Abstract
Infection of the gut by invasive bacterial pathogens leads to robust inflammatory responses that if left unchecked can lead to autoimmune disease and other sequelae. How the immune system controls inflammation and limits collateral damage to the host during acute bacterial infection is poorly understood. Here, we report that antibody-mediated neutralization of transforming growth factor β (TGF-β) prior to infection with the model enteric pathogen Yersinia enterocolitica reduces the mean time to death by 1 day (P = 0.001), leads to rapid colonization of the liver and lung, and is associated with exacerbation of inflammatory histopathology. During Yersinia enterocolitica infection CD4+ cells are the source of de novo TGF-β transcription in the Peyer's patches, mesenteric lymph nodes, and spleen. Correspondingly there is both antigen-specific and -independent expansion of CD4+ CD25+ Foxp3+ and TGF-β+ T-regulatory cells (T-regs) after Yersinia infection that is reduced in ovalbumin T-cell receptor-restricted OT-II mice. Functional inactivation of CD25 by anti-CD25 treatment results in more rapid death, dissemination of the bacteria to the liver and lungs, and exacerbated inflammatory histopathology, similar to what is seen during TGF-β neutralization. Altogether, these data suggest that TGF-β produced by T-regs is important in restricting bacteria during the acute phase of invasive bacterial infection of the gut. These data expand the roles of T-regs to include tempering inflammation during acute infection in addition to the well-established roles of T-regs in chronic infection, control of immune homeostasis, and autoimmune disease.
The intestinal tract is a complex organ of the immune system that interacts with the normal flora without initiating an inflammatory response, while being capable of a robust response when challenged with pathogens (42, 47). The mechanisms used to maintain immune homeostasis and to discriminate between normal flora and pathogens are poorly defined (42, 47). Because the gut is particularly sensitive to inflammation, once initiated, inflammation must be controlled and tempered to reduce immune pathology and associated chronic inflammatory sequelae (32). A number of invasive bacterial pathogens that infect the intestine including Yersinia, Salmonella, Listeria, and Shigella species generate a robust inflammatory response; yet currently, it is unclear how the gut tempers inflammatory responses against these invasive bacterial pathogens.
Yersinia enterocolitica is one of three species of Yersinia, along with Y. pseudotuberculosis and Y. pestis, which are human pathogens (16, 25). Normally, Yersinia enterocolitica is a food-borne pathogen that invades and colonizes intestinal tissues through the Peyer's patches (PP), often disseminating to mesenteric lymph nodes (MLN), resulting in self-limiting gastroenteritis and lymphadenitis. In rare cases, especially in the immunocompromised host, systemic disease can lead to infection of most organ systems, resulting in mortality rates of 50% (16). Yersinia enterocolitica has long served as a model enteric pathogen because the mouse model utilizes natural routes of infection, resulting in a disease spectrum that recapitulates most aspects of human disease (17-20).
All pathogenic Yersinia spp. have as a disease mechanism virulence genes contained on both plasmids and the chromosome to modulate the host environment to their advantage (22, 23, 41). These virulence factors allow the bacteria to gain key nutrients such as iron and to modify certain aspects of the immune response to infection, promoting the survival of the bacteria within the host. The host responds to infection by mobilizing the immune system to fight the infection, and, in the case of Y. enterocolitica, this host-pathogen interaction ultimately results in inflammation dominated by a CD4+ Th-1 cellular immune response that normally leads to sterilizing immunity (2, 4, 6, 7).
In the last 20 years, we have gained considerable insight into how the host responds to Y. enterocolitica infection and have identified many of the cells and molecules that elaborate the immune response to this infection. Use of the mouse model of Y. enterocolitica infection and pathogenesis has established a critical role for a number of proinflammatory cytokines, including interleukins-1α (IL-1α), -1β, -6, -12, and -18; tumor necrosis factor alpha (TNF-α); and gamma interferon (IFN-γ) (4-6, 10-14, 26, 28-30). However, how the host controls the acute phase of infection remains poorly understood (37, 39). Control of inflammation is better understood in chronic infectious disease, where emerging evidence suggests that T-regulatory cells (T-regs) are important regulators of pathogen-induced inflammation (37, 39).
T-regs are a subset of CD4+ T cells that maintain immune homeostasis at mucosal surfaces such as the gut (32). Regulatory T cells are critical in the maintenance of tolerance and the control of autoimmune disease, and until now they haven't been implicated in modulating inflammation during acute bacterial infections (24, 32). T-regs in general are phenotypically defined by the expression of CD4, CD25, and Foxp3 and predominantly suppress inflammation through the expression of the anti-inflammatory cytokines transforming growth factor β (TGF-β) and IL-10 as well as direct CTLA-4-mediated cell-to-cell inhibition. These cells are distinct from Foxp3-suppressive lymphocytes such as Tr1 and Th3 cells.
The role of TGF-β during Y. enterocolitica infection has not been definitively established. It has been previously reported that the administration of exogenous recombinant TGF-β to mice rendered them more resistant to Y. enterocolitica infection (13). Further, we reported that IL-6−/− mice have a hyperinflammatory response to Y. enterocolitica infection, which is characterized by decreased expression of TGF-β from antigen-stimulated lymphocytes (26).
In this study we investigate the role TGF-β during the host response to invasive gastrointestinal infections. We report a rapid antigen-dependent expansion of TGF-β-producing T-regs following gastrointestinal infection with the model invasive bacterial pathogen Yersinia enterocolitica. T-regs and TGF-β are important for controlling the systemic spread of Y. enterocolitica to the liver and the lung and for minimizing inflammation in those organs following infection. These data demonstrate a role for T-regs and TGF-β as a means to control systemic infection and inflammation during the acute phase of invasive gastrointestinal infection.
MATERIALS AND METHODS
Mice.
Female C57BL/6J, B6.129P2-Il10tm1Cgn/J (hereafter referred to as IL-10−/− mice), C57BL/6-Tg(TcrαTcrβ)425Cbn/J (hereafter referred to as OT-II mice), and C57BL/6J-IL-6tm1Kopf mice (hereafter referred to as IL-6−/− mice) 6 to 8 weeks of age were purchased from Jackson Laboratory and maintained in the barrier facility at the University of Texas Health Sciences Center at San Antonio (UTHSCSA). Mice were infected by oral gavage with 100 μl of bacterial culture. Mice were given free access to food and water throughout all experiments. Animals were sacrificed by carbon dioxide asphyxiation. The UTHSCSA committee on animal studies approved all animal experiments.
Bacteria.
The Y. enterocolitica strain used in this study (JB580v) is a virulent derivative of the serogroup 08 strain 8081 (33). Bacteria were grown overnight in LB broth at 26°C. Actual numbers of CFU were determined by serial dilutions of the overnight culture followed by plating on LB agar. The UTHSCSA institutional biosafety committee approved all animal experiments with pathogenic bacteria.
Survival curves and kinetics analysis.
