Abstract
Mycobacterium ulcerans is the causative agent of Buruli ulcer, a severe necrotizing skin disease that causes significant morbidity in Africa and Australia. Person-to-person transmission of Buruli ulcer is rare. Throughout Africa and Australia infection is associated with residence near slow-moving or stagnant water bodies. Although M. ulcerans DNA has been detected in over 30 taxa of invertebrates, fish, water filtrate, and plant materials and one environmental isolate cultured from a water strider (Gerridae), the invertebrate taxa identified are not adapted to feed on humans, and the mode of transmission for Buruli ulcer remains an enigma. Recent epidemiological reports from Australia describing the presence of M. ulcerans DNA in adult mosquitoes have led to the hypothesis that mosquitoes play an important role in the transmission of M. ulcerans. In this study we have investigated the potential of mosquitoes to serve as biological or mechanical vectors or as environmental reservoirs for M. ulcerans. Here we show that Aedes aegypti, A. albopictus, Ochlerotatus triseriatus, and Culex restuans larvae readily ingest wild-type M. ulcerans, isogenic toxin-negative mutants, and Mycobacterium marinum isolates and remain infected throughout larval development. However, the infections are not carried over into the pupae or adult mosquitoes, suggesting an unlikely role for mosquitoes as biological vectors. By following M. ulcerans through a food chain consisting of primary (mosquito larvae), secondary (predatory mosquito larva from Toxorhynchites rutilus septentrionalis), and tertiary (Belostoma species) consumers, we have shown that M. ulcerans can be productively maintained in an aquatic food web.
Infection with Mycobacterium ulcerans, the causative agent of Buruli ulcer (BU) disease, is associated with residence near stagnant and slow-moving water bodies in areas in which the disease is endemic (5, 36, 40, 45, 50). A plasmid-encoded macrolide toxin, mycolactone, is the primary virulence determinant of M. ulcerans (8, 41). Biting aquatic insects, such as several taxa in the Belostomatidae and Naucoridae families (Hemiptera), have been suggested as possible vectors of M. ulcerans in several laboratory experiments (16, 19, 20, 24, 31, 32); however, there is little empirical evidence from field studies to support the contention that these biting insects vector M. ulcerans to humans (2). In Melbourne, Australia, recent epidemiological evidence suggests that mosquitoes may serve as vectors in the transmission of BU disease (10, 11, 12, 34, 35). In this study, 957 pools consisting of over 11,000 mosquitoes of four different species were collected and tested by quantitative PCR (qPCR) for the presence of M. ulcerans DNA, and positive results were obtained from 48 of 957 pools tested (10). Of the 48 positive pools, 13 were positive for PCR directed against two insertion sequences (IS2404 and IS2606) as well as against sequence based on the ketoreductase domain of the mycolactone toxin genes. Because all of these target sequences are present multiple times in the genome, it was difficult to assign genome equivalents to these results. However, data from laboratory experiments suggested that 10 to 100 M. ulcerans isolates per mosquito were present in the positive pools. Epidemiological work also suggested a seasonal relationship between Buruli ulcer and mosquito-vectored diseases in Australia (12). These studies are extremely provocative and raise a number of questions for further work. What is the prevalence of M. ulcerans in other invertebrate taxa in the same environment? What is the infection rate in equal numbers of mosquitoes collected from areas in which the disease is not endemic? Is it possible to obtain physical evidence for the presence of M. ulcerans through microscopy or culture of mosquitoes in areas in which the disease is endemic, and, finally, what can we learn from laboratory studies concerning the interaction between mosquitoes and M. ulcerans?
The recent work from Australia suggesting that M. ulcerans is spread by mosquitoes is particularly significant because adult mosquitoes are the most important group of insects in the spread of human disease. They may serve as biological vectors that provide a major environment for pathogen replication, as in malaria or yellow fever, or as mechanical vectors that carry organisms between hosts without serving as a site of replication (1, 4, 7, 9, 38). Larval mosquitoes are common in habitats associated with BU disease, most notably lentic or standing water habitats, and feed by filtering particles in the water using labral head fans (21). Members of some genera (i.e., Anopheles) aggregate at the air-water interface in microlayers near plant stems and algal mats (27, 28, 46), where they feed on microorganisms such as bacteria and algae (47). Because of their collecting-filtering feeding mode, there is potential for larvae to consume M. ulcerans and concentrate mycobacteria through their feeding activities (22, 23).
In Ghana, the occurrence of M. ulcerans among invertebrate communities in lentic habitats has been documented from regions in Ga West and Ga East Districts in which the disease is endemic as well as those in which it is not endemic (2, 49) but not in geographically distinct areas in which the disease is not endemic such as the Volta region (49). M. ulcerans has been identified in a suite of environmental samples such as filtered water, biofilms, and algae as well as among a broad spectrum of invertebrate taxa, including both larval and adult mosquitoes (2, 11, 17, 49). However, the replication and trophic movement of M. ulcerans within these environmental samples and invertebrate communities have not been experimentally investigated. Conceptual models have been proposed that assume that the primary consumers of M. ulcerans (e.g., mosquito larvae, cladocerans, and chironomid larvae) may feed on bacteria and algae in biofilms, filter suspended matter from the water column, and then initiate the passage of M. ulcerans through an aquatic food web (2, 22, 31). This model predicts the movement of M. ulcerans through secondary and tertiary consumers and implies a complex trophic relationship in the ecology of M. ulcerans as well as an important role of aquatic invertebrates in the disease ecology of M. ulcerans.
In the studies reported here, we have explored the role of mosquitoes as biological or mechanical vectors of M. ulcerans, as well as the potential of mosquito larvae to play a central role in the movement of M. ulcerans through an aquatic food web. In order to investigate the ability of mosquito larvae to ingest and maintain M. ulcerans within their digestive tract as well as to persist throughout the mosquito development cycle, we took advantage of the fact that mosquito larvae naturally feed upon bacteria. Results presented here show that strains of M. ulcerans from Africa and Australia, as well as Mycobacterium marinum, were maintained at high levels in the larval mosquito gut for 6 days. However, neither M. ulcerans nor M. marinum was detected in adult mosquitoes that were infected in the larval stage. These results suggest that mosquitoes are unlikely to serve as biological vectors of M. ulcerans.
