Abstract
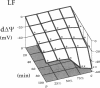
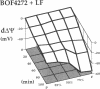
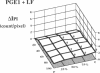
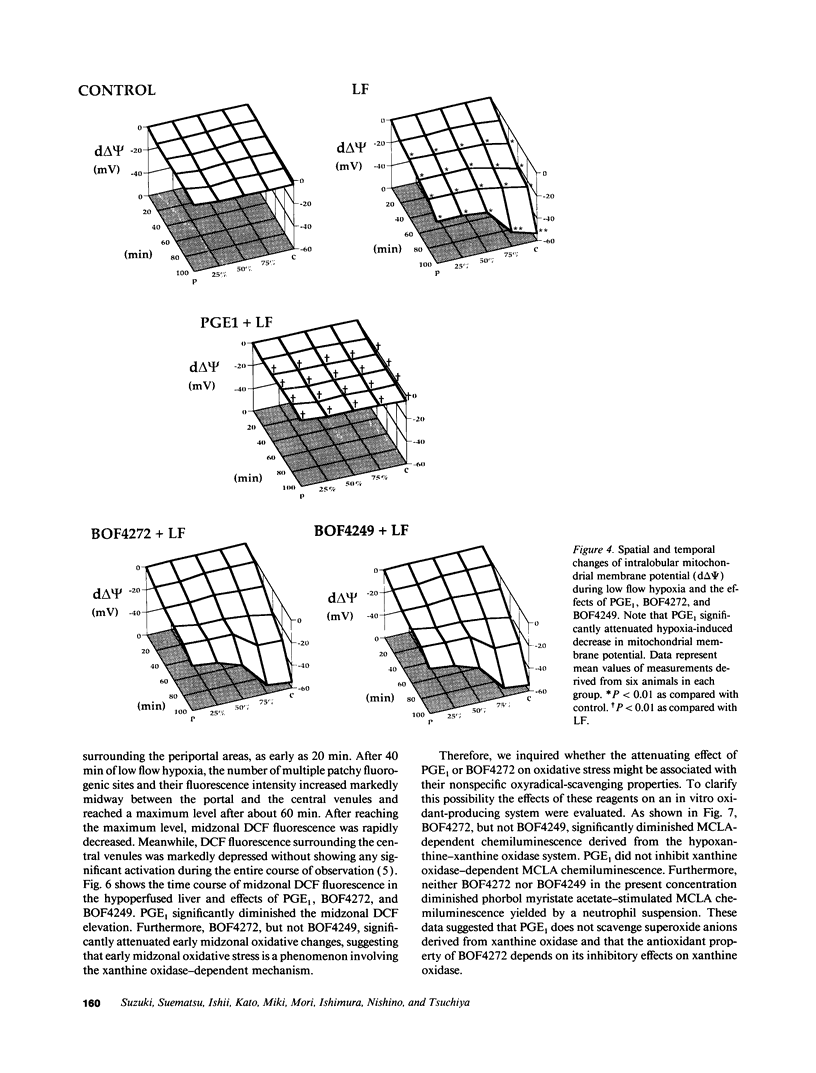
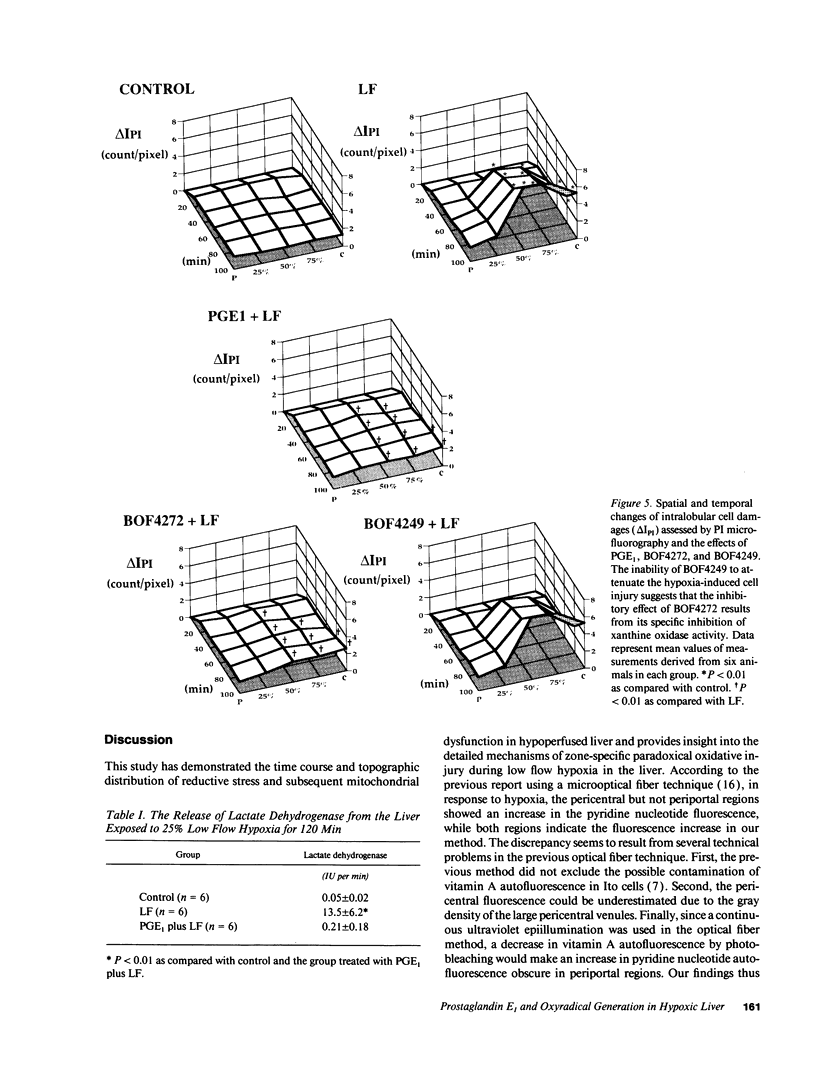
This study was designed to investigate the effects of prostaglandin E1 on reductive stress and the subsequent oxidative cell injury in hypoperfused rat liver. The intralobular heterogeneity of hepatocellular redox state, mitochondrial dysfunction, and intracellular hydroperoxide formation were visually monitored by digital microfluorography of pyridine nucleotide autofluorescence, rhodamine 123, and dichlorofluorescein fluorescence, respectively. Under the 25% low flow perfusion, pyridine nucleotide autofluorescence increased time-dependently and reached a steady state at 10 min among the entire lobules. The decrease in mitochondrial membrane potential was > 20 mV in all portions of the lobules at 60 min. The onset of hydroperoxide formation was observed at 40 min in the marginally oxygenated proximal portion of anoxic pericentral regions and the oxidative impact reached a maximum level at 60 min. Sodium (-)-8-(3-methoxy-4-phenylsulfinylphenyl) pyrazo [1,5-a]-1,3,5-triazine-4-olate monohydrate (BOF 4272), a novel xanthine oxidase inhibitor, suppressed the zone-specific oxidative changes without attenuating the increase in pyridine nucleotide autofluorescence and mitochondrial dysfunction. Pretreatment with prostaglandin E1 not only abrogated an early increase in pyridine nucleotide fluorescence and mitochondrial dysfunction induced by hypoperfusion but also diminished the subsequent midzonal oxidative injury. Since prostaglandin E1 has no oxyradical-scavenging action, the preventive effect of this reagent on the hypoxia-induced oxidative cell injury is attributable to the attenuation of mitochondrial dysfunction. These results suggest that, in low flow hypoxia, early reductive stress plays a key role in the initiation of xanthine oxidase-mediated midzonal oxidative changes, which may lead to subsequent centrilobular necrosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abecassis M., Falk J. A., Makowka L., Dindzans V. J., Falk R. E., Levy G. A. 16, 16 Dimethyl prostaglandin E2 prevents the development of fulminant hepatitis and blocks the induction of monocyte/macrophage procoagulant activity after murine hepatitis virus strain 3 infection. J Clin Invest. 