Bacteria were grown for 16 to 18 h in LB broth at 26°C. Survival curves were generated by infecting 10 mice with 1 × 107 to 5 × 107 CFU of bacteria and monitoring survival over a 14-day period. Data are a composite or representative of three independent experiments. Kinetics of infection analysis was determined by orally infecting mice with strain JB580v (33). At various times postinfection, 1, 3, 5, or 7 days, mice from each treatment group were sacrificed and dissected. Bacterial load recovered from the infected organs was determined by plating dilutions of the macerated tissues on LB plates containing 20 μg/ml nalidixic acid to select for Y. enterocolitica and reported as CFU per gram of tissue. Dead mice were excluded from bacterial burden analysis, as the exact time of death could not be determined. This analysis was done in triplicate.
Antibodies.
Hybridomas producing monoclonal antibodies, including anti-TGF-β clone 1D11, anti-CD4 clone GK1.5, and anti-CD25 clone PC 61, were purchased from ATCC. Hybridoma cells were grown in serum-free media containing monoclonal antibodies purified from the culture supernatant by protein G affinity chromatography. Purified antibody preparations were determined to have low endotoxin levels (<0.1 endotoxin unit [EU]/mg antibody) by Limulus assay. Cytokine or cell depletion was done as previously described (28); briefly, mice were injected intraperitoneally with 250 μg of purified antibody 1 day prior to infection and then every third day after infection. Purified mouse or rat IgG was purchased from Sigma and served as a control. Antibodies for flow cytometry were purchased from Life Span Biosciences, Serotech, eBiosciences, or BD Pharmingen and included anti-mouse CD16/32 (Fc block) clone 93, fluorescein isothiocyanate (FITC)-anti-mouse CD4 clone RM4-5, allophycocyanin (APC)-H7-anti-CD4 clone GK1.5, phycoerythrin (PE)-anti-CD8 clone 53-6.7, PE-CY7-anti-CD25 clone PC61.5, PE-anti-mouse CD25 clone PC61.5, APC-anti-mouse/human Foxp3 clone FJK-16S, FITC-anti-TGF-β1, or PE- or FITC-anti-F4/80 antigen. Antibodies used in in vitro T-cell stimulation assays were from BD Pharmingen and included anti-CD3ɛ clone 145-2C11 and anti-CD28 clone 37.51.
Flow cytometry.
For three-color analysis, tissues from 10 infected mice per time point (Peyer's patches, mesenteric lymph nodes, and spleens) were dissected and pooled. Subsequently, a single-cell suspension was made prior to blocking Fc receptors with anti-CD16/32. Cells were then stained for surface markers (CD4 and CD25) prior to being fixed and permeabilized for Foxp3 or TGF-β staining. Cells were analyzed on a FACSAria by excluding dead cells and gating on the lymphocyte population using forward and side light scattering. Foxp3-positive cells within the lymphocyte gate were gated on and analyzed for CD4 and CD25 expression or CD25 and TGF-β expression. Data are representative of two or three independent experiments. For five-color analysis, tissues from 10 infected mice per time point (Peyer's patches, mesenteric lymph nodes, and spleens) were dissected and pooled. Subsequently, a single-cell suspension was made prior to blocking Fc receptors with anti-CD16/32. Cells were then stained for surface markers (CD4, CD8, and CD25) prior to being fixed and permeabilized for Foxp3 or TGF-β staining. Cells were analyzed on a FACSAria by excluding dead cells and gating on the lymphocyte population using forward and side light scattering. Gating on the single-cell population by analyzing the forward and side scattering light area versus width further refined the lymphocyte gate. Foxp3-positive cells within the single-cell gate were gated on and analyzed for CD4 and CD8 expression. The Foxp3 cell population was overwhelmingly CD4+ T cells; thus, these cells were gated on and analyzed for the expression of CD25 and TGF-β. Data are representative of two or three independent experiments, and analysis was performed with the FACS-DIVA suite of programs.
In vitro T-cell stimulation.
Mice were infected orally with strain JB580v. On days 3 and 5 postinfection the mice were sacrificed and the PP, MLN, and spleens were removed. A single-cell suspension was made, and after red blood cell lysis CD4+ T cells were enriched by negative selection using magnetic cell separation. CD4+ cells were plated on tissue culture plates coated with anti-CD3ɛ, and costimulation was provided by adding anti-CD28 antibodies to the culture media. Cells were maintained in tissue culture for 3 days, and then the media were harvested to determine IL-17 levels. Enzyme-linked immunosorbent assays (ELISA) were performed using an ELISA kit from R&D Systems in accordance with the manufacturer's instructions to determine levels of mouse IL-17A.
Histopathology.
Mice receiving treatment were infected orally with strain JB580v. On days 1, 3, 5, and 7 postinfection the mice were sacrificed and the liver and lungs were removed. The tissues were fixed in 10% neutral buffered formaldehyde prior to being embedded in paraffin and stained with hematoxylin and eosin. Slides were investigated in a blind fashion by two independent investigators using criteria previously established (26-28). Representative images were captured digitally on a Zeiss Axioscope 2 upright microscope using the Axiovision suite of software. Dead mice were excluded from analysis.
Statistical analysis.
Student's t test or analysis of variance (ANOVA) was used to analyze data where appropriate. Survival curves were subjected to log rank analysis (Mantel-Cox test) using the Graphpad Prism suite of software. This test assigns statistical significance when P values are ≤0.05. Statistically significant comparisons are indicated where appropriate.
RESULTS
Neutralization of TGF-β leads to sensitivity to Y. enterocolitica infection.
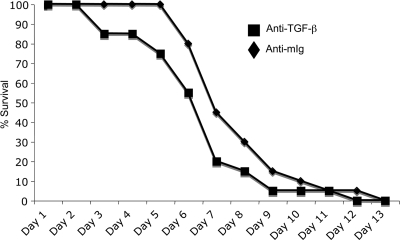
Previous studies indirectly suggested that TGF-β is important in the pathogenesis of Yersinia enterocolitica infection (13, 26). To test if TGF-β neutralization impacted the sensitivity of mice to Y. enterocolitica infection, mice were treated with a TGF-β-neutralizing antibody or normal mouse IgG as a control and then infected by gavage with 5 × 107 CFU Y. enterocolitica. Mice treated with anti-TGF-β antibodies develop outward signs of disease (ruffled fur and decreased movement) and die more rapidly than the control mice (Fig. 1), with 50% of the animals succumbing by day 6 compared to day 8 for the controls. There was not a statistically significant difference in the survival curves by log rank analysis, but there was a significant difference in the mean days to death, with animals treated with TGF-β-depleting antibodies dying on day 6.6 and controls on day 7.6 (P = 0.001, 4 independent experiments). These data suggest a role for TGF-β in controlling the progression of Y. enterocolitica infection.
FIG. 1.