We further developed a model for following the passage of M. ulcerans through a series of consumers to determine whether M. ulcerans could be passed up a trophic chain from primary to tertiary consumers. In this model, we conducted similar experiments using four species of nonpredatory mosquito larvae, Aedes aegypti (Linnaeus), Aedes albopictus (Skuse), Ochlerotatus triseriatus (Theobald), and Culex restuans (Theobald), as primary consumers. These larvae were infected with isogenic wild-type (WT) and toxin-negative isolates of M. ulcerans and of M. marinum, the closest relative to M. ulcerans (13, 14, 51). We have shown that M. ulcerans in mosquito larvae survive passage through secondary and tertiary consumers, thus providing the first laboratory evidence that M. ulcerans has the potential to move between and be maintained within different species in an aquatic food web.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study were WT M. ulcerans 1615 GFP (24) and an isogenic mycolactone-negative mutant, M. ulcerans 1615::TN118 GFP (24), and M. ulcerans V2 RFP, a fluorescently labeled clinical isolate from Australia (26). M. ulcerans 1615 GFP (ATCC 35840) is a well-characterized Malaysian human isolate with physical and biochemical properties very similar to those of the sequenced strain M. ulcerans Agy99 from Ghana (42). Transposon mutagenesis (37) was used to generate the mycolactone-negative mutant strain 1615::TN118 GFP, which has an insertion in the FabH-like ketosynthase III gene (MUP045). This strain produces neither the core nor the side chain of mycolactone, is not cytotoxic, and is avirulent. M. ulcerans strains were fluorescently labeled by introduction of a green fluorescent protein (GFP) or a red derivative (RFP) using the phage-integrating vector psm5 as described previously (24, 44). By using this method, the GFP/RFP gene is inserted into the chromosome of M. ulcerans in the phage attachment site (att) and has no effect on the virulence of the bacterium. M. marinum strain M, the M. marinum genome strain (42), and M. marinum 1218 obtained from the Trudeau Collection were used as controls. M. ulcerans and M. marinum were grown to mid-log phase in Middlebrook 7H9 (M7H9) medium supplemented with 10% oleic acid-albumin-dextrose enrichment (OADC) (Difco) and incubated at 32°C.
Invertebrate species and maintenance.
Mosquito larvae were collected from an urban setting in Knoxville, TN, using a standard 250-ml mosquito dipper. Larvae were collected from a suite of artificial container habitats that were populated with naturally breeding mosquitoes (e.g., trash can, bucket, and flower pots) or via baiting with gravid traps to collect egg rafts or first instars. Mosquito larvae and egg rafts were transferred to the lab for identification and maintained at 27°C. Four species of mosquito larvae were collected and identified by morphological characteristics: Culex restuans, Aedes aegypti, Ochlerotatus triseriatus, and Toxorhynchites rutilus septentrionalis. Twenty mosquito larvae of the first three species listed above and five larvae of Toxorhynchites rutilus septentrionalis were analyzed by PCR and microscopy to determine background levels of acid-fast bacteria in the native populations. The small number of negative controls for Toxorhynchites rutilus septentrionalis was due to the small number of total larvae obtained. None of these control mosquitoes were PCR positive for M. ulcerans. Although small numbers of larvae contained a few acid-fast staining bodies, none morphologically resembled mycobacterial species.
Larvae were maintained in 50-ml plastic containers and fed fish food ad libitum. Culex restuans and A. aegypti were used in experiments to determine the ability of M. ulcerans to survive throughout mosquito development from second-instar to adult stages. Predatory Toxorhynchites rutilus septentrionalis larvae served as secondary consumers for trophic experiments, and Ochlerotatus triseriatus larvae were reared to adults for the passive transfer of M. ulcerans experiment.
Members of an aquatic Belostoma sp. (Hemiptera: Belostomatidae) were collected with a dip net from a forested swamp near Millersville, PA, maintained alive in a cooler, and transported to the University of Tennessee. In the lab, the Belostoma sp. was maintained at 27′C with a 15-h photoperiod in individual 250-ml plastic containers with plastic plants for resting locations and fed midge larvae prior to M. ulcerans infections.
Invertebrate infections.
Larval infections were initiated by feeding second-instar mosquito larvae with a solution of M. ulcerans, because the first larval instars were too small and fragile to facilitate larval gut dissections and analysis for the presence of M. ulcerans. To establish an M. ulcerans infection, mosquito larvae were starved for 24 h to void larval guts of all food boluses and approximately 100 μl of an emulsified mixture of M. ulcerans (106 bacteria) was added to a petri dish stocked with 50 starved mosquito larvae. Larvae were allowed to feed for 1 h and transferred to clean petri dishes with fresh water changes at least once daily. A subsample of 10 larvae was collected 2 to 3 h post feeding, dissected, and analyzed by microscopy to determine the efficiency of infection. Water samples after the experiment were tested for the presence of M. ulcerans. This method nearly always resulted in 100% infection rate, with larval guts filled with large clumps of mycobacteria. To determine the maintenance of M. ulcerans throughout larval development, infected mosquito larvae were transferred to sterile dishes, maintained in replicates of 10 larvae/dish, and harvested for dissection once all larvae had molted into the next instar. Once all larvae had reached the designated instar, they were transferred to individual-well slides to avoid cross contamination. Contents from larval midguts were dissected using minuten pins and carefully teased from the body cavity in a well slide as described by Wallace and Merritt (48). The peritrophic matrix was removed from the gut contents prior to microscopic analysis in order to facilitate mycobacterial identification. Contents of the larval guts were examined using epifluorescence (GFP) microscopy (×20 to ×40 magnification) and acid-fast bacterial staining (×50 and ×100) to determine the presence of M. ulcerans within the midgut. At least 10 control uninfected larvae of all species were dissected and inspected for the presence of acid-fast bacilli. No acid-fast bacilli were found in any Culex restuans, Aedes aegypti, or Ochlerotatus triseriatus isolates. Round acid-fast bodies were detected in control Toxorhynchites rutilus septentrionalis; however, these were morphologically distinct from mycobacterial species and were not detected by fluorescence.
To follow the passage of M. ulcerans through the complete mosquito developmental cycle, one group of larvae were harvested at the pupal stage and another was allowed to emerge as adult mosquitoes. In most cases equal numbers of infected mosquitoes were harvested for analysis by microscopy and PCR at each developmental stage.
Trophic transmission of M. ulcerans.
