Abstract
Central to the understanding of infections by the waterborne pathogen Legionella pneumophila is its detection at the clonal level. Currently, multiple-locus variable-number tandem-repeat (VNTR) analysis (MLVA) of L. pneumophila isolates can be used as a tool for high-resolution genotyping. Since L. pneumophila is difficult to isolate, the isolation of outbreak strains often fails due to a viable but nonculturable (VBNC) state of the respective environmental population. Therefore, we developed a cultivation-independent approach to detect single clones in drinking water. This approach is based on the extraction of DNA from drinking water followed by PCR using a set of eight VNTR primer pairs necessary for MLVA genotyping of L. pneumophila. The PCR amplicons were analyzed by single-strand conformation polymorphism (SSCP) and capillary electrophoresis to obtain the respective MLVA profiles. Parallel to the high-resolution analysis, we used the same environmental DNA to quantify the number of L. pneumophila cells in drinking water using real-time PCR with 16S rRNA gene-targeted primers. We used a set of drinking water samples from a small-scale drinking water network to test our approach. With these samples we demonstrated that the developed approach was directly applicable to DNA obtained from drinking water. We were able to detect more L. pneumophila MLVA genotypes in drinking water than we could detect by isolation. Our approach could be a valuable tool to identify outbreak strains even after the outbreak has occurred and has the potential to be applied directly to clinical material.
Legionella pneumophila is a Gram-negative, facultative, intracellular pathogen that accounts for the majority of cases of Legionnaires' disease in Europe (16). It is also the causative agent of a milder form of infection, Pontiac fever (14). Legionellae are ubiquitous inhabitants of natural and human-made aquatic environments. They occur in bulk water and biofilms, where they replicate within protozoa, which can serve as a transmission vehicle and as a protective shell against disinfection or heat treatment (2, 6, 7). In drinking water supply systems (DWSSs), legionellae can survive in dead-end tubings, stagnated water in plumbings, or seldom-used facilities (2). The pathogen is transmitted via small droplets of water, e.g., aerosols from cooling towers, showerheads, or air conditioners. In the human lung, it is able to enter and replicate within alveolar macrophages, causing a severe pneumonia. Among the 48 species of the genus Legionella (1, 4, 21, 22), L. pneumophila is responsible for approximately 91% of all reported community-acquired cases of legionellosis. Among the 15 serogroups of L. pneumophila, serogroup 1 accounts for 84% of confirmed cases, as assessed by an international collaborative survey (40). Even among serogroup 1 isolates, a high level of genetic diversity was observed by several studies (12, 30, 35).
Epidemiological analyses of infections caused by L. pneumophila depend on the accurate identification of strains, preferably at the clonal level. Therefore, several typing methods have been implemented in the last years, e.g., MLST (multiple-locus sequence typing), which is based on DNA sequencing of multiple polymorphic DNA segments (13, 33). Recently, a multiple-locus variable-number tandem-repeat (VNTR) analysis (MLVA) was implemented by Pourcel et al. and approved by Eurosurveillance (30, 31). MLVA typing is used to determine the allele-related repeat size variation on different VNTR loci of L. pneumophila isolates. The method was further improved by adapting the eight-locus-comprising MLVA (MLVA-8) to capillary electrophoresis (CE) with the use of fluorescently labeled primers, thus providing a fast, reproducible, and low-cost genotyping method for L. pneumophila isolates (26). Upstream isolation procedures face several problems: especially in hot water with temperatures above 37°C, legionellae can lose culturability and enter a viable but nonculturable (VBNC) state (28). This VBNC state is mostly the reason why L. pneumophila cannot be isolated from aquatic environments that are suspected to be the source of infection (37). Additionally, cultivation of this fastidious bacterium is difficult due to its slow growth and overgrowing by competing bacteria on agar plates.
The aim of this study was to evaluate the utility of MLVAs directly on environmental DNA obtained from finished drinking water. Using DNA-based single-strand conformation polymorphism (SSCP) analysis, this method should (i) allow the identification of strains causing outbreaks, (ii) allow the monitoring of L. pneumophila strains present in a given sample at the clonal level without cultivation, and (iii) provide sequence information on VNTR markers obtained directly from the sequencing of SSCP gel bands, i.e., environmental DNA. To this end, we tested our approach with DNA from a set of drinking water samples from a small-scale drinking water network. We demonstrated that the method provides a reliable tool for the analysis of drinking water samples where the number of L. pneumophila cells present is relatively low and where isolation procedures did not succeed. Additionally, complete sequence information on the VNTR locus could be obtained from PCR amplicons separated on SSCP gels to identify the specific MLVA genotype.