Neutralization of TGF-β decreases the mean time to death following Y. enterocolitica infection. Mice were treated with 250 μg of anti-TGF-β or mouse Ig (mig) as a control prior to infection. Mice were then infected by gavage with 5 × 107 CFU of wild-type Y. enterocolitica (JB580v) and monitored for survival over a 14-day period. There was a significant difference in the mean days to death, with animals treated with TGF-β-depleting antibodies dying on day 6.6 and controls on day 7.6 (P = 0.001, n = 4 independent experiments). The data presented are a composite of 3 independent experiments with 5 to 10 animals/treatment group.
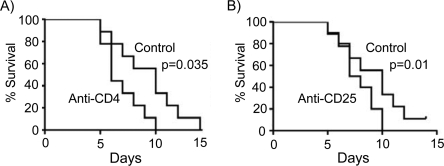
Mice immunodepleted of CD4 T cells or treated with anti-CD25 antibodies are sensitive to infection.
The major TGF-β-producing CD4+ T cells are T-regulatory (T-reg) cells (15), suggesting that animals impaired for T-reg cell function will be sensitive to infection. To test this, we first confirmed a role for CD4+ T cells in our model as described for other models of Y. enterocolitica infection (2, 5-7). Mice were immunodepleted of CD4+ T cells and then challenged with an oral Y. enterocolitica infection. Consistent with other studies, mice lacking CD4+ T cells are sensitive to Y. enterocolitica infection (P = 0.035, control mice versus GK1.5-treated mice) (Fig. 2A). This is not a surprising result since many T-cell subsets express the CD4 coreceptor, including Th-1 cells, which are critical in the control of Y. enterocolitica infection (2, 4, 6, 12, 14). Natural T-regs can be distinguished from other T-cell subsets by the high levels of expression of the IL-2 receptor (CD25) on their surfaces under basal conditions (32). To test for a role for T-regs during Y. enterocolitica infection, we treated mice with anti-CD25 antibodies that functionally inactivate CD25 and thus T-reg cells prior to infection (35). Consistent with neutralization of TGF-β and CD4+ T cells, mice functionally impaired for CD25 activity are more sensitive to Y. enterocolitica infection (P = 0.01, control mice versus PC61.5-treated mice) and these animals die at a faster rate than control animals (Fig. 2B). Altogether, these data suggest a role for CD4+ T cells, TGF-β, and/or T-regs in the control of Y. enterocolitica infection.
FIG. 2.
Mice immunodepleted of CD4 T cells or treated with anti-CD25 antibodies are sensitive to Y. enterocolitica infection. (A) Mice were treated with anti-CD4 antibodies or rat IgG as a control and then infected by gavage with 5 × 107 CFU of wild-type Y. enterocolitica (JB580v) and monitored for survival over a 14-day period. There was a significant (P = 0.035) difference between the treated and the control animals, as determined by log rank analysis. (B) Mice were treated with anti-CD25 antibodies or rat IgG as a control and then infected by gavage with 5 × 107 CFU of wild-type Y. enterocolitica (JB580v) and monitored for survival over a 14-day period. There was a significant (P = 0.01) difference between the treated and the control animals, as determined by log rank analysis. Data are representative of two independent experiments with 10 animals per treatment group/experiment.
Colonization of the Peyer's patches, mesenteric lymph nodes, and spleens is normal after TGF-β neutralization.
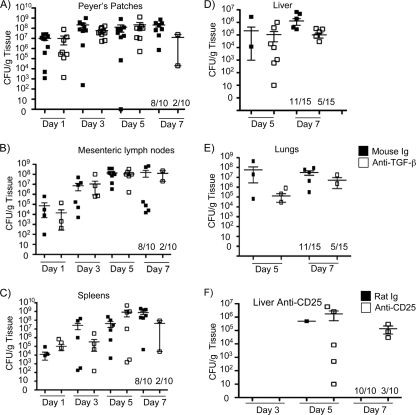
Neutralization of TGF-β renders mice more sensitive to Y. enterocolitica infection, leading to a more rapid time to death (Fig. 1). Based on other studies (26), it would be reasonable to predict that animals treated with anti-TGF-β antibodies would have higher bacterial burdens in the Peyer's patches, lymph nodes, and spleens. However, similar bacterial burdens were present in these tissues over the course of the experiment (Fig. 3A, B, and C). It should be noted that, consistent with our survival studies, 80% of the anti-TGF-β-treated animals destined for sacrifice on day 7 died prior to day 7 compared to 20% of the control animals. It is likely that animals treated with the TGF-β-neutralizing antibody that died had very high tissue burdens of bacteria; however, this was not tested directly. These data suggest that decreased concentrations of functional TGF-β in the Peyer's patches, mesenteric lymph nodes, and spleens have little effect on the ability of Y. enterocolitica to colonize and replicate in these tissues, yet these mice are more sensitive to infection.
FIG. 3.
Colonization of the Peyer's patches, mesenteric lymph nodes, and spleens of anti-TGF-β-treated mice is similar to that of controls. (A to C) Mice were treated with 250 μg of anti-TGF-β or mouse IgG as a control and then infected by gavage with 5 × 107 CFU of wild-type Y. enterocolitica. On days 1, 3, 5, and 7 postinfection Peyer's patches, mesenteric lymph nodes, and spleens were harvested and CFU/g tissue was determined. Data are presented as means ± standard errors of the means (SEM) and are the composite of two independent experiments with 5 mice/treatment group/day/experiment. Fractions on the x axis represent the numbers of mice surviving to that time point. (D and E) Mice were treated with 250 μg of anti-TGF-β or mouse IgG as a control and then infected by gavage with 3 × 107 CFU of wild-type Y. enterocolitica. On days 3, 5, and 7 postinfection livers were harvested and CFU/g tissue was determined. No bacteria were detected in the livers 3 days postinfection. (F) Mice were treated with 250 μg of anti-CD25 or rat IgG as a control and then infected by gavage with 3 × 107 CFU of wild-type Y. enterocolitica. On days 5 and 7 postinfection lungs were harvested and CFU/g tissue was determined. Data represent a composite of two independent experiments with 5 to 10 mice/treatment group/day/experiment.
Mice treated with TGF-β-neutralizing antibodies or T-reg-inhibiting antibodies are defective for controlling systemic dissemination of Y. enterocolitica.