In these studies, mosquito larvae (Aedes aegypti) were primary consumers, predatory mosquito larvae (Toxorhynchites rutilus septentrionalis) were secondary consumers, and predatory water bugs (Belostomatidae) were tertiary consumers. To demonstrate transfer of M. ulcerans between primary and secondary consumers, fourth-instar Aedes aegypti (n = 50) were infected with M. ulcerans 1615 GFP as previously described. A subset of A. aegypti was analyzed by microscopy to confirm the initial infection rate. Six predatory mosquito larvae (Toxorhynchites rutilus septentrionalis) were fed five M. ulcerans-infected Aedes aegypti larvae. Because of the difficulty in obtaining large numbers of Toxorhynchites rutilus septentrionalis, only three were used as uninfected controls. Infected Toxorhynchites rutilus septentrionalis larvae were analyzed 24 h postfeeding by microscopy and PCR analysis to determine the infection rate. To test for movement of M. ulcerans from secondary to tertiary consumers, predatory water bugs (a Belostoma sp.) (n = 12) were fed one infected Toxorhynchites larva. After feeding, each Belostoma bug was removed to a fresh container, sacrificed, dissected, and analyzed for the presence of M. ulcerans at designated time intervals. Belostoma bugs were sacrificed at 2, 14, 21, 28, and 35 days, dissected, and analyzed for the presence of M. ulcerans by microscopy. Ten uninfected Aedes aegypti mosquito larvae and belostomatid bugs were analyzed as controls.
All mosquito larvae and predatory water bugs were analyzed for the presence or absence of M. ulcerans using epifluorescence microscopy, acid-fast staining, and enoyl reductase PCR (ER-PCR). Internal organs, consisting of salivary gland and guts, were analyzed by microscopy as previously described. Because water bugs grab their prey with their raptorial forelegs, dissected forelegs were also analyzed separately for the presence of M. ulcerans using PCR. The size of the raptorial forearms made microscopic examination impossible.
Model for mechanical transmission.
In order to investigate whether infected mosquitoes could transmit an infection through superficial contact, Ochlerotatus triseriatus mosquitoes (n = 40) were maintained in a 12-by-12-by-12-inch mesh cage postemergence. A slurry of emulsified M. ulcerans (107 M. ulcerans/ml) and glucose solution was mixed and poured onto sterile cotton balls for a mosquito sugar meal. Mosquitoes were allowed to feed for 2 days on cotton balls saturated with glucose and M. ulcerans solution. At this time, the M. ulcerans-contaminated cotton balls were removed, sterile glucose-saturated cotton balls were placed in the cage, and mosquitoes were allowed to feed for an additional 2 days. Mosquitoes were analyzed by microscopy and PCR at 2 days to determine infection and at 4 days after secondary feeding to determine infection. Sterile cotton balls were analyzed by PCR to evaluate the transfer of M. ulcerans.
Detection of M. ulcerans in insect tissues.
At each time point, infected insects were dissected, and individual organs were homogenized in 200 μl of 1 M Tris-HCl buffer (pH 7.5). Wet mounts of each homogenate were viewed using a fluorescent microscope (Nikon Eclipse E400) equipped with a standard epifluorescent attachment filter set for the detection of the fluorescent-labeled bacteria. Slides were also stained for acid-fast bacilli using a modified Kinyoun's carbol fuchsin stain (BBL). Acid-fast bacilli (AFB) were viewed with a light microscope (Olympus BX51/BX52). Although AFB microscopy provided better visualization of M. ulcerans morphology than fluorescent microscopy, the presence of fluorescently labeled M. ulcerans was the criterion for microscopic confirmation of M. ulcerans in mosquitoes. Culture of M. ulcerans from infected larvae was not attempted due to the large repertoire of fast-growing bacteria and fungi in the larvae. However, cultures were made from adult mosquito salivary glands. For bacterial culture, salivary glands were dissected and homogenized and 10-fold homogenate dilutions were plated on Middlebrook 7H9 (M7H9) medium supplemented with 10% oleic acid-albumin-dextrose enrichment (OADC; Difco) and incubated at 32°C. Large external insect morphological structures such as legs and other exoskeletal components could not be viewed easily by microscopy and were analyzed primarily by PCR for the presence of M. ulcerans as previously described (24).
DNA extraction and PCR analysis.
DNA was extracted from insect and larval homogenates with the UltraClean soil DNA extraction kit (Mo Bio Laboratories) according to the manufacturer's instruction. The enoyl reductase (ER) domain of the polyketide gene (mlsA) encoding the mycolactone core was the PCR target for ER-PCR used to determine the presence of mycobacterial DNA in insect tissues as previously described (24). Briefly, five microliters of each DNA sample was amplified with the ER primer pair 5′-GAGATCGGTCCCGACGTCTAC-3′ and 5′-GGCTTGACTCATGTCACGTAAG-3′ in 50-μl PCR mixtures using the GoTaq polymerase buffer system (Promega). Each reaction mixture contained 36.7 μl double-distilled water, 5 μl GoTaq green master mix (400 μl of each deoxynucleoside triphosphate, 3 mM MgCl2, blue and yellow dyes), 1 μM forward and reverse primers, 1.5 U of GoTaq polymerase, and 5 μl of DNA template. Cycling was performed in a Mastercycler gradient thermal cycler (Eppendorf) as follows: 95°C for 5 min; 35 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min; and 72°C for 10 min. Nine microliters of each reaction mixture was analyzed on 1.5% agarose gels in 1 μM Tris-acetate-EDTA stained with 1 μg/ml ethidium bromide for visualization of amplicons.
RESULTS
Mycobacterium ulcerans persists in the midgut of Culex restuans throughout the mosquito larval developmental cycle.
M. ulcerans was readily grazed upon by C. restuans larvae. All larval guts were packed with M. ulcerans following a 24-hour feeding period (Fig. 1). A high infection rate was maintained throughout the infection period at each successive instar (nine of nine for second instars, seven of eight for third instars, and five of five for fourth instars). Despite the heavy infection, larval development proceeded normally to pupation and emergence of adults.
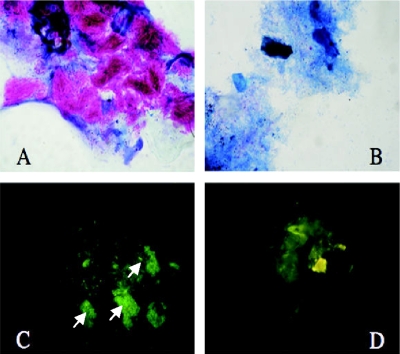
FIG. 1.
Mosquito infection experiments: (A) Acid-fast bacterium (AFB)-positive stain of M. ulcerans packed in larval midgut of Culex restuans larva (magnification, ×240); (B) AFB-negative-control gut (magnification, ×240); (C) detection of M. ulcerans 1615 GFP (white arrows) in larval gut (magnification, ×480); (D) control gut showing no GFP (magnification, ×480).
Survival of M. ulcerans is strain independent and does not require mycolactone.
