythrocyte agar plates containing 0.1% or 1.0% glucose and then incubated at 37°C for 1 day. (B) Cells were grown for 20 h at 37°C in MOPS-BHI medium containing different concentrations of glucose (0.1 to 2%). (C) UNS38, 38 EM, creE, and creS cells were grown for 15 h at 37°C in MOPS-BHI medium containing 0.1% glucose and 1.0% glycerol (Gly), fructose (Fru), maltose (Mal), or sucrose (Suc). Then, the OD600 of the cultures was measured, and standardized amounts of the culture supernatants were analyzed by 12% SDS-PAGE. Anti-ILY monoclonal antibody was used as a probe for immunodetection of ILY.
We further examined the correlation between the amount of ILY secreted and the glucose or other carbohydrate concentration in the culture medium by immunoblotting analysis (Fig. 6B and C). UNS38 EMr and cre mutants were cultured in MOPS-BHI medium containing the indicated concentrations of glucose or other carbohydrates. The amount of ILY secreted into the culture supernatant reduced according to increasing glucose/utilizable carbohydrate concentration in the UNS38 EMr cells. Upon addition of more than 0.5% glucose/utilizable carbohydrate to the culture medium, only a weak ILY signal was detected. Conversely, an ILY signal was detected for both the cre mutants even under high glucose/utilizable carbohydrate concentrations, as previously observed for ΔccpA cells. These data strongly suggested that the cre site of the ily promoter region repressed ily expression through the association with CcpA. To confirm this possibility, we examined the relative amount (ily/gyrB) of ily in UNS38 EMr, UNS38 creE, and UNS38 creS cells under the low glucose condition (0.1%) and the high glucose condition (1.0%) by using qRT-PCR (Fig. 3). Identical levels of ily expression were observed in UNS38 EMr, UNS38 creE, and UNS38 creS under the low glucose condition. In contrast, with UNS38 EMr under the high glucose condition a considerable reduction in ily expression was observed, to only 1% of that observed with the low glucose level. The levels of ily expressed by UNS38 creE and UNS38 creS were significantly higher (P < 0.02) than those expressed by UNS38 EMr under the high glucose condition, although these were only 11% (UNS38 creE) and 9% (UNS38 creS) of the level of ily expressed by UNS38 EMr cultured under the low glucose condition. We also analyzed the amount of secreted ILY by performing EIA on culture supernatants that were identical to those used for the qRT-PCR experiment (Table. 3). A significant amount of ILY was detected using EIA from culture supernatants under the low glucose condition in UNS38 EMr, UNS38 creE, and UNS38 creS, although ILY could not be detected in UNS38 EMr under the high glucose condition. UNS38 creE and UNS38 creS could secrete a detectable amount of ILY in the culture medium even under the high glucose condition, although these amounts were 33% (UNS38 creE) and 27% (UNS38 creS) of the amount of ILY observed in the culture supernatant of UNS38 EMr under the low glucose condition. These data showed that the cre site of the ily promoter region could repress ily expression, although cre mutants showed only partial derepression of ily by CCR. Overall our data suggest that CcpA might control ily expression via two pathways: direct repression by binding the cre site and indirect repression controlling the amount of transcriptional regulator for ily.
Electrophoretic mobility shift assay with cre or mutated cre DNA fragments.
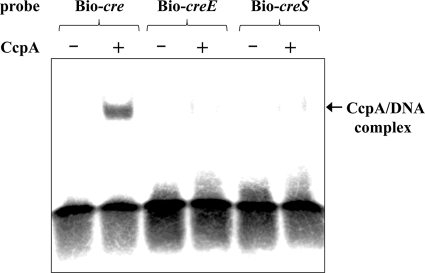
To demonstrate that CcpA directly interacts with the cre region of the ily promoter, we performed electrophoretic mobility shift assays with a purified, histidine-tagged S. intermedius CcpA protein (Fig. 7). CcpA requires HPr-(Ser-46-P) for binding to the cre site in vivo (38). Fructose-1,6-bisphosphate is an allosteric effector of CcpA and enhances the binding of CcpA to the fructan hydrolase (fruA) gene promoter region carrying intact cre of S. mutans (1). However, several reports have shown that although there is slightly low affinity, CcpA can recognize and bind to the cre without HPr-(Ser-46-P) and fructose-1,6-bisphosphate in vitro (1, 2). Therefore, we performed the assays without HPr-(Ser-46-P) and fructose-1,6-bisphosphate. In addition, to avoid nonspecific binding of the biotin-labeled probes to CcpA, we carried out every experiment in the presence of 2 μM nonspecific double-stranded oligonucleotide; this amount was 400-fold higher than that present in the biotin-labeled probes. As shown in Fig. 7, CcpA induced a shift in the mobility of the DNA fragment containing the intact cre sequence (Bio-cre). However, when fragments carrying a mutated cre sequence (Bio-creE or Bio-creS) were used, a dramatic reduction was observed in the ability of CcpA to induce a shift. These data clearly showed that ily expression can be directly controlled by the binding of CcpA to the cre site of the ily promoter region.