Data presented above demonstrate that animals treated with TGF-β-neutralizing antibodies or T-reg-inhibiting anti-CD25 antibodies are more sensitive to infection (Fig. 1 and 2B). Yet, when the colonization of the Peyer's patches, mesenteric lymph nodes, and spleen is examined, no difference in bacterial burdens or the kinetics of infection is found (Fig. 3A, B, and C). We and others have documented that following oral infection of mice with Y. enterocolitica the Peyer's patches, mesenteric lymph nodes, and occasionally the spleen are efficiently colonized (17, 25-27, 38). This is consistent with human infection, which usually results in a self-limiting infection of the Peyer's patches and mesenteric lymph nodes. In the mouse, colonization of other organs is more stochastic and is often associated with terminal disease or immune compromise. We hypothesized that the sensitivity of the anti-TGF-β- and anti-CD25-treated animals was due to more rapid systemic spread of Y. enterocolitica in these animals. To test this hypothesis, mice were treated with anti-TGF-β, anti-CD25, or control antibodies; subsequently they were orally infected with Y. enterocolitica, and then bacterial burdens in the livers and lungs were determined. Further, livers and lungs were harvested and processed for histopathology. Neutralization of TGF-β or inhibition of CD25 leads to rapid colonization of the liver, with 50% of the anti-TGF-β-treated animals having heavy colonization of the liver at day 5 postinfection compared to 13% of the controls. By day 7 postinfection, only 30% of the anti-TGF-β-treated animals still survived and all five had heavy colonization of the liver. In comparison, 73% of the control animals survived to day 7 and only a one-third of them had detectable bacterial burdens in the liver (Fig. 3D). Colonization of the lungs was more variable, but it followed the same trend as the liver colonization (Fig. 3E). Treatment with anti-CD25 antibodies gave very similar results (Fig. 3F). Colonization of the liver was never detected at day 3 postinfection in any of the experimental groups. Colonization of the lungs in the anti-CD25-treated animals by CFU determination was not examined. Altogether, these data suggest that TGF-β and a functional IL-2r (CD25) help to control systemic dissemination of Y. enterocolitica.
Histopathology of the lungs and livers of mice treated with anti-TGF-β or anti-CD25 antibodies.
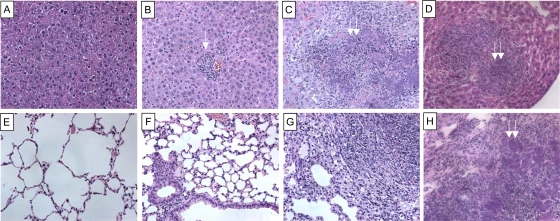
In addition to more rapid dissemination of Y. enterocolitica to the liver, there were also profound changes in the histopathology of the livers and lungs of the mice treated with anti-TGF-β or anti-CD25 antibodies compared to controls. The majority of the control mice had no detectable changes in histopathology in the liver or lungs. The few control animals that had detectable lesions displayed small focal inflammatory lesions in the liver and congestion in the lungs, most commonly on days 5 and 7 postinfection (Fig. 4B and F). Animals treated with anti-TGF-β or anti-CD25 antibodies had lesions in the liver that ranged from small focal inflammatory lesions to large microabscesses, with evidence of overt bacterial growth as early as day 5 postinfection (Fig. 4C and D). In the lungs of the mice treated with anti-TGF-β or anti-CD25 antibodies, changes in histopathology ranged from edema and congestion to lobular consolidated pneumonia with large, focal microabscesses (Fig. 4G and H). In both the liver and the lungs of the mice treated with anti-TGF-β or anti-CD25 antibodies there is evidence for a strong cellular inflammatory response to infection. These data suggest that a lack of TGF-β and functional IL-2r (CD25) early during infection leads to a rapid systemic spread of Y. enterocolitica, which may account for the sensitivity of these mice to infection.
FIG. 4.
Treatment of mice with anti-TGF-β or anti-CD25 antibodies leads to changes in inflammatory histopathology. Mice were treated with 250 μg of anti-TGF-β, anti-CD25, or appropriate control IgG and then infected by gavage with 3 × 107 CFU of wild-type Y. enterocolitica. On days 3, 5, and 7 postinfection livers and lungs were harvested, embedded in paraffin, and stained with hematoxylin and eosin. (A) Normal liver. (B) Liver from a mouse treated with mouse IgG and infected for 5 days. Note the small inflammatory lesion (white arrow). These lesions were present in 10% of the livers from this group. (C) Liver from a mouse treated with anti-TGF-β and infected for 5 days. Note the large microabscess and associated inflammatory lesions with overt bacterial growth (double arrows). These lesions were present in 50% of the livers from this group. (D) Liver from a mouse treated with anti-CD25 and infected for 5 days. Note the large microabscess and associated inflammatory lesions with overt bacterial growth (double arrows). These lesions were present in 50% of the livers from this group. (E) Normal lung. (F) Lung from a mouse treated with mouse IgG and infected for 5 days. (G) Lung from a mouse treated with anti-TGF-β and infected for 5 days. The image is from the consolidated right lower lobe. Note the large microabscess and associated inflammatory lesions with overt bacterial growth. These lesions were present in 50% of the lungs from this group. (H) Lung from a mouse treated with anti-CD25 and infected for 5 days. The image is from the consolidated left lower lobe. Note the large microabscess and associated inflammatory lesions with overt bacterial growth (double arrows). These lesions were present in 50% of the lungs from this group. All images are representative of two independent experiments with 5 mice/treatment group/time point/experiment. Original magnification, ×20.
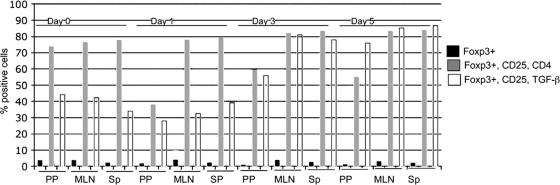
In vivo expansion of CD4+ CD25+ Foxp3+ T cells and CD25+ Foxp3+ TGF-β+ T cells following Y. enterocolitica infection.
Our data indicate that TGF-β produced by CD4+ cells and functional CD25 are important for controlling the systemic spread of Y. enterocolitica, strongly suggesting a role for T-regs in the host response to Y. enterocolitica infection. T-regs can be phenotypically defined by the surface expression of CD4, CD25, and the lineage-specific transcription factor Foxp3. To investigate the dynamics of natural T-reg populations following Y. enterocolitica infection, we infected mice and then determined the percentages of T-regs in the Peyer's patches, mesenteric lymph nodes, and spleens using flow cytometry. Single-cell suspensions from infected tissues were stained with antibodies against T-reg markers (CD4, CD25, Foxp3, and TGF-β), and then the Foxp3+ cells were analyzed for CD4 and CD25 expression or CD25 and TGF-β expression. At the initial site of infection, the Peyer's patches, there is an ∼50% decrease in CD4+ CD25+ Foxp3+ lymphocytes on day 1 postinfection relative to uninfected controls. This corresponds to an ∼50% decrease in the percentage of Foxp3+ cells in the total lymphocyte population present in the infected mice versus the uninfected control (3.82% versus 1.74%). At the same time point (day 1) there is a corresponding decrease in the CD25+ TGF-β+ Foxp3+ cells (Fig. 5). The percentages of CD4+ CD25+ Foxp3+ and CD25+ TGF-β+ Foxp3+ lymphocytes in the Peyer's patches increase at days 3 and 5 postinfection, while the total percentages of Foxp3+ lymphocytes remain relatively constant, 0.95% and 1.24%, respectively. The decrease in T-reg numbers is consistent with the known cytotoxicity of Yersinia for immune cells (34), and the later decrease in Foxp3+ cells in the Peyer's patches may be related to the severe tissue damage that occurs later in infection. Importantly, even though there is a decrease in the total Foxp3+ cell population following infection, there is an increase in the percentage of CD4+ CD25+ Foxp3+ and CD25+ TGF-β+ Foxp3+ lymphocytes, consistent with expansion of these cell populations. In the mesenteric lymph nodes and spleens there is a more subtle increase in CD4+ CD25+ Foxp3+ cells but there is a 2- to 3-fold increase in the population of CD25+ Foxp3+ TGF-β-expressing lymphocytes in these tissues (Fig. 5).