Because earlier work (16) suggested that the M. ulcerans toxin mycolactone was required for growth of M. ulcerans in aquatic hemipterae, we tested whether mycolactone was required for survival in mosquito larvae. Both M. ulcerans 1615 GFP and the isogenic mycolactone-negative mutant M. ulcerans 1615::TN118 GFP were maintained in infected larvae for 6 days (Table 1). In addition, M. marinum, which is a non-toxin-producing potential progenitor of M. ulcerans, was also capable of prolonged survival in mosquito larvae. To address the hypotheses that mosquitoes might be involved in the transmission of M. ulcerans in Australia (11, 12) and that bacterial strain specificity might be an issue, M. ulcerans isolated from a patient in Australia (MU V2RFP) was included in these studies. A nearly 100% infection rate was maintained within the mosquito larval gut by all strains tested, irrespective of mycolactone phenotype or geographic origin (Table 1), illustrating that this characteristic is likely to be broadly shared among M. ulcerans isolates (Table 1). Some larvae progressed to pupation during the course of infection, resulting in a smaller number of infected larvae at 6 days.
TABLE 1.
Persistence of M. ulcerans in the midguts of larval Culex restuans mosquitoes throughout larval development
| Days p.i.a | Strain | No. of positive samples/total no. of samplesb |
|---|---|---|
| 1 | M. ulcerans 1615 GFP | 10/10 |
| M. ulcerans 1615::TN118 GFP | 10/10 | |
| M. ulcerans V2 RFP | 10/10 | |
| M. marinum M | 8/10 | |
| 4 | M. ulcerans 1615 GFP | 10/10 |
| M. ulcerans 1615::TN118 GFP | 10/10 | |
| M. ulcerans V2 RFP | 10/10 | |
| M. marinum M | 8/10 | |
| 6 | M. ulcerans 1615 GFP | 5/5 |
| M. ulcerans 1615::TN118 GFP | 5/5 | |
| M. ulcerans V2 RFP | 5/5 | |
| M. marinum M | 5/5 |
p.i., postinfection.
Samples scored positive based on detection of fluorescently labeled mycobacteria and acid-fast microscopy (M. marinum strain M).
M. ulcerans present in infected larvae are not maintained through pupation and development of the adult.
During larval mosquito development, the peritrophic matrix of the midgut region is shed during each molt, as well as when the mosquito larva pupates and emerges as an adult mosquito. Previous studies have shown an enormous decrease in bacterial flora during mosquito development, and support for transstadial transfer of bacteria from larvae to adult mosquitoes is relatively sparse for bacterial flora (15). In order to determine whether M. ulcerans acquired through larval grazing persisted in adult mosquitoes, larvae that fed on M. ulcerans were allowed to pupate and develop into adult mosquitoes. Because of the possibility that some bacteria might remain in the water in field situations from which adult mosquitoes emerged, both internal and external mosquito morphology were assayed for the presence of M. ulcerans by microscopy, PCR, and culture.
Although M. ulcerans DNA could be detected in many adult mosquitoes by PCR (Table 2), mycobacteria were not detected by microscopic examination of the adult mosquito homogenate and cultures from adults were consistently negative for M. ulcerans, although other bacterial species were isolated in pure culture (data not shown). Salivary glands and gut tissue from dissected mosquitoes were negative by both PCR and microscopy (Table 2). To determine whether the external parts of the adult mosquito could become contaminated through contact with infected water, legs and external parts of several mosquitoes were dissected and analyzed by PCR. M. ulcerans DNA was detected in both external compartments (exoskeleton and legs) analyzed. Because the external compartments of several mosquitoes were positive by PCR, we conducted a second study in which water was changed twice a day during the experiment. Under these conditions we failed to detect M. ulcerans in pupae or adult mosquitoes by PCR or microscopic methods. In addition, PCR from external compartments (body) were also PCR negative.
TABLE 2.
Presence of M. ulcerans in dissected compartments of adult Culex restuans mosquitoes infected as larvae
| Compartment | Strain | No. of positive samples/total no. of samples |
|
|---|---|---|---|
| Microscopya | PCRb | ||
| Mosquito homogenate | M. ulcerans 1615 GFP | 0/4 | 1/6 |
| M. ulcerans 1615::TN118 GFP | 0/4 | 2/7 | |
| M. ulcerans V2 RFP | 0/4 | 4/8 | |
| M. marinum M | 0/4 | 3/8 | |
| Salivary gland/gut | M. ulcerans 1615 GFP | 0/2 | 0/2 |
| M. ulcerans V2 RFP | 0/6 | 0/6 | |
| Exoskeleton | M. ulcerans 1615 GFP | ND | 2/4 |
| M. ulcerans 1615::TN118 GFP | ND | 3/3 | |
| M. ulcerans V2 RFP | ND | 2/4 | |
| M. marinum M | ND | 3/5 | |
| Legs | M. ulcerans 1615 GFP | ND | 3/4 |
| M. ulcerans 1615::TN118 GFP | ND | 2/3 | |
| M. ulcerans V2 RFP | ND | 2/4 | |
| M. marinum M | ND | 3/5 | |
Presence of bacteria determined by fluorescent microscopy and acid-fast staining (M. marinum strain M). ND, not determined.
Detection of M. ulcerans based on detection of the enoyl reductase domain of mycolactone; detection of M. marinum based on amplification of the esat6 gene.
M. ulcerans-infected mosquito larvae pass the infection up a food chain.
The fact that M. ulcerans remains viable in mosquito larvae and aquatic hemipterans (23) for an extended period of time suggested that viable M. ulcerans might be passed up the food chain from mosquito larvae to belostomatids. This is a natural route of infection, since many mosquito larvae filter feed on bacteria and belostomatids actively feed on mosquito larvae in the wild (4). Two days after belostomatids consumed M. ulcerans-infected mosquito larvae, the bacteria could be readily detected in the dissected salivary glands and gut of all six Belostoma bugs (Table 3). Bacteria could still be detected in over 80% of the belostomatids 3 weeks after feeding, although numbers decreased over time. Even though microscopic analysis of external skeletal parts was hampered by the chitinous exoskeleton, M. ulcerans was present on the raptorial arms from all six Belostoma 14 days after feeding. Ten uninfected control belostomatids were negative by PCR and microscopy (Table 3).
TABLE 3.
