Abstract
The effects of nitrogen oxides on anammox bacteria are not well known. Therefore, anammox bacteria were exposed to 3,500 ppm nitric oxide (NO) in the gas phase. The anammox bacteria were not inhibited by the high NO concentration but rather used it to oxidize additional ammonium to dinitrogen gas under conditions relevant to wastewater treatment.
Nitric oxide (NO) has several different roles in bacteria, fungi, and mammals (24). In nitrogen cycle bacteria, it acts as an intermediate and cell communication/signal transduction molecule. On the other hand, NO is a highly reactive and toxic compound that contributes to ozone depletion and air pollution (5). Due to its reactive nature, many bacteria employ an arsenal of proteins (those encoded by norVW, as well as bacterial globins, heme proteins, etc.) that are used to detoxify NO to the less-reactive and more-stable nitrous oxide (N2O) (24). Still, N2O is a very effective greenhouse gas and an unfavorable constituent in the off-gases from nitrification/denitrification nitrogen removal systems (4). The presence of gene(s) encoding cytochrome cd1 nitrite reductase (EMBL accession no. CAJ74898), flavorubredoxin NorVW (accession no. CAJ73918 and CAJ73688), and bacterial hemoglobin (accession no. CAJ72702) in the genome of Kuenenia stuttgartiensis led to the proposal that NO also plays this dual role (metabolic versus toxic) in anammox bacteria (Fig. 1) (10, 20). This has ramifications for both application and metabolism of anammox bacteria. The source of NO in an anammox reactor could be the activity of other community members (ammonium-oxidizing or denitrifying bacteria) or high concentrations of nitrite in the influent wastewater stream. Full-scale anammox reactors typically contain a significant population of ammonium-oxidizing bacteria (AOB). In the single nitritation-anammox reactors, these carry out the conversion of 50% of the ammonium in the wastewater to nitrite (6). It has been shown that AOB may produce significant amounts of NO (2, 7), and recently it was reported that NO and N2O could be emitted from these reactors up to 0.005 and 1.2% of the total nitrogen load to the reactor, respectively (6, 23). NO may inhibit the anammox bacteria and could also be further reduced to N2O in these reactors (6, 23). It is presently unknown whether anammox bacteria contribute to the NO or N2O emissions, although it has been suggested previously that anammox bacteria do not produce N2O under physiologically relevant conditions (10). Nevertheless, if conversion of NO could be coupled to anaerobic ammonium oxidation, the toxic air pollutant NO would facilitate further removal of ammonium in full-scale anammox bioreactors. In the present study, we investigated the effect of very high NO fluxes on anammox bacteria.
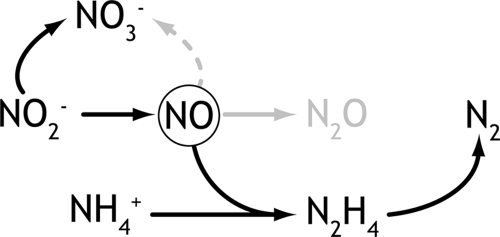
FIG. 1.
The hypothetical anammox pathway with possible routes of NO removal. Solid black arrows: anammox pathway, including nitrite oxidation to nitrate; gray arrow, possible detoxification pathway to N2O (not observed in the bioreactor); dashed gray arrow, NO oxidation to nitrite/nitrate (not possible under anoxic conditions).
NO has been described many times as a potent inhibitor of nitrogen cycle bacteria; aerobic ammonium oxidizers, nitrite oxidizers, and denitrifiers were all inhibited by concentrations as low as a few micromolar units (1, 18, 24). In a previous study, it was suggested that “Candidatus Brocadia anammoxidans” could tolerate up to 600 ppm NO (approximately 1 mg NO·day−1 NO load) (16). In the reported experiments, without direct measurement of nitrous oxide (N2O) in the effluent gas stream, it was postulated that NO was reduced to N2O (16). In the present study, we used a carefully monitored sequencing batch reactor (SBR) to further our understanding of the effect and fate of NO in a laboratory-scale anammox reactor under conditions which are relevant in wastewater treatment plants.