1987 Sep;80(3):881–889. doi: 10.1172/JCI113147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass C. A., Narciso J., Gollan J. L. Enhanced activity of the free radical producing enzyme xanthine oxidase in hypoxic rat liver. Regulation and pathophysiologic significance. J Clin Invest. 1991 Feb;87(2):424–431. doi: 10.1172/JCI115013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Ames B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983 Oct 1;134(1):111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Emaus R. K., Grunwald R., Lemasters J. J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986 Jul 23;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Flarsheim C. E., Dawson T. L., Nieminen A. L., Herman B., Lemasters J. J. Swelling, reductive stress, and cell death during chemical hypoxia in hepatocytes. Am J Physiol. 1989 Aug;257(2 Pt 1):C347–C354. doi: 10.1152/ajpcell.1989.257.2.C347. [DOI] [PubMed] [Google Scholar]
- Greig P. D., Woolf G. M., Sinclair S. B., Abecassis M., Strasberg S. M., Taylor B. R., Blendis L. M., Superina R. A., Glynn M. F., Langer B. Treatment of primary liver graft nonfunction with prostaglandin E1. Transplantation. 1989 Sep;48(3):447–453. doi: 10.1097/00007890-198909000-00020. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Maidt L., Poyer L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(II). Biochem J. 1990 Jul 1;269(1):169–174. doi: 10.1042/bj2690169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H., Kleinwaechter C., Wendel A. NADH-dependent reductive stress and ferritin-bound iron in allyl alcohol-induced lipid peroxidation in vivo: the protective effect of vitamin E. Chem Biol Interact. 1992 Jan;81(1-2):57–68. doi: 10.1016/0009-2797(92)90026-h. [DOI] [PubMed] [Google Scholar]
- Kato S., Kawase T., Alderman J., Inatomi N., Lieber C. S. Role of xanthine oxidase in ethanol-induced lipid peroxidation in rats. Gastroenterology. 1990 Jan;98(1):203–210. doi: 10.1016/0016-5085(90)91311-s. [DOI] [PubMed] [Google Scholar]