Transfer of M. ulcerans 1615 GFP from primary consumers (Aedes aegypti larvae) to secondary consumers (aquatic hemipteran Belostoma)
| Process | Time period | No. of positive samples/total no. of samplesa |
|||
|---|---|---|---|---|---|
| Gut | Salivary gland | Legs | Head | ||
| Microscopy | 48 h | 6/6 | 6/6 | ||
| 14 days | 6/6 | 1/6 | |||
| 21 days | 5/6 | 1/6 | |||
| 28 days | 2/3 | 0/3 | |||
| 35 days | 3/4 | 3/4 | |||
| ER-PCR | 14 days | 6/6 | 5/6 | 6/6 | 1/1 |
| 21 days | 0/6 | 1/6 | 1/6 | ||
| 28 days | 1/3 | 2/3 | 2/3 | 3/3 | |
| 35 days | 0/3 | 0/3 | 0/3 | 0/3 | |
M. ulcerans positivity based on fluorescent microscopy and acid-fast staining.
In order to further document the potential for predatory water bugs to serve as dispersal vectors and/or reservoirs for M. ulcerans in an aquatic environment, we extended this experiment to investigate the potential for passage of M. ulcerans through three trophic levels. In this experiment, five M. ulcerans-infected mosquito larvae (Aedes aegypti) served as prey for each predatory mosquito larva (Toxorhynchites rutilus septentrionalis). At 24 h postingestion, the infection rate for secondary consumers (Toxorhynchites rutilus septentrionalis) was 100% (Table 4). Microscopic analysis of Toxorhynchites guts 24 h after feeding showed large masses of M. ulcerans that were also successfully transferred to tertiary consumers, Belostoma bugs, when they were fed on infected Toxorhynchitis larvae (Table 4). Although the efficiency of transfer between secondary and tertiary consumers was not as good as that between primary and secondary consumers, M. ulcerans was detected in the salivary glands (two of six) and guts (three of six) of the Belostoma bugs 21 days after feeding. Thus, these experiments demonstrate successful transfer of M. ulcerans through a biologically relevant food web.
TABLE 4.
Transfer of M. ulcerans through primary (Aedes aegypti), secondary (Toxorhynchites rutilus septentrionalis), and tertiary (Belastoma spp.) consumers
| Consumer | Days postingestion | No. of positive samples/total no. of samplesa |
|---|---|---|
| Primary | ||
| Aedes aegypti | 1 | 10/10 |
| Uninfected controls | 1 | 0/5 |
| Secondary | ||
| Toxorhynchites rutilus septentrionalis | 1 | 6/6 |
| Uninfected controls | 1 | 0/3 |
| Tertiary | ||
| Belastoma spp. | 1 | 4/6 |
| Uninfected controls | 1 | 0/5 |
| Belastoma spp. | 21 | 3/6 |
| Uninfected controls | 21 | 0/5 |
M. ulcerans positivity based on fluorescent microscopy and acid-fast staining.
Transmission of M. ulcerans by adult mosquitoes through mechanical contact.
There is considerable evidence for the mechanical transmission of bacterial pathogens by insect vectors. In order to test whether mosquitoes could serve as mechanical vectors of M. ulcerans, attempts were made to feed adult mosquitoes on a glucose solution in a shallow container. However, we were unable to get the mosquitoes to feed under these conditions. As a result, we developed a method where mosquitoes successfully fed on a cotton ball saturated with an M. ulcerans-contaminated glucose solution. Although mosquitoes readily fed under these conditions, M. ulcerans was not detected in the salivary gland or midgut of dissected adult mosquitoes (Table 5). However, M. ulcerans DNA could be detected in 3 of 3 whole mosquito homogenates as well as on 4 of 10 insect bodies and 1 of 10 appendage samples, suggesting that M. ulcerans could be transferred by feeding to external compartments (Table 5). Despite the presence of M. ulcerans DNA on the external areas of some adult mosquitoes, M. ulcerans was not transferred to the second set of glucose-saturated cotton balls through feeding, suggesting an inability to mechanically move M. ulcerans among cotton ball substrates (Table 6).
TABLE 5.
Presence of M. ulcerans in adult Ochlerotatus triseriatus mosquitoes introduced to M. ulcerans-contaminated glucose-saturated cotton balls
| Sample | No. of positive samples/total no. of samples |
|
|---|---|---|
| Microscopy | ER-PCR | |
| Midgut/salivary gland | 0/17 | 0/17 |
| Adult whole mosquitoes | NDa | 3/3 |
| Bodies | ND | 4/10 |
| Legs and wings | ND | 1/10 |
| Control adult bodies | ND | 0/5 |
| Control legs/wings | ND | 0/5 |
ND, external compartments could not be evaluated by microscopy.
TABLE 6.
Detection of M. ulcerans in Ochlerotatus triseriatus between contaminated cotton balls by sample type and assaya
| Mosquito no. infectedb | Detection in sample type by indicated assayc |
|||||||
|---|---|---|---|---|---|---|---|---|
| Proboscis |
Salivary gland |
Gut |
Body |
|||||
| PCR | AFB | PCR | AFB | PCR | AFB | PCR | AFB | |
| 1 | − | − | − | − | − | − | − | − |
| 2 | − | − | − | − | − | − | − | − |
| 3 | − | − | − | − | − | − | − | − |
| 4 | − | − | − | + | − | − | − | − |
| 5 | − | − | − | − | − | − | − | − |
| 6 | − | − | − | − | − | − | +w | − |
| 7 | + | − | − | − | − | − | − | − |
| 8 | − | − | − | +w | − | − | − | − |
| 9 | +w | − | +w | − | +w | − | +w | − |
| 10 | − | − | − | − | − | − | − | − |
When infected mosquitoes were fed on cotton balls for 4 days and PCR testing was done, 0 of 5 mosquitoes fed on uninfected cotton balls were positive, 5 of 5 mosquitoes fed on M. ulcerans-contaminated cotton balls were positive, and 0 of 10 sterile cotton balls fed on by infected mosquitoes after contact with M. ulcerans-contaminated cotton balls were positive.
In uninfected controls, 0 of 10 samples of each sample type were positive.
+, positive; −, negative; +w, weak band.
DISCUSSION
This paper provides strong quantitative evidence for the interaction between mosquitoes and M. ulcerans and the potential impact of this interaction on the ecology of M. ulcerans within proposed conceptual food web dynamics (22). A number of papers have shown an association between M. ulcerans and invertebrate taxa in environmental aquatic samples (2, 6, 16-20, 31, 33, 49), and several experimental studies have confirmed that M. ulcerans can be maintained in predaceous aquatic insects for an extensive period of time (16, 24). Although a potential role for predaceous aquatic hemipterae such as Belostomatidae and Naucoridae as possible vectors of M. ulcerans (16-20, 24, 31, 39) has attracted considerable attention, the fact that these species are not hematophagous and bite humans only accidentally casts doubt on the relevance of these associations for transmission of M. ulcerans. In addition, Benbow et al. (2) reported no associations between populations of these insects and M. ulcerans.