MATERIALS AND METHODS
Strains and growth conditions.
Reference strain Legionella pneumophila subsp. pneumophila strain Philadelphia DSMZ7513T was provided by Molecular Diagnostics Center, Orihuela, Spain. All other reference strains (L. pneumophila strain Corby, L. pneumophila strain Lens, Legionella jamestowniensis, L. jordanis, L. cincinnatiensis, and L. feeleii) were kindly provided by Michael Steinert from the Institute of Microbiology at the Technical University of Braunschweig. All reference strains were grown on solid buffered charcoal yeast extract (BCYE) medium supplemented with l-cysteine and ferric pyrophosphate, with or without antibiotics (Oxoid, Basingstoke, United Kingdom).
Sampling of drinking water and isolation of Legionella sp. strains.
Hot and cold drinking water samples were collected from several sources (for details, see Table 1) on the campus of the Helmholtz Centre for Infection Research (HZI) in Braunschweig, Germany, on 23 June 2009. In addition, cooling tower water samples (bulk water) were obtained on 10 July 2009. Legionella sp. strains were isolated from the samples according to ISO 11731-2 (German monitoring method for the isolation and enumeration of Legionella organisms in water intended for human use) (15a). In brief, 10 to 1,000 ml (mostly 250 ml) of the water sample (or an appropriate dilution) was filtered onto a 0.45-μm HABG filter (mixed cellulose esters, black, with counting grid; Millipore, Schwalbach, Germany) and treated with 20 ml of acidic buffer (0.2 mol HCl-KCl solution [pH 2.2]) for 5 min. After a washing step with 10 ml of sterile distilled water, the filter was transferred onto GVPC (BCYE medium with glycine vancomycin polymyxin cycloheximide) or MWY (BCYE medium with antimicrobial agents, glycine, and differential dyes) solid medium (Mibius, Düsseldorf, Germany) and incubated at 36°C ± 1°C for 3 to 10 days. Legionella colonies appeared as gray or white round colonies with ground-glass opacity when observed after 3 days of culture. All Legionella-like colonies were picked from this medium and subcultured onto BCYE medium (Mibius, Düsseldorf, Germany) with and without antibiotics and onto sheep blood agar plates (Mibius, Düsseldorf, Germany). Organisms growing on charcoal agar but not on blood agar were tested by PCR with Legionella genus-specific primers Lgsp17F and Lgsp28R (see below). Positive colonies were subcultured onto BCYE medium and further characterized by complete sequencing of the 16S rRNA gene.
TABLE 1.
Characteristics of Legionella sp. isolates obtained on the HZI campus
| Isolate | Source of isolation | Isolation date | Isolation medium | L. pneumophila PCR result | Isolation vol (ml) (dilution) | 16S rRNA gene sequence analysis result (∼1,450-bp amplicon) |
|---|---|---|---|---|---|---|
| SK1 | Scullery, D building | 23 June 2009 | MWY | + | 250 | L. pneumophila strain Corby |
| SK2 | Scullery, D building | 23 June 2009 | MWY | + | 250 | L. pneumophila strain Corby |
| Y7 | Y building, technical room | 23 June 2009 | MWY | + | 250 | L. pneumophila strain Corby |
| Y8 | Y building, technical room | 23 June 2009 | MWY | + | 250 | L. pneumophila strain Corby |
| GZ1 | GZ building, toilet 3.015 | 23 June 2009 | MWY | − | 250 | L. anisa |
| GZ2 | GZ building, toilet 3.015 | 23 June 2009 | MWY | − | 250 | L. anisa |
| KT1 | Heat exchange water tower | 10 July 2009 | GVPC | + | 250 (1:100) | L. pneumophila Philadelphia/L. pneumophila Paris |
| KT2 | Heat exchange water tower | 10 July 2009 | GVPC | + | 250 (1:100) | L. pneumophila Philadelphia/Paris/Corby |
| KT3 | Heat exchange water tower | 10 July 2009 | GVPC | + | 250 (1:100) | L. pneumophila Philadelphia/L. pneumophila Paris |
| KT7 | Heat exchange water tower | 10 July 2009 | MWY | + | 250 (1:100) | L. pneumophila Philadelphia/L. pneumophila Paris |
| KT8 | Heat exchange water tower | 10 July 2009 | MWY | + | 250 (1:100) | L. pneumophila Philadelphia/L. pneumophila Paris |
| KT9 | Heat exchange water tower | 10 July 2009 | MWY | + | 250 (1:100) | L. pneumophila Philadelphia/L. pneumophila Paris |
| KT10 | Heat exchange water tower | 10 July 2009 | MWY | + | 250 (1:100) | L. pneumophila Philadelphia/L. pneumophila Paris |
| KT11 | Heat exchange water tower | 10 July 2009 | MWY | + | 250 (1:100) | L. pneumophila Philadelphia/L. pneumophila Paris |
| KT12 | Heat exchange water tower | 10 July 2009 | MWY | + | 250 (1:100) | L. pneumophila Philadelphia/L. pneumophila Paris |
For the long-term storage of the drinking water samples and DNA extraction, bacteria were harvested from 3 to 5 liters of drinking water by filtration through a filter sandwich consisting of a 0.2-μm-pore-size polycarbonate filter (90-mm diameter) (Nuclepore; Whatman, Maidstone, United Kingdom) with a precombusted glass fiber filter on top (90-mm diameter) (GF/F; Whatman) according to a method described previously by Eichler et al. (8). These filter sandwiches were stored at −70°C until further analysis.