An SBR (working volume, 3.5 liters) consisting of approximately 80% of the anammox bacterium “Candidatus Brocadia fulgida” and no detectable aerobic ammonium oxidizers (determined by fluorescence in situ hybridization (FISH) as described previously [15]) was used in the present study. Before the first introduction of NO into the reactor, the influent (synthetic wastewater) (21) was supplied to the reactor at a flow rate of 1.4 ml·min−1 with nitrite and ammonium concentrations (assayed as previously described [9]) at 45 and 39 mM, respectively (corresponding to a total of 2,370 mg N·day−1). All nitrite was consumed in the reactor, while 2 mM ammonium was still present in the effluent. For every 1 mol of ammonium, 1.22 mol of nitrite was consumed, similar to the previously determined anammox stoichiometry (19). NO was first introduced at a concentration of 400 to 600 ppm in the gas phase at a flow rate of 10 ml/min (CLD 700EL chemiluminescence NOx analyzer, detection limit of 0.1 ppm NO, with 15 ml/min Ar/CO2 as the dilution gas [a load of 25 to 28 mg NO·day−1]; EcoPhysics, Michigan). During this period, 45% (±6%) of the supplied NO was removed from the system. Initially, there was no detectable change in the ammonium and nitrite removal efficiencies and no detectable nitrous oxide (N2O) in the flue gas (analyzed with an Agilent 6890 gas chromatograph). It is most likely that NO was converted to N2, but the increase in the N2 concentrations in the off-gas was below the detection limit (1,000 ppm).
At day 49, the influent NO concentration was increased to 3,500 ppm (640 mg NO·day−1 load). Simultaneously, the stirring speed of the reactor was increased from 200 to 600 rpm to enable better mass transfer to the flocculent anammox biomass. The increase in the stirring speed did not result in any disturbance in the floc size and settling ability of the biomass but did lead to a much higher level of NO removal (128 mg NO·day−1) by the anammox bacteria. The converted NO could theoretically be converted to N2O via detoxification enzymes or coupled to ammonium oxidation (Fig. 1). Surprisingly, there was no change in the nitrite removal capacity of the bioreactor, suggesting that NO was not a substrate preferred over nitrite. Nitrate concentrations (assayed according to the method in reference 9) were stable around 7.2 mM (±0.7 mM). Theoretically, as anammox bacteria reduce NO, they could oxidize a larger proportion of nitrite to nitrate (Fig. 1) to increase their capacity for CO2 fixation; however, such an increase in nitrate production was not observed (or could not be discriminated by the method used [sensitivity, 100 μM]). During this phase of the experiment, the effluent ammonium concentration gradually decreased to below the detection limit (Fig. 2). There was only a minimal N2O (0.6 ppm) emission from the system, and the total N2 production increased from 3,060 to 3,680 mg N2·day−1. This indicated that NO reduction was coupled to the catabolism of the anammox bacteria rather than being detoxified by anammox or other community members. To the best of our knowledge, this was the first time that such a high load of NO was not found to be toxic to the nitrogen cycle bacteria. In a previous study, an NO load of 1 mg NO·day−1 was reported to be toxic to anammox bacteria, most probably due to the fact that the experiments were conducted with biomass that had a 100-fold lower cell density and 10-fold lower activity compared to the current enrichment cultures. Furthermore, the NO conversion in the current experiments was stoichiometrically coupled to ammonium oxidation and not converted to N2O, indicating that the previously reported N2O emissions from full-scale anammox bioreactors originated not with the anammox bacteria but rather with other community members as hypothesized previously (8).
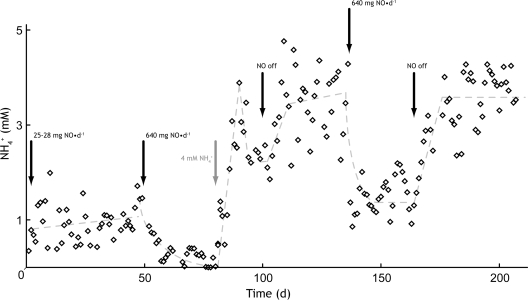
FIG. 2.
Ammonium concentration in the effluent of the anammox bioreactor. Dashed lines indicate the trend of effluent ammonium concentration during different phases of the reactor operation. Black arrows indicate the manipulations to influent NO stream, and the gray arrow points to an increase in the influent ammonium concentration. d, day.
To determine if there could be more NO-dependent ammonium removal, the influent ammonium concentration was first increased to 41 mM (day 80) and then to 43 mM (day 81). This resulted in a slow but gradual increase in the effluent ammonium concentration, and additional ammonium did not appear to be completely converted, most probably due to NO mass transfer limitations. As a result of the higher level of ammonium removal, the observed anammox stoichiometry in the reactor decreased from 1.22 to 0.91 (nitrite/ammonium). Between days 95 and 131, the NO supply to the reactor was turned off, which resulted in an average ammonium concentration of 3.3 mM (±0.9 mM) in the effluent. Following this period, on day 132, the NO load on the reactor was increased back to 640 mg NO·day−1 (Fig. 2). As a result, the effluent ammonium concentration gradually decreased again to an average of 1.5 mM (±0.36 mM). The highest level of NO removal achieved in this period was 371 mg NO·day−1. When the NO supply was turned off on day 165, ammonium concentrations increased back to 3.5 mM (±0.71 mM).