- Kurokawa T., Nonami T., Harada A., Nakao A., Sugiyama S., Ozawa T., Takagi H. Effects of prostaglandin E1 on the recovery of ischemia-induced liver mitochondrial dysfunction in rats with cirrhosis. Scand J Gastroenterol. 1991 Mar;26(3):269–274. doi: 10.3109/00365529109025041. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., Ji S., Thurman R. G. Centrilobular injury following hypoxia in isolated, perfused rat liver. Science. 1981 Aug 7;213(4508):661–663. doi: 10.1126/science.7256265. [DOI] [PubMed] [Google Scholar]
- Marotto M. E., Thurman R. G., Lemasters J. J. Early midzonal cell death during low-flow hypoxia in the isolated, perfused rat liver: protection by allopurinol. Hepatology. 1988 May-Jun;8(3):585–590. doi: 10.1002/hep.1840080325. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y., Tsutsui H., Miyajima K., Sakagami Y., Seki S., Kobayashi K., Yamamoto S., Morisawa S. The protective effects of prostaglandin E1 in an experimental massive hepatic cell necrosis model. Hepatology. 1987 Nov-Dec;7(6):1184–1188. doi: 10.1002/hep.1840070603. [DOI] [PubMed] [Google Scholar]
- Nakano M. Determination of superoxide radical and singlet oxygen based on chemiluminescence of luciferin analogs. Methods Enzymol. 1990;186:585–591. doi: 10.1016/0076-6879(90)86154-n. [DOI] [PubMed] [Google Scholar]
- Nishino T., Tamura I. The mechanism of conversion of xanthine dehydrogenase to oxidase and the role of the enzyme in reperfusion injury. Adv Exp Med Biol. 1991;309A:327–333. doi: 10.1007/978-1-4899-2638-8_74. [DOI] [PubMed] [Google Scholar]
- Sato S., Tatsumi K., Nishino T. A novel xanthine dehydrogenase inhibitor (BOF-4272). Adv Exp Med Biol. 1991;309A:135–138. doi: 10.1007/978-1-4899-2638-8_30. [DOI] [PubMed] [Google Scholar]
- Shimmura S., Tsubota K., Oguchi Y., Fukumura D., Suematsu M., Tsuchiya M. Oxiradical-dependent photoemission induced by a phacoemulsification probe. Invest Ophthalmol Vis Sci. 1992 Sep;33(10):2904–2907. [PubMed] [Google Scholar]
- Stachura J., Tarnawski A., Ivey K. J., Mach T., Bogdal J., Szczudrawa J., klimczyk B. Prostaglandin protection of carbon tetrachloride-induced liver cell necrosis in the rat. Gastroenterology. 1981 Aug;81(2):211–217. [PubMed] [Google Scholar]