FIG. 5.
Y. enterocolitica infection leads to expansion of CD4+ CD25+ Foxp3+ cells and CD25+ TGF-β+ Foxp3+ T cells. Shown is flow cytometric analysis of T-regs in the mesenteric lymph nodes 1, 3, and 5 days after infection with Y. enterocolitica. The lymphocyte population was identified by light scattering and gated. Foxp3+ cells within that gate were analyzed for CD4 and CD25 or TGF-β and CD25 expression. Black bars represent total Foxp3+ cells in the lymphocyte gate. Gray bars represent Foxp3+ CD25+ CD4+ T cells, and white bars represent Foxp3+ CD25+ TGF-β+ T cells. Data are representative of two independent experiments with cells pooled from 10 mice/group/time point/experiment. Sp, spleens.
TGF-β-expressing lymphocytes are CD4+ CD25+ Foxp3+ and not CD8+ Foxp3+ T cells.
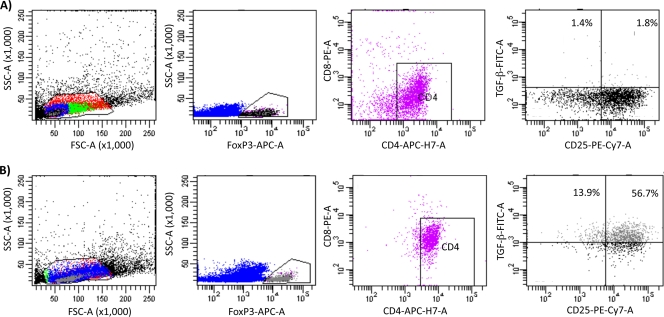
To further test the cellular source of TGF-β following Y. enterocolitica infection, we performed additional five-color flow cytometry analysis. Mice were infected and single cell suspensions were made as described above except that in this analysis the single-cell lymphocyte gate was analyzed for the expression of Foxp3 and CD4 versus CD-8, CD25, and TGF-β. Consistent with our three-color analysis CD4+ CD25+ Foxp3+ cells are the source of TGF-β (Fig. 6 and Table 1). After infection there were essentially no CD-8+ Foxp3+ CD25+ cells producing TGF-β (Fig. 6 and data not shown). Further, we investigated the possibility that F4/80+ macrophages could be a source of cells producing TGF-β. However, we did not detect any macrophages producing TGF-β following infection (data not shown). Altogether, these data demonstrate an increase in T-reg cells and TGF-β-producing T-reg cells following Y. enterocolitica infection.
FIG. 6.
TGF-β is produced by Foxp3+ CD4+ CD25+ T cells and not Foxp3+ CD8+ T cells following Y. enterocolitica infection. Shown is flow cytometric analysis of T-regs in the mesenteric lymph nodes 5 days after infection with Y. enterocolitica. The single-cell lymphocyte population was identified by light scattering and gated. Foxp3+ cells within that gate were analyzed for CD4 versus CD8, and, since the Foxp3+ population was exclusively CD4+, these cells were gated on and analyzed for TGF-β and CD25 expression. (A) Representative fluorescence-activated cell sorter (FACS) data from the mesenteric lymph nodes of uninfected mice. (B) Representative FACS data from the mesenteric lymph nodes of mice infected for 3 days with Y. enterocolitica. Data are representative of two independent experiments with cells pooled from 10 mice/group/time point/experiment. See Table 1 for a summary of these data. FSC, forward scatter; SSC, side scatter.
TABLE 1.
Percentages of CD4+ CD25+ Foxp3+ TGF-β+ cells
| Tissue | % of CD4+ CD25+ Foxp3+ TGF-β+ cells on day: |
||
|---|---|---|---|
| 0 | 3 | 5 | |
| Peyer's patches | 4.7 | 57.8 | 70.8 |
| Mesenteric lymph nodes | 2.6 | 69.9 | 70.6 |
| Spleen | 3.0 | 58.3 | 56.7 |
We previously discovered that antigen-stimulated splenocytes from IL-6−/− mice produced less TGF-β than control splenocytes, consistent with the hyperinflammatory phenotype of these mice following infection (26). Consistent with our previous data, IL-6−/− mice have 10% fewer TGF-β-positive T-regs in the Peyer's patches and spleen after infection than wild-type control animals (data not shown). The numbers of TGF-β-positive T-regs in the mesenteric lymph nodes were comparable between the wild-type animals and the IL-6−/− animals. These data further confirm older data illustrating the role of IL-6 in regulating inflammation after Y. enterocolitica infection through the action of TGF-β and other cytokines.
In addition to TGF-β, T-regs produce another potent anti-inflammatory cytokine, IL-10. The role of IL-10 in the pathogenesis of Y. enterocolitica infection is controversial (1, 44, 45), but we hypothesized that, if T-reg-mediated suppression of inflammation was an important aspect of the host response to infection, then the host might compensate for an IL-10 deficiency by increasing the numbers of TGF-β-producing T-regs. To test this hypothesis, IL-10−/− mice and control mice were infected with Y. enterocolitica and then the percentages of CD4+ CD25+ Foxp3+ cells and CD25+ TGF-β+ Foxp3+ lymphocytes present in the Peyer's patches, mesenteric lymph nodes, and spleens of the mice were determined by flow cytometry and compared (data not shown). Consistent with our hypothesis, there is a 53% increase in CD4+ CD25+ Foxp3+ cells in the Peyer's patches on days 1 and 5 postinfection and a 56% to 86% increase in CD4+ Foxp3+ TGF-β+ cells in the Peyer's patches on days 1, 3, and 5 after infection of the IL-10−/− mice. Percentages of CD4+ CD25+ Foxp3+ cells and CD25+ TGF-β+ Foxp3+ lymphocytes present in the mesenteric lymph nodes and spleens of the IL-10−/− mice were comparable to those for the control mice except that there were 50% fewer CD25+ TGF-β+ Foxp3+ lymphocytes present in the mesenteric lymph nodes and spleens of IL-10−/− mice on day 1 postinfection.
Expansion of T-regulatory cells during Y. enterocolitica infection is predominantly antigen dependent.