Of significant interest, however, are the recent epidemiological reports and correlative studies suggesting that mosquitoes may serve as vectors of M. ulcerans disease in Australia (11, 12). Mosquitoes are well-known biological vectors of several viral, protozoan, and helminthic diseases such as dengue fever, malaria, and filariasis, respectively (4, 7). The complex biology involved in the movement of organisms from the gut following ingestion to the salivary gland for transmission is well appreciated in the case of plasmodium and microfilarian pathogens. While this phenotype has been documented in the case of mosquito-transmitted viral diseases such as dengue, Rift Valley, and yellow fevers (3, 12, 25), there has been no evidence that mosquitoes are biological vectors of any bacterial disease. Studies conducted in Australia (11, 12) provide epidemiological evidence for the role of mosquitoes as possible vectors for M. ulcerans by demonstrating the presence of M. ulcerans DNA in a small percentage (<5%) of field-captured adult mosquitoes. However, much more work is required to prove vector competency. The demonstration of pathogen DNA in association with an insect is only the first step in demonstrating vector competency. For example, other pathogens have been found within the midgut of mosquitoes such as West Nile virus (1, 9) or externally attached via lab experimentation, e.g., Bacterium tularense, the causative agent for tularemia (29); however, these correlations fall short of providing proof for the role of mosquitoes in transmitting infection to humans. It is important to note that if M. ulcerans were transmitted to humans via mosquitoes, humans would represent a dead end, since the bacterium does not replicate at 37°C and has never been identified in the blood of human patients.
This is the first study to examine the maintenance of M. ulcerans throughout the mosquito developmental cycle. A major finding reported here is that although M. ulcerans is maintained throughout larval development, it is not carried through the developmental cycle into pupal or adult mosquito stages. The same results were found using four separate species of mosquito larvae, including one species closely related to the Australian species associated with M. ulcerans DNA. Nonetheless, it is possible that differences in host specificity could limit the relevance of these studies to the Australian environment.
Our findings on M. ulcerans in mosquito larvae are in partial agreement with recent reports from Australia (43). Both studies provide evidence for the survival of M. ulcerans in mosquito larvae. In the Australian study, the infection was not studied throughout the mosquito developmental cycle. One difference between our work and that of Tobias et al. (43) is that Tobias found that M. ulcerans survived much better in Aedes comptorhynchus mosquito larvae than did a nonpigmented variant of the closely related non-toxin-producing M. marinum species. In contrast, we find that the survival phenotype of WT M. marinum in Aedes aegypti mosquito larvae is identical to that of M. ulcerans; both species survived in high numbers throughout the larval developmental cycle. In addition, we directly tested the contribution of the mycolactone toxin to survival in mosquito larvae by comparing isogenic WT M. ulcerans and mycolactone-negative M. ulcerans in our infection model and found no difference in survival. A second difference between our study and that of Tobias et al. is that our larvae progressed through the developmental cycle in less than 2 weeks, whereas in the Tobias study mosquitoes were maintained as larvae for 5 weeks.
Failure to detect M. ulcerans in mosquito pupae may have been due in part to difficulties in dissecting pupae, in which cell differentiation can make extraction of organs difficult. However, the degradation of the entire larval midgut prior to pupation would likely result in a major loss of bacteria which had not evolved specific strategies for maintenance (23). Our results regarding the sparse bacterial population of adult mosquitoes are consistent with results from others (15). Because isolation of bacteria in pure culture from adult mosquitoes is greatly facilitated by the sparse flora in the salivary gland, we believe we would have been successful in isolating M. ulcerans had organisms been present.
Although PCR-based detection of pathogen DNA in environmental samples is a well-established technique for identifying pathogen-host interactions, we and others suggest caution in interpreting data based on PCR analysis of whole-insect homogenates (30). Evidence presented here shows that the external parts of the mosquito are readily contaminated as they emerge from pupae and that positive PCR results do not necessarily reflect colonization.
Even though the results reported here on mechanical transmission of M. ulcerans are negative, we cannot rule out the possibility of mechanical transmission of M. ulcerans to humans by either mosquitoes or other invertebrates (3, 25). This area deserves further investigation. The efficiency of mechanically vectored infections is pathogen dependent. However, in most cases a small number of organisms are transferred.
Our studies provide strong evidence for the significance of mosquito larvae as potential reservoirs of M. ulcerans, consistent with field studies documenting M. ulcerans DNA positivity rates in larval mosquitoes and many other aquatic invertebrate taxa (2, 5, 31-33, 49). We show that M. ulcerans survives passage through primary, secondary, and tertiary consumers. Although the bacterial load in the tertiary consumers (belostomatid bugs) is lower than that in secondary (mosquito larva) consumers, it is clear that M. ulcerans survives through sequential passage in three hosts covering a period of nearly 3 weeks.
Empirical data from studies in Ghana have shown that M. ulcerans is present in some aquatic habitats but absent from others (2, 49). Can mobile invertebrates, such as mosquitoes or Belostoma bugs, transfer the pathogen between water bodies? Our preliminary results suggest this is a possibility. While adult mosquitoes are unlikely to play a large role as dispersal vectors of M. ulcerans within the environment, mosquito larvae may play a significant role. It is possible that passage of M. ulcerans from mosquito larvae up the food chain to flying aquatic insects such as belostomatid adults may provide an important mechanism for spread of M. ulcerans in the environment.
In summary, evidence presented in this paper makes it unlikely that mosquitoes are biological vectors of M. ulcerans via vertical transmission of bacterium from larva to adult. However, the ability of M. ulcerans for prolonged survival and passage up the food chain suggests that M. ulcerans-infected mosquito larvae may play an important role in the maintenance and distribution of M. ulcerans in aquatic environments.
Acknowledgments
This work was supported in part, by grants from PASSHE Faculty Professional Development Council, Millersville University Faculty Grants and Sabbatical Leave Program, the World Health Organization, the Optimus Foundation, and the NIH. The study described was supported by grant number R01TW007550 from the Fogarty International Center through the NIH/NSF Ecology of Infectious Diseases Program and by grant number R03AI062719.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.
Footnotes
Published ahead of print on 30 July 2010.
REFERENCES
- 1.Anderson, J. F., T. G. Andreadis, C. R. Vossbrinck, S. Tirrell, E. M. Wakem, R. A. French, A. E. Garmendia, and H. J. Van Kruiningen. 1999. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science 286:2331-2333. [DOI] [PubMed] [Google Scholar]
- 2.Benbow, M. E., H. Williamson, R. Kimbirauskas, M. D. McIntosh, R. Kolar, C. Quaye, F. Akpabey, D. Boakye, P. Small, and R. W. Merritt. 2008. Aquatic invertebrates as unlikely vectors of Buruli ulcer disease. Emerg. Infect. Dis. 14:1247-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chihota, C. M., L. F. Rennie, R. P. Kitching, and P. S. Mellor. 2001. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiol. Infect. 126:317-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins, F. H., and R. K. Washino. 1985. Insect predators, p. 25-41. In H. C. Chapman (ed.), Biological control of mosquitoes. American Mosquito Control Association, Fresno, CA.