- Suematsu M., Kato S., Ishii H., Asako H., Yanagisawa T., Suzuki H., Oshio C., Tsuchiya M. Intralobular heterogeneity of carbon tetrachloride-induced oxidative stress in perfused rat liver visualized by digital imaging fluorescence microscopy. Lab Invest. 1991 Feb;64(2):167–173. [PubMed] [Google Scholar]
- Suematsu M., Kurose I., Asako H., Miura S., Tsuchiya M. In vivo visualization of oxyradical-dependent photoemission during endothelium-granulocyte interaction in microvascular beds treated with platelet-activating factor. J Biochem. 1989 Aug;106(2):355–360. doi: 10.1093/oxfordjournals.jbchem.a122857. [DOI] [PubMed] [Google Scholar]
- Suematsu M., Oda M., Suzuki H., Kaneko H., Watanabe N., Furusho T., Masushige S., Tsuchiya M. Intravital and electron microscopic observation of Ito cells in rat hepatic microcirculation. Microvasc Res. 1993 Jul;46(1):28–42. doi: 10.1006/mvre.1993.1033. [DOI] [PubMed] [Google Scholar]
- Suematsu M., Oshio C., Miura S., Tsuchiya M. Real-time visualization of oxyradical burst from single neutrophil by using ultrasensitive video intensifier microscopy. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1106–1110. doi: 10.1016/0006-291x(87)90522-5. [DOI] [PubMed] [Google Scholar]
- Suematsu M., Suzuki H., Ishii H., Kato S., Hamamatsu H., Miura S., Tsuchiya M. Topographic dissociation between mitochondrial dysfunction and cell death during low-flow hypoxia in perfused rat liver. Lab Invest. 1992 Oct;67(4):434–442. [PubMed] [Google Scholar]
- Suematsu M., Suzuki H., Ishii H., Kato S., Yanagisawa T., Asako H., Suzuki M., Tsuchiya M. Early midzonal oxidative stress preceding cell death in hypoperfused rat liver. Gastroenterology. 1992 Sep;103(3):994–1001. doi: 10.1016/0016-5085(92)90034-v. [DOI] [PubMed] [Google Scholar]
- Suematsu M., Suzuki M., Kitahora T., Miura S., Suzuki K., Hibi T., Watanabe M., Nagata H., Asakura H., Tsuchiya M. Increased respiratory burst of leukocytes in inflammatory bowel diseases--the analysis of free radical generation by using chemiluminescence probe. J Clin Lab Immunol. 1987 Nov;24(3):125–128. [PubMed] [Google Scholar]