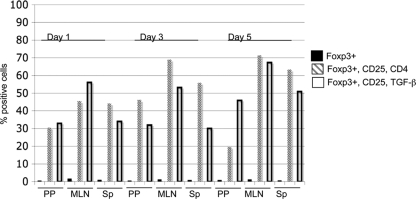
The rapid expansion of T-regs after Y. enterocolitica infection could suggest a nonspecific polyclonal expansion of T cells as opposed to a T-cell receptor (TCR) antigen-dependent clonal expansion. On the other hand, tissue damage releasing self peptides or pathogen-specific antigens may lead to the expansion of T-regs following Y. enterocolitica infection. To test if T-reg expansion after Y. enterocolitica infection is dependent on antigen-specific clonal expansion, we infected OT-II mice with Y. enterocolitica and measured changes in CD4+ CD25+ Foxp3+ cells and CD25+ TGF-β+ Foxp3+ lymphocytes. OT-II mice express a transgenic T-cell receptor that recognizes the peptide comprising amino acids 323 to 339 from chicken ovalbumin in a CD4/major histocompatibility complex class III-A b (MHC-III-A b)-dependent manner (8). CD4 T cells from OT-II mice can't undergo clonal expansion except in the context of chicken ovalbumin. Following Y. enterocolitica infection, there is a limited expansion of CD4+ CD25+ Foxp3+ cells and CD25+ TGF-β+ Foxp3+ T cells in the OT-II mice, suggesting a minor MHC-TCR-independent expansion of the T cells (Fig. 7) in response to infection. There are ∼2/3 fewer Foxp3+ cells in the total lymphocyte population and small increases in CD25+ CD4+ Foxp3+ and CD25+ TGF-β+ Foxp3+ cells following Y. enterocolitica infection. The small percentages of OT-II T-regs expanding after Y. enterocolitica infection may reflect those T cells that are responding to bacterial danger signals such as lipopolysaccharide. These data are consistent with the major expansion of CD25+ CD4+ Foxp3+ and CD25+ TGF-β+ Foxp3+ cells in wild-type mice being an antigen-dependent expansion of T-regs following Y. enterocolitica infection, with a minor contribution from a pathogen-associated molecular pattern such as a Toll-like receptor ligand. Altogether these data suggest that T-regs may play an important role early during infection of the gut to limit the extent of acute infection.
FIG. 7.
Expansion of T-regulatory cells during Y. enterocolitica infection is predominantly antigen dependent. Shown is flow cytometric analysis of T-regs in the mesenteric lymph nodes of OT-II mice 1, 3, and 5 days after infection with Y. enterocolitica. The lymphocyte population was identified by light scattering and gated. Foxp3+ cells within that gate were analyzed for CD4 and CD25 or TGF-β and CD25 expression. Black bars represent total Foxp3+ cells in the lymphocyte gate. Gray stippled bars represent Foxp3+ CD25+ CD4+ T cells, and white bars represent Foxp3+ CD25+ TGF-β+ T cells. Data are representative of two independent experiments with cells pooled from 10 mice/group/time point/experiment.
In vitro-stimulated T cells from mice treated with TGF-β-neutralizing antibodies or T-reg-inhibiting antibodies produce less IL-17A.
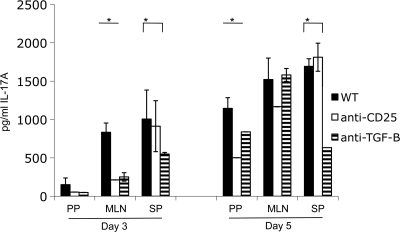
The T-cell response to infection is highly orchestrated to elicit the appropriate response and to limit inappropriate inflammation. It has been clear for 2 decades that a strong Th-1 polarized T-cell response is important for controlling Y. enterocolitica (3, 4, 6). However, recently, Th-17 cells have been shown to be important for controlling a Salmonella infection, and IL-17 is highly induced following Y. enterocolitica infection of gut tissues (29, 40). Given the central importance of Th-17 cells in controlling extracellular bacterial infections and the requirement of TGF-β for the differentiation of Th-17 cells, we hypothesized that one reason for defective control of systemic infection following anti-TGF-β or -CD25 treatment might be a defect in Th-17 responses. To test this hypothesis, mice were treated with anti-TGF-β or anti-CD25 antibodies and infected with 5 × 107 CFU of Y. enterocolitica and then 3 or 5 days postinfection CD4+ T cells were purified from the PP, MLN, and spleens. The cells were stimulated in vitro with anti-CD3ɛ and anti-CD-28 antibodies, and after 3 days of stimulation an ELISA measured IL-17A levels in the culture supernatant. As shown in Fig. 8, antibody-mediated inhibition of TGF-β or CD25 decreases the amount of IL-17A secreted from T cells isolated from the mesenteric lymph nodes and spleens 3 days postinfection. Further, at 5 days postinfection, T cells isolated from the Peyer's patches and spleens secreted less IL-17A after treatment with anti-TGF-β and anti-CD25 or anti-TGF-β, respectively. Altogether, these data suggest that functional T-regs influence effector T-cell responses during a Y. enterocolitica infection.
FIG. 8.
Blocking T-reg function inhibits Th-17 responses after Y. enterocolitica infection. Mice were treated with TGF-β- or CD25-blocking antibodies and were then infected by gavage with 5 × 107 CFU of Y. enterocolitica. On days 3 and 5 postinfection CD4+ T cells from the PP, MLN, and spleen were enriched for by negative magnetic separation. Cells were plated on tissue culture plates coated with anti-CD3ɛ, and the media were supplemented with anti-CD-28 antibodies. After 3 days of in vitro stimulation/costimulation, culture supernatants were harvested and the levels of IL-17A were determined by ELISA. WT, wild type (control). *, P ≤ 0.05.
DISCUSSION
The interaction of invasive bacterial pathogens of the gut such as Yersinia with the host causes a potent inflammatory response and tissue damage, leading to significant human morbidity and mortality (16). Several lines of evidence suggest that even during the acute response to infection tempering the inflammatory response can be beneficial to the host. For example, previous work indicates that IL-6−/− mice have a hyperinflammatory response to Y. enterocolitica infection that results in a more rapid death of the animals and tissue pathologies that reflect exacerbated inflammatory changes (26). These studies also demonstrated an IL-6-dependent defect in TGF-β secretion from stimulated splenocytes that correlated with an older observation that mice treated with recombinant TGF-β were more resistant to Y. enterocolitica infection (13, 26). TGF-β is a potent anti-inflammatory cytokine (36); in fact, it wasn't possible to use TGF-β−/− mice for these studies because the animals die 2 to 3 weeks after birth from systemic inflammation (43). Altogether, these data suggest that cytokine-mediated regulation of the extent of inflammation during an invasive bacterial infection is a crucial aspect of the host response.
Cytokine-mediated immune regulation is often carried out by CD4 + helper T cells (Th), which polarize the immune system to appropriately respond to pathogen challenge. There is strong experimental evidence supporting a central role for T cells in clearing Y. enterocolitica infection, especially IFN-γ-producing Th-1 cells. However, in the last 10 years, a number of newly discovered CD4+ T-cell subsets (T-reg and Th-17) have emerged as key players in the pathogenesis of diseases ranging from infectious disease to autoimmune disease (37, 39, 48, 49). T-reg cells are critical to the maintenance of immune tolerance and prevention of unwanted inflammatory responses at mucosal surfaces such as the gut, and, not surprisingly, T-regs have been implicated in the pathogenesis of a variety of chronic infectious diseases (32, 37). Several studies have also implicated T-regs in the modulation of memory responses following an acute infection, but little information on the direct role of T-regs during the host response to an acute bacterial infection is available.