For enumeration of total bacteria, formaldehyde-fixed drinking water samples (2% final concentration) were stained with Sybr green I dye (1:10,000 final dilution; Molecular Probes, Invitrogen) for 15 min at room temperature in the dark. Five-milliliter portions were filtered onto 0.2-μm-pore-size Anodisc filters (Whatman) and mounted with Citifluor onto microscopic glass slides according a protocol described previously Weinbauer et al. (39). Slides were either analyzed directly with epifluorescence microscopy or stored frozen (−20°C) until examination. For epifluorescence microscopy, a microscope (Axioplan; Zeiss) with suitable fluorescence filters was used, and the slides were examined by using a 100-fold magnification. For each filter, either 10 photographs were taken and image sections of a defined size (0.642 mm by 0.483 mm) were analyzed by using Image J software from MacBiophotonics (http://www.macbiophotonics.ca/) or 30 fields (0.125 mm by 0.125 mm) were counted by eye.
Heterotrophic plate counts (HPCs) were done in triplicate by using an aliquot of the drinking water sample and the spread plate technique with R2A agar (Oxoid) plates. Incubation was carried out at two different temperatures according to German drinking water regulations (36°C for 48 h and 22°C for 72 h) (10).
DNA extraction, PCR, and real-time PCR.
For the extraction of DNA from the filter sandwiches, a modified DNeasy protocol (Qiagen, Hilden, Germany) was used. In brief, sandwich filters were cut into pieces and incubated with enzymatic lysis buffer (20 mM Tris-HCl, 2 mM EDTA, 1.2% Triton X-100 [pH 8.0]) containing 10 mg/ml lysozyme (Sigma) for 60 min in a 37°C water bath. After the addition of AL buffer from the kit, the samples were incubated at 78°C in a shaking water bath for 20 min. After filtration through a polyamide mesh with a 250-μm pore size, absolute ethanol was added to the filtrate (ratio of filtrate to ethanol of 2:1), and the mixture was applied onto the spin column of the kit. After this step, the protocol was done according to the manufacturer's instructions. DNA was eluted from the column with DNase/RNase-free water and stored at −20°C. Quantification of DNA was carried out by using Picogreen (double-stranded DNA [dsDNA] quantification; Molecular Probes, Invitrogen) according to a method described previously by Weinbauer et al. (39). For Legionella sp. isolates, genomic DNA was isolated from agar plates using the standard DNeasy procedure for Gram-negative bacteria (Qiagen, Hilden, Germany).
Different PCRs were carried out as follows. For the classification of Legionella-like colonies obtained from the HZI sampling, a Legionella genus-specific PCR using primers Lgsp17F (5′-GGCCTACCAAGGCGACGATCG-3′) and Lgsp28R (5′-CACCGGAAATTCCACTACCCTCTC-3′) and a Legionella pneumophila-specific PCR using primers Lp-16S_246-248F (CCTGGGCTTAACCTGGGAC) and Lp-16S_246-248R (CTTAGAGTCCCCACCATCACAT) were applied. For the sequencing of the complete 16S rRNA gene of Legionella isolates, primers 27F (AGAGTTTGATCMTGGCTCAG) and 1492R (TACGGYTACCTTGTTACGACTT) were used. Each amplification was carried out by using 0.2 to 2 ng of DNA template in a final volume of 50 μl under the conditions described in Table 2. Amplification was achieved by using HotStar Taq DNA polymerase (Qiagen, Hilden, Germany).
TABLE 2.