- 5.Duker, A. A., F. Portaels, and M. Hale. 2006. Pathways of Mycobacterium ulcerans infection: a review. Environ. Int. 32:567-573. [DOI] [PubMed] [Google Scholar]
- 6.Eddyani, M., D. Ofori-Adjei, G. Teugels, D. De Weirdt, D. Boakye, W. M. Meyers, and F. Portaels. 2004. Potential role for fish in transmission of Mycobacterium ulcerans disease (Buruli ulcer): an environmental study. Appl. Environ. Microbiol. 70:5679-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eldridge, B. F. 2005. Mosquitoes, the Culicidae, p. 108. In W. C. Marquardt (ed.), Biology of disease vectors. Elsevier Academic Press, New York, NY.
- 8.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. L. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 9.Hayes, E. B., N. Komar, R. S. Nasci, S. P. Montgomery, D. R. O'Leary, and G. L. Campbell. 2005. Epidemiology and transmission of West Nile virus. Emerg. Infect. Dis. 11:1167-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, P. D. R., J. Azuolas, C. J. Lavender, E. Wishart, T. P. Stinear, J. A. Hayman, L. Brown, G. Jenkins, and J. A. M. Fyfe. 2007. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg. Infect. Dis. 13:1653-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, P. D. R., T. Stinear, P. L. Small, G. Pluschke, R. W. Merritt, F. Portaels, K. Huygen, J. A. Hayman, and K. Asiedu. 2005. Buruli ulcer (M. ulcerans infection): new insights, new hope for disease control. PLoS Med. 2:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, P. D. R., and C. J. Lavender. 2009. Correlation between Buruli ulcer and vector-borne notifiable diseases, Victoria, Australia. Emerg. Infect. Dis. 15:614-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Käser, M., S. Rondini, M. Naegeli, T. Stinear, F. Portaels, U. Certa, and G. Pluschke. 2007. Evolution of two distinct phylogenetic lineages of the emerging human pathogen Mycobacterium ulcerans. BMC Evol. Biol. 7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Käser, M., J. Hauser, P. Small, and G. Pluschke. 2009. Large sequence polymorphisms unveil the phylogenetic relationship of environmental and Mycobacterium ulcerans and pathogenic mycobacteria related to Mycobacterium ulcerans. Appl. Environ. Microbiol. 75:5667-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindh, J. 2007. Identification of bacteria associated with malaria mosquitoes—their characterization and potential use. Ph.D. dissertation. Stockholm University, Stockholm, Sweden.
- 16.Marsollier, L., R. Robert, J. Aubry, J. P. Saint Andre, H. Kouakou, P. Legras, A. L. Manceau, C. Mahaza, and B. Carbonnelle. 2002. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 68:4623-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsollier, L., T. Stinear, J. Aubry, J. P. Saint Andre, R. Robert, P. Legras, A. Manceau, C. Audrain, S. Bourdon, H. Kouakou, and B. Carbonnelle. 2004. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl. Environ. Microbiol. 70:1097-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsollier, L., T. Severin, J. Aubry, R. W. Merritt, J. P. Saint Andre, P. Legras, A. L. Manceau, A. Chauty, B. Carbonnelle, and S. T. Cole. 2004. Aquatic snails, passive hosts of Mycobacterium ulcerans. Appl. Environ. Microbiol. 70:6296-6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsollier, L., J. Aubry, E. Coutanceau, J. P. Andre, P. L. Small, G. Milon, P. Legras, S. Guadagnini, B. Carbonnelle, and S. T. Cole. 2005. Colonization of the salivary glands of Naucoris cimicoides by Mycobacterium ulcerans requires host plasmatocytes and a macrolide toxin, mycolactone. Cell. Microbiol. 7:935-943. [DOI] [PubMed] [Google Scholar]
- 20.Marsollier, L., J. P. Andre, W. Frigui, G. Reysset, G. Milon, B. Carbonnelle, J. Aubry, and S. T. Cole. 2007. Early trafficking events of Mycobacterium ulcerans within Naucoris cimicoides. Cell. Microbiol. 9:347-355. [DOI] [PubMed] [Google Scholar]
- 21.Merritt, R. W., R. H. Dadd, and E. D. Walker. 1992. Feeding behavior, natural food and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 37:349-376. [DOI] [PubMed] [Google Scholar]
- 22.Merritt, R. W., M. E. Benbow, and P. L. C. Small. 2005. Unraveling an emerging disease associated with disturbed aquatic environments: the case of Buruli ulcer. Front. Ecol. Environ. 3:323-331. [Google Scholar]
- 23.Moll, R. M., W. S. Romoser, M. C. Modrazakowski, A. C. Moncayo, and K. Lerdthusnee. 2001. Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J. Med. Entomol. 38:29-32. [DOI] [PubMed] [Google Scholar]
- 24.Mosi, L., H. Williamson, J. R. Wallace, R. W. Merritt, and P. L. C. Small. 2008. Persistent association of Mycobacterium ulcerans with West African predaceous insects of the family Belostomatidae. Appl. Environ. Microbiol. 74:7036-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motha, M. X., J. R. Eggerton, and A. W. Sweeney. 1984. Some evidence of mechanical transmission of reticuloendotheliosus virus by mosquitoes. Avian Dis. 28:858-868. [PubMed] [Google Scholar]
- 26.Mve-Obiang, A., R. E. Lee, F. Portaels, and P. L. C. Small. 2003. Heterogeneity of mycolactones produced by clinical isolates of Mycobacterium ulcerans: implications for virulence. Infect. Immun. 71:774-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orr, B. K., and V. H. Resh. 1989. Experimental test of the influence of aquatic macrophyte cover on the survival of Anopheles larvae. J. Am. Mosq. Control Assoc. 5:579-585. [PubMed] [Google Scholar]
- 28.Orr, B. K., and V. H. Resh. 1991. Interactions among aquatic vegetation, predators, and mosquitoes: implications for management of Anopheles mosquitoes in a freshwater marsh. Proc. Calif. Mosq. Vector Control Assoc. 58:214-220. [Google Scholar]
- 29.Philip, C. B., G. E. Davis, and R. R. Parker. 1932. Experimental transmission of tularemia by mosquitoes. Ann. Intern. Med. 6:705. [Google Scholar]