FIG. 7.
Electrophoretic mobility shift assay results of biotin-labeled DNA fragments containing wild-type (Bio-cre) or mutated (Bio-creE or Bio-creS) cre sequences. Labeled DNA fragments (5 nM) were incubated with (+) or without (-) purified N-his CcpA (100 nM) and then electrophoresed on a 4% nondenaturing acrylamide gel. The labeled DNA was detected by chemiluminescence.
DISCUSSION
ILY is a major virulence factor that is essential for cytotoxicity and invasion of human cells by S. intermedius. To determine the expression control mechanisms of ily, we analyzed the nucleotide sequence of the ily promoter region and found a highly homologous region to the cre consensus sequence. Therefore, we produced ΔccpA and cre mutant strains to investigate whether ily expression is controlled by CcpA-dependent CCR. We showed that the ΔccpA mutant did not induce catabolite repression of ily by glucose/utilizable carbohydrates, and cre mutants could partially restore catabolite repression of ily. ILY is a member of the CDCs, and cdc genes are found in many Gram-positive pathogens; however, the direct regulation of cdc genes by CcpA has so far not been reported. Indeed, the cre consensus sequence has not been found up to 500 bp upstream from the translation start site of cdc genes such as pneumolysin from Streptococcus pneumoniae P1031, suilysin from Streptococcus suis P1/7, listerolysin O from Listeria monocytogenes EGD-e, perfringolysin O from Clostridium perfringens strain 13, or vaginolysin from Gardnerella vaginalis ATCC 14019 (data not shown). Because localization of the cdc genes in the genome sequence of the regulatory element and promoter region is generally not conserved even in streptococci, CCR of the ily gene seems to have evolved exclusively in S. intermedius.
Most of the streptococcal genes regulated by CcpA revealed in the S. pyogenes M1 MGAS5005 genome by using transcriptome analysis are similar to the genes reported in a study on Bacillus subtilis, many of which are known to be involved or putatively involved in carbohydrate transport and metabolism (20). Carbohydrate catabolism genes mediating transcriptional control by CcpA are thought to have an important function in regulating the pathogenicity of streptococci. Genome-wide studies such as signature-tagged mutagenesis screens have identified the genes involved in basic metabolic processes, including the catabolism of complex carbohydrates, as crucial to the pathogenesis of disease caused by many streptococci (33). In addition, the numerous genes encoding proteins with known or putative carbohydrate metabolism functions are upregulated during GAS and S. pneumoniae infections (13, 28). Several recent reports have indicated that CcpA regulates not only the gene for catabolism but also some genes for virulence factors, including streptolysin S of GAS (20, 34). The broad-spectrum cytolysin streptolysin S is a homologue of bacteriocin, which encodes sagA and localizes in the sagA-I operon of the GAS chromosome (6); sagA encodes a 53-amino-acid protoxin and then converts to a 30-amino-acid active cytolysin by modification of the SagBCD trimeric complex and cleavage of the leader sequence (22, 24). Streptolysin S is a major pathogenic factor and is believed to have important functions related to the pathogenesis of streptococcal necrotizing soft tissue infection. Therefore, the fact that both unrelated cytolysins, ILY and streptolysin S, are similarly controlled by CcpA with synchronization of expression of metabolic genes for carbohydrate utilization is suggestive of some important function of ILY in pathogenesis by S. intermedius.
BHI medium, a nutrient-rich medium, is commonly used for S. intermedius culture. We compared the growth of wild-type strain and ΔccpA in BHI medium containing 0.1% or 1.0% glucose and observed a prolonged lag phase and slower doubling time under both conditions (Fig. 4A and B). A similar but not identical phenotype was observed in ΔccpA from GAS, which grew normally in a nutrient-rich medium; however, a prolonged lag phase was observed in a chemically defined medium with glucose and slower doubling time in a chemically defined medium with maltose (34). These results suggest that CcpA functions to control growth rate by monitoring the extracellular glucose concentration. Addition of 1.0% preferred carbohydrate such as maltose and sucrose into MOPS-BHI medium markedly decreased S. intermedius ILY secretion, as observed with glucose; however, such an effect was not observed in ΔccpA (Fig. 2C). In contrast, cre mutants showed only partial derepression of ily repression by glucose (Fig. 3 and Table 3). CcpA might control ily expression via two pathways: direct repression by binding the cre site and indirect repression controlling the amount of transcriptional regulator for ily. Although these data do not unequivocally demonstrate the influence of CCR/CcpA in real S. intermedius infections, they do give grounds to hypothesize that S. intermedius has two modes of growth: a rapid growth and lower-virulence mode under preferred carbohydrate-abundant conditions and a slow-growing and highly virulent mode under carbohydrate-limited conditions. It may be that the changing growth phase according to the environment would be a sound strategy for long-term parasitism by using ILY to procure nutrients from the host cells.