PCR programs used in this study
| PCR | Initial denaturation temp (°C) (time [min]) | Denaturation temp (°C) (time [s]) | Annealing temp (°C) (time [s]) | Elongation temp (°C) (time [s]) | Final elongation temp (°C) (time [min]) |
|---|---|---|---|---|---|
| Legionella genus specific | 95 (15) | 95 (30) | 66.5 (30) | 72 (30) | 72 (10) |
| Legionella pneumophila | 95 (15) | 95 (45) | 60.0 (45) | 72 (45) | 72 (20) |
| Legionella 16S rRNA gene | 95 (15) | 95 (90) | 55.0 (40) | 72 (90) | 72 (10) |
For the quantification of the number of Legionella pneumophila genes in drinking water samples, real-time PCR using Sybr green I chemistry (Roche Diagnostics, Germany) was implemented. For real-time PCR, Legionella pneumophila-specific primers Lp-16S_246-248F and Lp-16S_246-248R (see above) were used. On the Light Cycler 480 real-time PCR machine (Roche Diagnostics, Germany), the 16S rRNA gene was used to determine the number of L. pneumophila genomes in an approach utilizing a standard of genomic DNA from L. pneumophila strain Philadelphia (DSMZ7513T) and a detection limit of approximately 0.4 Legionella pneumophila cells per PCR assay.
MLVA-8 and capillary electrophoresis.
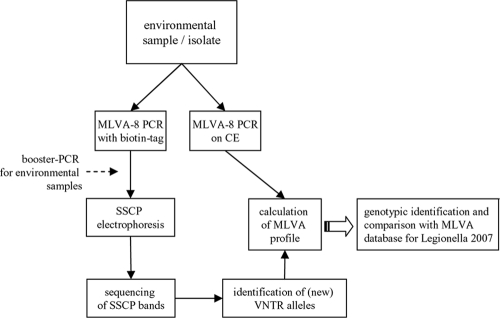
MLVA-8 single PCRs were carried out by using the primer sets described previously by Pourcel et al. (31) and the diagram shown in Fig. 1. PCR mixtures of 50 μl contained 1× reaction buffer (Qiagen, Hilden, Germany), 2.5 U of HotStar Taq DNA polymerase, 0.2 to 2 ng of template DNA, 125 nmol MgCl2, 7.5 nmol each deoxynucleoside triphosphate (dNTP), and 0.02 nmol each primer. Forward primers for PCR were synthesized with a biotin tag on the 5′ end (MWG Operon, Ebersberg, Germany). Each PCR was carried out by using an initial denaturation step for 15 min at 95°C and a total of 35 cycles (30 s at 95°C, 30 s at 65°C, and 30 s at 72°C), followed by a final elongation step for 10 min at 72°C. For PCRs of environmental DNA, a booster PCR was performed using 1 μl of the first amplification in a second PCR (50 μl).
FIG. 1.
Flow chart of the analytical setup. Either an isolate or an environmental sample was analyzed simultaneously by MLVA-8 for CE and SSCP electrophoresis. For CE, PCR was done by using fluorescently labeled primers to enable the determination of the repeat numbers in the alleles through an analysis of the peaks in the electropherograms. Subsequently, a comparison with the MLVA database for Legionella (2007) was conducted (http://bacterial-genotyping.igmors.u-psud.fr/Legionella2006/help.htm) to identify single clones. For the SSCP analysis of the samples, PCR amplification with 5′-biotin-tagged forward primers was applied. Bands originating from the SSCP fingerprints were cut out and analyzed by sequencing. The sequence information could then be converted into the repeat profile.