Recently, Rudensky and coworkers reported that T-regs maintain Th-17 homeostasis in the gut utilizing a STAT-3-dependent mechanism that was distinct from that used by TGF-β (21). These studies also highlighted the interaction of different classes of T cells to maintain tolerance of the gut. It would not be unreasonable to predict that similar control mechanisms are in place to control inflammation generated in response to acute bacterial infections of the gut.
Here we report a role for T-regs during the host response to acute invasive infection of the gastrointestinal tract by Y. enterocolitica. By 3 days postinfection there is a rapid expansion of CD4+ CD25+ Foxp3+ T cells and a 2- to 3-fold increase in TGF-β+ CD25+ Foxp3+ T cells yet there is no increase in CD8+ Foxp3+ TGF-β-producing T cells or macrophages producing TGF-β. T-reg cells are critical for controlling dissemination to extraintestinal sites of infection based on the more rapid liver colonization and the subsequent rapid death of mice treated with anti-TGF-β or anti-CD25 antibodies. Active TGF-β transcription in the Peyer's patches, mesenteric lymph nodes, and spleen following infection is dependent on the presence of CD4+ cells, suggesting that T cells are the major source of this cytokine during infection (Fig. 6). The importance of CD4+ T cells is well established as an immune control mechanism during Yersinia infection, but, to our knowledge, this is the first report demonstrating a protective role for a CD4+ T-cell subset other than Th-1 for Yersinia (6, 7).
The protective role of T-regs is presumably secondary to that provided by Th-1 or Th-17 T cells given that all of the control animals in these experiments developed disease and in many cases succumbed to infection. Here, we provide evidence for the generation of CD4+ Th-17 cells following Y. enterocolitica infection that is consistent with the presence of IL-6, IL-1, and TGF-β in infected tissues. However, in a situation where an animal is deficient in TGF-β or CD25 function, bacteria disseminate to the liver and lung more rapidly and the majority of animals succumb to disease several days before the control animals. Interestingly, we show that animals treated with antibodies that block TGF-β or CD25 function have a diminished Th-17 response following Y. enterocolitica infection. These data are consistent with a T-reg response during acute infection impacting the appropriate Th-17 effector T-cell response. However these data should be interpreted with some caution as Th-17 cells express IL-2r and it is possible that anti-CD25 treatment might impact IL-17 production by blocking IL-2r on these cells. These data do demonstrate a clear Th-17 response during Y. enterocolitica infection, further expanding the known CD4+ effector responses elicited during this infection.
While CD25 can be expressed on other CD4+ effector T cells once they are activated, the kinetics of liver and lung colonization in the mice treated with anti-CD25 antibodies suggest a target T cell expressing high levels of CD25 at days 0 to 3 postinfection during a primary immune response, which is most consistent with T-regs. Interestingly, another potent anti-inflammatory cytokine produced by T-regs is IL-10, which has a controversial role in the pathogenesis of Y. enterocolitica infection, with some studies suggesting that deficiency in IL-10 leads to resistance and others demonstrating that IL-10 deficiency has no impact on Yersinia infection (1, 44, 45). The different phenotypes observed with IL-10 versus TGF-β deficiency may be reflective of distinct roles during the host response or a difference due to genetic versus antibody-mediated deficiency. Altogether, these data would suggest that T-regs, through the action of TGF-β, help to limit bacterial dissemination to the liver and lung by an unknown mechanism.
A number of recent studies have suggested that the dissemination of Yersinia from the gut to other organs is more complex than previously appreciated, finding that Peyer's patches are not absolutely required for infection through the gut and that pools of bacteria in the gut can seed the deeper tissues (9, 31). Given these revelations, it is not unreasonable to predict that the host has evolved a means to temper inflammation during an acute bacterial infection as a means to diminish tissue damage and limit dissemination of the infection.
In the case of invasive bacterial pathogens of the gut, natural T-regulatory cells appear to figure prominently in this role. Studies with ovalbumin (OVA)-restricted mice suggest that a fraction of the increases in T-regs after Y. enterocolitica infection are independent of clonal expansion and may be a response to pathogen-associated antigens recognized through the Toll-like receptors expressed on these cells (46). Likewise, these same data would suggest that the expansion of T-regs in the Yersinia-infected C57BL/6 mice is predominantly due to antigen-dependent expansion of these cells. Currently it is not clear if these cells expand due to a pathogen-associated antigen or in response to a self antigen released as a result of the severe tissue damage caused by these infections.
Altogether, these studies reveal a previously unrecognized role for T-regs in the acute phase of the host response to invasive bacterial pathogens of the gut. Although T-regs are unlikely to be directly involved in antimicrobial activities in a manner analogous to Th-1 cells, they likely control inflammation, thus limiting tissue damage and direct access to the circulation, thereby indirectly impacting the dissemination of bacteria. The robust colonization of the livers by day 5 postinfection in mice treated with anti-TGF-β or anti-CD25 antibodies suggests that these mice develop bacteremia more rapidly than control animals. Additionally, during acute infections, T-regs may play an important role in regulating immune homeostasis, limiting the extent of inflammation and/or assisting in the return to basal status once infection has been resolved. These data supply further evidence for mechanisms of immune control of acute inflammation during invasive bacterial infection.
Acknowledgments
The National Institutes of Health through grants AI067716 and AI060789 awarded to P.H.D. supported this work. Flow cytometry data collection and analysis were done in the Institutional Flow Cytometry core facility, partially supported by National Cancer Institute grant P30 CA54174.
We thank Ben Daniels for assistance with flow cytometry.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 28 June 2010.