In conclusion, we have shown that ILY production is modulated by CcpA-mediated CCR. Further studies elucidating the meaning of simultaneous regulation of carbohydrate catabolism genes and ily in S. intermedius would help to clarify the strategy for survival, infectivity, and pathogenesis in humans.
Acknowledgments
We thank A. Kominami for technical assistance.
This work was supported by KAKENHI [Grants-in-Aid for Scientific Research (C) 18592004 and 19590449] from the Ministry of Education, Culture, Sports, Science, and Technology of the Japanese Government.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 12 July 2010.
REFERENCES
- 1.Abranches, J., M. M. Nascimento, L. Zeng, C. M. Browngardt, Z. T. Wen, M. F. Rivera, and R. A. Burne. 2008. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J. Bacteriol. 190:2340-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almengor, A. C., T. L. Kinkel, S. J. Day, and K. S. McIver. 2007. The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the group A streptococcus. J. Bacteriol. 189:8405-8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billington, S. J., B. H. Jost, and J. G. Songer. 2000. Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol. Lett. 182:197-205. [DOI] [PubMed] [Google Scholar]
- 4.Claridge, J. E. III., S. Attorri, D. M. Musher, J. Hebert, and S. Dunbar. 2001. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin. Infect. Dis. 15:1511-1515. [DOI] [PubMed] [Google Scholar]
- 5.Cossart, P., M. F. Vicente, J. Mengaud, F. Baquero, J. C. Perez-Diaz, and P. Berche. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta, V., S. M. Myskowski, L. A. Kwinn, D. N. Chiem, N. Varki, R. G. Kansal, M. Kotb, and V. Nizet. 2005. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol. Microbiol. 56:681-695. [DOI] [PubMed] [Google Scholar]
- 7.Deutscher, J. 2008. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 11:87-93. [DOI] [PubMed] [Google Scholar]
- 8.Ellemor, D. M., R. N. Baird, M. M. Awad, R. L. Boyd, J. I. Rood, and J. J. Emmins. 1999. Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene. Infect. Immun. 67:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita, Y. 2009. Carbon catabolite control of the metabolic network in Bacillus subtilis. Biosci. Biotechnol. Biochem. 73:245-259. [DOI] [PubMed] [Google Scholar]
- 10.Gamer, J., H. Bujard, and B. Bukau. 1992. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor σ32. Cell 69:833-842. [DOI] [PubMed] [Google Scholar]
- 11.Görke, B., and J. Stülke. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6:613-624. [DOI] [PubMed] [Google Scholar]
- 12.Gotoh, O. 1999. Multiple sequence alignment: algorithms and applications. Adv. Biophys. 36:159-206. [DOI] [PubMed] [Google Scholar]
- 13.Graham, M. R., K. Virtaneva, S. F. Porcella, D. J. Gardner, R. D. Long, D. M. Welty, W. T. Barry, C. A. Johnson, L. D. Parkins, F. A. Wright, and J. M. Musser. 2006. Analysis of the transcriptome of group A Streptococcus in mouse soft tissue infection. Am. J. Pathol. 169:927-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirst, R. A., A. Kadioglu, C. O'Callaghan, and P. W. Andrew. 2004. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin. Exp. Immunol. 138:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, J. A., H. G. Pietersen, E. E. Stobberingh, and P. B. Soeters. 1995. Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius: clinical relevance, hemolytic and serologic characteristics. Am. J. Clin. Pathol. 104:547-553. [DOI] [PubMed] [Google Scholar]
- 16.Jerng, J. S., P. R. Hsueh, L. J. Teng, L. N. Lee, P. C. Yang, and K. T. Luh. 1997. Empyema thoracis and lung abscess caused by viridans streptococci. Am. J. Respir. Crit. Care Med. 156:1508-1514. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman, G. E., and J. Yother. 2007. CcpA-dependent and -independent control of beta-galactosidase expression in Streptococcus pneumoniae occurs via regulation of an upstream phosphotransferase system-encoding operon. J. Bacteriol. 189:5183-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, J. H., and G. H. Chambliss. 1997. Contacts between Bacillus subtilis catabolite regulatory protein CcpA and amyO target site. Nucleic. Acids Res. 25:3490-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, J. H., Y. K. Yang, and G. H. Chambliss. 2005. Evidence that Bacillus catabolite control protein CcpA interacts with RNA polymerase to inhibit transcription. Mol. Microbiol. 56:155-162. [DOI] [PubMed] [Google Scholar]