During the course of the experiment, the biodiversity of the reactor was monitored using FISH and 16S rRNA gene sequence analysis as described previously (15) with probes specific to eubacteria (3), Planctomycetes (13), anammox bacteria (15), “Ca. Brocadia fulgida” (11), and a variety of aerobic ammonium-oxidizing bacteria (12, 22). Before the experiments started and throughout the cultivation of the anammox bacteria with NO, the only detectable anammox species (with FISH and 16S rRNA gene sequence analysis) was “Candidatus Brocadia fulgida.”
In the present study, we showed that 2 mM ammonium (4.5% of the influent concentration) could be removed by anammox bacteria via direct coupling to NO reduction. These observations support the proposal of NO as an intermediate of the anammox reaction and have two consequences for application of the anammox process for nitrogen removal. First, we obtained strong indications that previously reported N2O emissions (6, 8) from full-scale anammox reactors were not generated by anammox bacteria. In our experiments, even under a very high load of NO, there was hardly any detectable N2O in the effluent gas stream. The competition for nitrogen oxides by denitrifying and anammox bacteria needs further study but may ultimately be used to design operational conditions that would reduce or even prevent NO and N2O emissions from full-scale nitritation-anammox reactors. Second, by implementing the results of this study, in the future the anammox process could be designed to remove NO from flue gases. Since NO is mostly emitted together with O2, this could be achieved by the combination of anammox and aerobic ammonium-oxidizing bacteria, for example, with CANON (completely autotrophic nitrogen removal over nitrite)- or OLAND (oxygen-limited autotrophic nitrification-denitrification)-type reactor systems (14, 17).
Acknowledgments
This research was supported by the Foundation for Applied Research (STW project 06252). M. S. M. Jetten is supported by an Advanced ERC grant (no. 232987).
We acknowledge Marc Strous for discussions.
Footnotes
Published ahead of print on 30 July 2010.
REFERENCES
- 1.Carr, G. J., and S. J. Ferguson. 1990. Nitric-oxide formed by nitrite reductase of Paracoccus denitrificans is sufficiently stable to inhibit cytochrome-oxidase activity and is reduced by its reductase under aerobic conditions. Biochim. Biophys. Acta 1017:57-62. [DOI] [PubMed] [Google Scholar]
- 2.Colliver, B. B., and T. Stephenson. 2000. Production of nitrogen oxide and dinitrogen oxide by autotrophic nitrifiers. Biotechnol. Adv. 18:219-232. [DOI] [PubMed] [Google Scholar]
- 3.Daims, H., A. Bruehl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 4.Fux, C., and H. Siegrist. 2004. Nitrogen removal from sludge digester liquids by nitrification/denitrification or partial nitritation/anammox: environmental and economical considerations. Water Sci. Technol. 50:19-26. [PubMed] [Google Scholar]
- 5.Johnston, H. 1971. Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust. Science 173:517-522. [DOI] [PubMed] [Google Scholar]
- 6.Kampschreur, M. J., R. Poldermans, R. Kleerebezem, W. R. L. van der Star, R. Haarhuis, W. R. Abma, M. S. M. Jetten, and M. C. M. van Loosdrecht. 2009. Emission of nitrous oxide and nitric oxide from a full-scale single-stage nitritation-anammox reactor. Water Sci. Technol. 60:3211-3217. [DOI] [PubMed] [Google Scholar]
- 7.Kampschreur, M. J., N. C. G. Tan, R. Kleerebezem, C. Picioreanu, M. S. M. Jetten, and M. C. M. van Loosdrecht. 2008. Effect of dynamic process conditions on nitrogen oxides emission from a nitrifying culture. Environ. Sci. Technol. 42:429-435. [DOI] [PubMed] [Google Scholar]
- 8.Kampschreur, M. J., W. R. L. van der Star, H. A. Wielders, J. W. Mulder, M. S. M. Jetten, and M. C. M. van Loosdrecht. 2008. Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res. 42:812-826. [DOI] [PubMed] [Google Scholar]