REFERENCES
- 1.Auerbuch, V., and R. R. Isberg. 2007. Growth of Yersinia pseudotuberculosis in mice occurs independently of Toll-like receptor 2 expression and induction of interleukin-10. Infect. Immun. 75:3561-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth, I. B., M. Beer, E. Bohn, S. H. E. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., M. Beer, P. Hantschmann, S. Preger, U. Vogel, B. Heymer, and J. Heesemann. 1993. The cellular immune response against Yersinia enterocolitica in different inbred strains of mice: evidence for an important role of T lymphocytes. Int. J. Med. Microbiol. Virol. Parasitol. Infect. Dis. 278:383-395. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth, I. B., and J. Heesemann. 1992. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med. Microbiol. Immunol. 181:333-338. [DOI] [PubMed] [Google Scholar]
- 5.Autenrieth, I. B., V. Kempf, T. Sprinz, S. Preger, and A. Schnell. 1996. Defense mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autenrieth, I. B., A. Tingle, A. Reske-Kunz, and J. Heesemann. 1992. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect. Immun. 60:1140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autenrieth, I. B., U. Vogel, S. Preger, B. Heymer, and J. Heesemann. 1993. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect. Immun. 61:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnden, M. J., J. Allison, W. R. Heath, and F. R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76:34-40. [DOI] [PubMed] [Google Scholar]
- 9.Barnes, P. D., M. A. Bergman, J. Mecsas, and R. R. Isberg. 2006. Yersinia pseudotuberculosis disseminates directly from a replicating bacterial pool in the intestine. J. Exp. Med. 203:1591-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beuscher, H. U., U.-P. Rausch, I. G. Otterness, and M. Röllinghoff. 1992. Transition from interleukin 1β (IL-1β) to IL-1α production during maturation of inflammatory macrophages in vivo. J. Exp. Med. 175:1793-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beuscher, H. U., F. Rodel, A. Forsberg, and M. Rollinghoff. 1995. Bacterial evasion of the host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect. Immun. 63:1270-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohn, E., J. Heesemann, S. Ehlers, and I. B. Autenrieth. 1994. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect. Immun. 62:3027-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohn, E., E. Schmitt, C. Bielfeldt, A. Noll, R. Schulte, and I. B. Autenrieth. 1998. Ambiguous role of interleukin-12 in Yersinia enterocolitica infection in susceptible and resistant mouse strains. Infect. Immun. 66:2213-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohn, E., A. Sing, R. Zumbihl, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heesemann, and I. B. Autenrieth. 1998. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J. Immunol. 160:299-307. [PubMed] [Google Scholar]
- 15.Bommireddy, R., and T. Doetschman. 2007. TGFbeta1 and Treg cells: alliance for tolerance. Trends Mol. Med. 13:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter, P. B. 1975. Oral Yersinia enterocolitica infection of mice. Am. J. Pathol. 81:703-705. [PMC free article] [PubMed] [Google Scholar]
- 18.Carter, P. B. 1975. Pathogenicity of Yersinia enterocolitica for mice. Infect. Immun. 11:164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter, P. B., and F. M. Collins. 1974. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect. Immun. 9:851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter, P. B., and F. M. Collins. 1974. The route of enteric infection in normal mice. J. Exp. Med. 139:1189-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhry, A., D. Rudra, P. Treuting, R. M. Samstein, Y. Liang, A. Kas, and A. Y. Rudensky. 2009. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326:986-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 24.Curotto de Lafaille, M. A., and J. J. Lafaille. 2009. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30:626-635. [DOI] [PubMed] [Google Scholar]
- 25.Dube, P. 2009. Interaction of Yersinia with the gut: mechanisms of pathogenesis and immune evasion. Curr. Top. Microbiol. Immunol. 337:61-91. [DOI] [PubMed] [Google Scholar]
- 26.Dube, P. H., S. A. Handley, J. Lewis, and V. L. Miller. 2004. Protective role of interleukin-6 during Yersinia enterocolitica infection is mediated through the modulation of inflammatory cytokines. Infect. Immun. 72:3561-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dube, P. H., S. A. Handley, P. A. Revell, and V. L. Miller. 2003. The rovA mutant of Yersinia enterocolitica displays differential degrees of virulence depending on the route of infection. Infect. Immun. 71:3512-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dube, P. H., P. A. Revell, D. D. Chaplin, R. G. Lorenz, and V. L. Miller. 2001. A role for IL-1 alpha in inducing pathologic inflammation during bacterial infection. Proc. Natl. Acad. Sci. U. S. A. 98:10880-10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Handley, S. A., P. Dube, and V. L. Miller. 2006. Histamine signalling through the H2 receptor in the Peyer's patch is important for controlling Yersinia enterocolitica infection. Proc. Natl. Acad. Sci. U. S. A. 103:9268-9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handley, S. A., P. H. Dube, P. A. Revell, and V. L. Miller. 2004. Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect. Immun. 72:1645-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handley, S. A., R. D. Newberry, and V. L. Miller. 2005. Yersinia enterocolitica invasin-dependent and invasin-independent mechanisms of systemic dissemination. Infect. Immun. 73:8453-8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izcue, A., J. L. Coombes, and F. Powrie. 2009. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27:313-338. [DOI] [PubMed] [Google Scholar]
- 33.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O:8 and construction of a transformable R−M+ mutant. Gene 136:271-275. [DOI] [PubMed] [Google Scholar]
- 34.Koberle, M., A. Klein-Gunther, M. Schutz, M. Fritz, S. Berchtold, E. Tolosa, I. B. Autenrieth, and E. Bohn. 2009. Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model. PLoS Pathog. 5:e1000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohm, A. P., J. S. McMahon, J. R. Podojil, W. S. Begolka, M. DeGutes, D. J. Kasprowicz, S. F. Ziegler, and S. D. Miller. 2006. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 176:3301-3305. [DOI] [PubMed] [Google Scholar]
- 36.Letterio, J. J., and A. B. Roberts. 1998. Regulation of immune responses by TGF-β. Annu. Rev. Immunol. 16:137-161. [DOI] [PubMed] [Google Scholar]
- 37.Majlessi, L., R. Lo-Man, and C. Leclerc. 2008. Regulatory B and T cells in infections. Microbes Infect. 10:1030-1035. [DOI] [PubMed] [Google Scholar]
- 38.Pepe, J. C., and V. L. Miller. 1993. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc. Natl. Acad. Sci. U. S. A. 90:6473-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccirillo, C. A. 2008. Regulatory T cells in health and disease. Cytokine 43:395-401. [DOI] [PubMed] [Google Scholar]
- 40.Raffatellu, M., R. L. Santos, D. E. Verhoeven, M. D. George, R. P. Wilson, S. E. Winter, I. Godinez, S. Sankaran, T. A. Paixao, M. A. Gordon, J. K. Kolls, S. Dandekar, and A. J. Baumler. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14:421-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revell, P. A., and V. L. Miller. 2001. Yersinia virulence: more than a plasmid. FEMS Microbiol. Lett. 205:159-164. [DOI] [PubMed] [Google Scholar]
- 42.Round, J. L., and S. K. Mazmanian. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9:313-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shull, M. M., I. Ormsby, A. B. Kier, S. Pawlowski, R. J. Diebold, M. Yin, and A. C. Sidman. 1992. Targeted disruption of the mouse transforming growth factor beta 1 gene results in multifocal inflammatory disease. Nature 359:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10 deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 45.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Maren, W. W., J. F. Jacobs, I. J. de Vries, S. Nierkens, and G. J. Adema. 2008. Toll-like receptor signalling on Tregs: to suppress or not to suppress? Immunology 124:445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Wijk, F., and H. Cheroutre. 2009. Intestinal T cells: facing the mucosal immune dilemma with synergy and diversity. Semin. Immunol. 21:130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou, L., M. M. Chong, and D. R. Littman. 2009. Plasticity of CD4+ T cell lineage differentiation. Immunity 30:646-655. [DOI] [PubMed] [Google Scholar]
- 49.Zhou, L., I. I. Ivanov, R. Spolski, R. Min, K. Shenderov, T. Egawa, D. E. Levy, W. J. Leonard, and D. R. Littman. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8:967-974. [DOI] [PubMed] [Google Scholar]