- 20.Kinkel, T. L., and K. S. McIver. 2008. CcpA-mediated repression of streptolysin S expression and virulence in the group A streptococcus. Infect. Immun. 76:3451-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. W., D. A. Mitchell, A. L. Markley, M. E. Hensler, D. Gonzalez, A. Wohlrab, P. C. Dorrestein, V. Nizet, and J. E. Dixon. 2008. Discovery of a widely distributed toxin biosynthetic gene cluster. Proc. Natl. Acad. Sci. U. S. A. 105:5879-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz, R., and H. Bujard. 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic. Acids Res. 25:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell, D. A., S. W. Lee, M. A. Pence, A. L. Markley, J. D. Limm, V. Nizet, and J. E. Dixon. 2009. Structural and functional dissection of the heterocyclic peptide cytotoxin streptolysin S. J. Biol. Chem. 284:13004-13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miwa, Y., A. Nakata, A. Ogiwara, M. Yamamoto, and Y. Fujita. 2000. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 28:1206-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagamune, H., C. Ohnishi, A. Katsuura, K. Fushitani, R. A. Whiley, A. Tsuji, and Y. Matsuda. 1996. Intermedilysin, a novel cytotoxin specific for human cells secreted by Streptococcus intermedius UNS46 isolated from a human liver abscess. Infect. Immun. 64:3093-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagamune, H., R. A. Whiley, T. Goto, Y. Inai, T. Maeda, J. M. Hardie, and H. Kourai. 2000. Distribution of the intermedilysin gene among the anginosus group streptococci and correlation between intermedilysin production and deep-seated infection with Streptococcus intermedius. J. Clin. Microbiol. 38:220-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orihuela, C. J., J. N. Radin, J. E. Sublett, G. Gao, D. Kaushal, and E. I. Tuomanen. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72:5582-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer, M. 2001. The family of thiol-activated, cholesterol-binding cytolysins. Toxicon 39:1681-1689. [DOI] [PubMed] [Google Scholar]
- 30.Pecharki, D., F. C. Petersen, and A. A. Scheie. 2008. LuxS and expression of virulence factors in Streptococcus intermedius. Oral Microbiol. Immunol. 23:79-83. [DOI] [PubMed] [Google Scholar]
- 31.Ruoff, K. L. 1988. Streptococcus anginosus (“Streptococcus milleri”): the unrecognized pathogen. Clin. Microbiol. Rev. 1:102-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumacher, M. A., G. S. Allen, M. Diel, G. Seidel, W. Hillen, and R. G. Brennan. 2004. Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P. Cell. 118:731-741. [DOI] [PubMed] [Google Scholar]
- 33.Shelburne, S. A., M. T. Davenport, D. B. Keith, and J. M. Musser. 2008. The role of complex carbohydrate catabolism in the pathogenesis of invasive streptococci. Trends Microbiol. 16:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelburne, S. A. III., D. Keith, N. Horstmann, P. Sumby, M. T. Davenport, E. A. Graviss, R. G. Brennan, and J. M. Musser. 2008. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc. Natl. Acad. Sci. U. S. A. 105:1698-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sierig, G., C. Cywes, M. R. Wessels, and C. D. Ashbaugh. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group a streptococci. Infect. Immun. 71:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sukeno, A., H. Nagamune, R. A. Whiley, S. I. Jafar, J. Aduse-Opoku, K. Ohkura, T. Maeda, K. Hirota, Y. Miyake, and H. Kourai. 2005. Intermedilysin is essential for the invasion of hepatoma HepG2 cells by Streptococcus intermedius. Microbiol. Immunol. 49:681-694. [DOI] [PubMed] [Google Scholar]
- 37.Takamatsu, D., M. Osaki, and T. Sekizaki. 2001. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid 45:101-113. [DOI] [PubMed] [Google Scholar]
- 38.Warner, J. B., and J. S. Lolkema. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67:475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weickert, M. J., and G. H. Chambliss. 1990. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 87:6238-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiley, R. A., D. Beighton, T. G. Winstanley, H. Y. Fraser, and J. M. Hardie. 1992. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J. Clin. Microbiol. 30:243-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiley, R. A., H. Fraser, J. M. Hardie, and D. Beighton. 1990. Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus strains within the “Streptococcus milleri group.” J. Clin. Microbiol. 28:1497-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]