- 30.Pizarro, J. C., D. E. Lucero, and L. Stevens. 2007. PCR reveals significantly higher rates of Trypanosoma cruzi infection than microscopy in the Chagas vector, Triatoma infestans: high rates found in Chuquisaca, Bolivia. BMC Infect. Dis. 7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portaels, F., P. Elsen, A. Guimaraes-Peres, P. A. Fonteyne, and W. M. Meyers. 1999. Insects in the transmission of Mycobacterium ulcerans infection. Lancet 353:986. [DOI] [PubMed] [Google Scholar]
- 32.Portaels, F., K. Chemlal, P. Elsen, P. D. Johnson, J. A. Hayman, J. Hibble, R. Kirkwood, and W. M. Meyers. 2001. Mycobacterium ulcerans in wild animals. Rev. Sci. Tech. 20:252-264. [DOI] [PubMed] [Google Scholar]
- 33.Portaels, F., W. M. Meyers, A. Ablordey, A. G. Castro, K. Chemlal, P. de Rijk, P. Elsen, K. Fissette, A. G. Fraga, R. Lee, E. Mahrous, P. L. C. Small, P. Stragier, E. Torrado, A. Van Aerde, M. T. Silva, and J. Pedrosa. 2008. First cultivation and characterization of Mycobacterium ulcerans from the environment. PLoS Negl. Trop. Dis. 2:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quek, T. Y. J., E. Athan, M. J. Henry, J. A. Pasco, J. Redden-Hoare, A. Hughes, and P. D. R. Johnson. 2007. Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg. Infect. Dis. 13:1661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quek, T. Y. J., M. J. Henry, J. A. Pasco, D. P. O'Brien, P. D. R. Johnson, A. Hughes, A. C. Cheng, J. Redden-Hoare, and E. Athan. 2007. Mycobacterium ulcerans infection: factors influencing diagnostic delay. Med. J. Aust. 187:561-563. [DOI] [PubMed] [Google Scholar]
- 36.Raghunathan, P. L., E. A. Whitney, K. Asamoa, Y. Stienstra, T. H. Taylor, Jr., G. K. Amofah, D. Ofori-Adjei, K. Dobos, J. Guarner, S. Martin, S. Pathak, E. Klutse, S. Etuaful, W. T. van der Graaf, T. S. van der Werf, C. H. King, J. W. Tappero, and D. A. Ashford. 2005. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin. Infect. Dis. 40:1445-1453. [DOI] [PubMed] [Google Scholar]
- 37.Rubin, E. J., B. J. Akerley, V. N. Novik, D. J. Lampe, R. N. Husson, and J. J. Mekalanos. 1999. In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc. Natl. Acad. Sci. U. S. A. 96:1645-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Service, M. 1989. Demography and vector-borne diseases. CRC Press, Boca Raton, FL.
- 39.Silva, M. T., F. Portaels, and J. Pedrosa. 2007. Aquatic insects and Mycobacterium ulcerans: an association relevant to Buruli ulcer control? PLoS Med. 4:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stienstra, Y., W. T. van der Graaf, G. J. te Meerman, T. H. The, L. F. de Leij, and T. S. van der Werf. 2001. Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors. Trop. Med. Int. Health 6:554-562. [DOI] [PubMed] [Google Scholar]
- 41.Stinear, T. P., A. Mve-Obiang, P. L. Small, W. Frigui, M. J. Pryor, R. Brosch, G. A. Jenkin, P. D. Johnson, J. K. Davies, R. E. Lee, S. Adusumilli, T. Garnier, S. F. Haydock, P. F. Leadlay, and S. T. Cole. 2004. Giant plasmid encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc. Natl. Acad. Sci. U. S. A. 101:1345-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stinear, T. P., T. Seemann, S. Pidot, W. Frigui, G. Reysset, T. Garnier G. Meurice, D. Simon, C. Bouchier, L. Ma, M. Tichit, J. L. Porter, J. Ryan, P. D. R. Johnson, J. K. Davies, G. A. Jenkins, P. L. C. Small, L. M. Jones, F. Tekaia, F. Laval, M. Daffé, J. Parkhill, and S. T. Cole. 2007. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 17:192-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobias, N. J., T. Seemann, S. J. Pidot, J. L. Porter, L. Marsollier, E. Marion, F. Letournet, T. Zakir, J. Azuolas, J. R. Wallace, H. Hong, J. K. Davies, B. P. Howden, P. D. R. Johnson, G. A. Jenkins, and T. P. Stinear. 2009. Mycolactone gene expression is controlled by strong SigA-like promoters with utility in studies by Mycobacterium ulcerans and Buruli ulcer. PLoS Negl. Trop. Dis. 3:e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valdivia, R. H., A. E. Hromockyj, D. Monack, L. Ramakrishnan, and S. Falkow. 1996. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene 173:47-52. [DOI] [PubMed] [Google Scholar]
- 45.van der Werf, T. S., Y. Stienstra, R. C. Johnson, R. Phillips, O. Adjei, B. Fleischer, M. H. Wansbrough-Jones, P. D. Johnson, F. Portaels, W. T. van der Graaf, and K. Asiedu. 2005. Mycobacterium ulcerans disease. Bull. World Health Organ. 83:785-791. [PMC free article] [PubMed] [Google Scholar]
- 46.Walker, E. D., E. J. Olds, and R. W. Merritt. 1988. Gut analysis of mosquito (Diptera: Culicidae) using DAPI stain and epifluorescence microscopy. J. Med. Entomol. 25:544-551. [DOI] [PubMed] [Google Scholar]
- 47.Walker, E. D., and R. W. Merritt. 1993. Bacterial enrichment in the surface microlayer of an Anopheles quadrimaculatus (Diptera: Culicidae) larval habitat. J. Med. Entomol. 30:1050-1052. [DOI] [PubMed] [Google Scholar]
- 48.Wallace, J. R., and R. W. Merritt. 1999. Influence of microclimate, food, and predation on Anopheles quadrimaculatus. Environ. Entomol. 32:233-239. [Google Scholar]
- 49.Williamson, H. R., M. E. Benbow, K. D. Nguyen, D. C. Beachboard, R. K. Kimbirauskas, M. D. McIntosh, C. Quaye, E. O. Ampadu, D. Boakye, R. W. Merritt, and P. L. Small. 2008. Distribution of Mycobacterium ulcerans in Buruli ulcer endemic and non-endemic aquatic Mycobacterium ulcerans and mosquitoes sites in Ghana. PLoS Negl. Trop. Dis. 2:e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. 2001. Buruli ulcer: diagnosis of Mycobacterium ulcerans disease. World Health Organization, Geneva, Switzerland.
- 51.Yip, M. J., J. L. Porter, J. A. M. Fyfe, C. J. Lavender, F. Portaels, M. Rhoades, H. Kator, A. Colorni, G. A. Jenkins, and T. Stinear. 2007. Evolution of Mycobacterium ulcerans and other mycolactone-producing mycobacteria from a common Mycobacterium marinum progenitor. J. Bacteriol. 189:2021-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]