- 9.Kartal, B., M. Koleva, R. Arsov, W. van der Star, M. S. M. Jetten, and M. Strous. 2006. Adaptation of a freshwater anammox population to high salinity wastewater. J. Biotechnol. 126:546-553. [DOI] [PubMed] [Google Scholar]
- 10.Kartal, B., M. M. M. Kuypers, G. Lavik, J. Schalk, H. J. M. Op den Camp, M. S. M. Jetten, and M. Strous. 2007. Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ. Microbiol. 9:635-642. [DOI] [PubMed] [Google Scholar]
- 11.Kartal, B., L. van Niftrik, J. Rattray, J. de Vossenberg, M. C. Schmid, J. S. S. Damste, M. S. M. Jetten, and M. Strous. 2008. Candidatus ‘Brocadia fulgida’: an autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiol. Ecol. 63:46-55. [DOI] [PubMed] [Google Scholar]
- 12.Mobarry, B. K., M. Wagner, V. Urbain, B. E. Rittmann, and D. A. Stahl. 1996. Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl. Environ. Microbiol. 62:2156-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neef, A., R. I. Amann, H. Schlesner, and K.-H. Schleifer. 1998. Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology 144:3257-3266. [DOI] [PubMed] [Google Scholar]
- 14.Pynaert, K., S. Wyffels, R. Sprengers, P. Boeckx, O. Van Cleemput, and W. Verstraete. 2002. Oxygen-limited nitrogen removal in a lab-scale rotating biological contactor treating an ammonium-rich wastewater. Water Sci. Technol. 45:357-363. [PubMed] [Google Scholar]
- 15.Schmid, M., U. Twachtmann, M. Klein, M. Strous, S. Juretschko, M. S. M. Jetten, J. W. Metzger, K.-H. Schleifer, and M. Wagner. 2000. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst. Appl. Microbiol. 23:93-106. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt, I., C. Hermelink, K. van de Pas-Schoonen, M. Strous, H. J. M. Op den Camp, J. G. Kuenen, and M. S. M. Jetten. 2002. Anaerobic ammonia oxidation in the presence of nitrogen oxides (NOx) by two different lithotrophs. Appl. Environ. Microbiol. 68:5351-5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sliekers, A. O., N. Derwort, J. L. C. Gomez, M. Strous, J. G. Kuenen, and M. S. M. Jetten. 2002. Completely autotrophic nitrogen removal over nitrite in one single reactor. Water Res. 36:2475-2482. [DOI] [PubMed] [Google Scholar]
- 18.Starkenburg, S. R., D. J. Arp, and P. J. Bottomley. 2008. Expression of a putative nitrite reductase and the reversible inhibition of nitrite-dependent respiration by nitric oxide in Nitrobacter winogradskyi Nb-255. Environ. Microbiol. 10:3036-3042. [DOI] [PubMed] [Google Scholar]
- 19.Strous, M., J. G. Kuenen, and M. S. M. Jetten. 1999. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microbiol. 65:3248-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strous, M., E. Pelletier, S. Mangenot, T. Rattei, A. Lehner, M. W. Taylor, M. Horn, H. Daims, D. Bartol-Mavel, P. Wincker, V. Barbe, N. Fonknechten, D. Vallenet, B. Segurens, C. Schenowitz-Truong, C. Medigue, A. Collingro, B. Snel, B. E. Dutilh, H. J. M. Op den Camp, C. van der Drift, I. Cirpus, K. T. van de Pas-Schoonen, H. R. Harhangi, L. van Niftrik, M. Schmid, J. Keltjens, J. van de Vossenberg, B. Kartal, H. Meier, D. Frishman, M. A. Huynen, H. W. Mewes, J. Weissenbach, M. S. M. Jetten, M. Wagner, and D. Le Paslier. 2006. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440:790-794. [DOI] [PubMed] [Google Scholar]
- 21.Van de Graaf, A. A., P. deBruijn, L. A. Robertson, M. S. M. Jetten, and J. G. Kuenen. 1996. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142:2187-2196. [Google Scholar]
- 22.Wagner, M., G. Rath, R. Amann, H. P. Koops, and K.-H. Schleifer. 1995. In-situ identification of ammonia-oxidizing bacteria. Syst. Appl. Microbiol. 18:251-264. [Google Scholar]
- 23.Weissenbacher, N., I. Takacs, S. Murthy, M. Fuerhacker, and B. Wett. 2010. Gaseous nitrogen and carbon emissions from a full-scale deammonification plant. Water Environ. Res. 82:169-175. [DOI] [PubMed] [Google Scholar]
- 24.Zumft, W. G. 1993. The biological role of nitric oxide in bacteria. Arch. Microbiol. 160:253-264. [DOI] [PubMed] [Google Scholar]