In the PCR for MLVA-8 for capillary electrophoresis, forward primers were labeled with VIC, PET, 6-carboxyfluorescein (FAM), and NED (Applied Biosystems, Foster City, CA) (Table 3). All reverse primers were synthesized unlabeled (MWG Operon, Ebersberg, Germany). After amplification, 1 μl (PCR for isolates) or up to 10 μl (in situ samples) of the PCR products of Lpms1b (here Lpms1), Lpms3, Lpms33, and Lpms35 (panel I) or Lpms13, Lpms17, Lpms19b (here Lpms19), and Lpms34 (panel II) was pooled, purified using the MinElute PCR cleanup kit (Qiagen, Hilden, Germany), and diluted 1:50 or 1:100 with distilled water. In the wells of a 96-well microtiter plate, 1 μl of the pooled and diluted PCR product mix was added to 8.85 μl of HiDi (highly deionized) formamide (Applied Biosystems) and 0.15 μl GeneScan 1200LIZ size standard (Applied Biosystems) containing 68 single-stranded labeled fragments in the range of 20 to 1,200 bp. The samples were denatured for 3 min at 95°C in a thermoblock, cooled on ice for at least 1 min, and spun briefly for 500 rpm in a Multifuge 1 centrifuge (Heraeus, Germany). Fragment analysis was performed with a 3130xl sequencer (Applied Biosystems) equipped with 50-cm capillaries by using a POP-7 polymer with the recommended running parameters for the GeneScan LIZ1200 size standard (running period of 2.5 h, running voltage of 8.5 kV, and injection voltage of 15 kV). Each L. pneumophila minimicrosatellite (Lpms) locus was identified by color and assigned a size by GeneMapper software, version 3.7 (Applied Biosystems), using settings for microsatellite analysis. The number of repeats in the alleles was estimated by subtracting the invariable flanking region from the amplicon size divided by the repeat unit length, as determined for reference strain Philadelphia according to a method described previously by Pourcel et al. (31).
TABLE 3.
Legionella pneumophila MLVA-8 setup for capillary electrophoresis
| Primera | Dye | Color | Panel | Repeat length (bp) | Total flanking region (bp) |
|---|---|---|---|---|---|
| Lpms1b | VIC | Green | I | 45 | 205 |
| Lpms3 | FAM | Blue | I | 96 | 173 |
| Lpms33 | NED | Yellow | I | 125 | 102 |
| Lpms35 | PET | Red | I | 18 | 148 |
| Lpms13 | NED | Yellow | II | 24 | 164 |
| Lpms17 | VIC | Green | II | 39 | 200 |
| Lpms19b | FAM | Blue | II | 21 | 89 |
| Lpms34 | PET | Red | II | 125 | 84 |
Primer designations according to Pourcel et al. (31).
SSCP electrophoresis.
For the preparation of single-stranded DNA (ssDNA) from the PCR amplicons, a variant of a protocol described previously by Eichler et al. (9) was applied. Briefly, magnetic streptavidin-coated beads (Promega, Madison, WI) were used to prepare ssDNA from the PCR amplicons. For SSCP analysis, 25 ng of the obtained ssDNA was mixed with gel loading buffer (95% formamide, 10 mM NaOH, 0.25% bromphenol blue, 0.25% xylene cyanol) in a final volume of 7 μl. After incubation for 3 min at 95°C, the ssDNA samples were stored on ice, loaded onto a nondenaturing polyacrylamide-like gel (0.6 × MDE gel solution; Cambrex BioScience, Rockland, ME), and electrophoretically separated at 20°C at 400 V for 20 h on a Macrophor sequencing apparatus with 55-cm glass plates (Pharmacia Biotech, Germany). The gel was silver stained according to a method described previously by Bassam et al. (3). Dried SSCP gels were digitized by using an Epson Expression 1600 Pro scanner.
Reamplification and sequencing of ssDNA bands from SSCP gels.
Sequence information was obtained according to a protocol described previously by Eichler et al. (9). Briefly, ssDNA bands were excised from SSCP acrylamide gels and boiled in Tris buffer (10 mM Tris-HCl, 5 mM MgCl2, 5 mM KCl, 0.1% Triton X-100 [pH 9]). Five microliters of the solution was used in a reamplification PCR with the unbiotinylated Lpms primers described above. After checking on a 2% agarose gel, the amplicons were purified and subsequently sequenced by cycle sequencing (ABI Prism BigDye Terminator cycle sequencing ready-reaction kit; Applied Biosystems, Foster City, CA). Before analysis on an ABI Prism 3100 genetic analyzer, the products were purified by using the BigDye Terminator purification kit (Qiagen, Hilden, Germany). The obtained sequence information was used to calculate Lpms locus repeats comparable to the data obtained by capillary electrophoresis (see above).
Nucleotide sequence accession numbers.
Sequences retrieved from SSCP gels of strain-specific MLVA profiles were deposited in the GenBank/EMBL/DDBJ database under accession numbers GU598121 (band 1) to GU598174 (band 54).
RESULTS
Molecular quantification of L. pneumophila and isolation of Legionella sp. strains.
During 1 years, drinking water from the tap of room D0.04 was analyzed by using Legionella genus-specific and Legionella pneumophila-specific PCR screening. To estimate the abundances of single L. pneumophila genotypes in our drinking water samples and to determine the efficiency of the developed assay, we additionally applied a Legionella pneumophila-specific real-time PCR approach for quantification. We compared the numbers of genomes with heterotrophic plate counts on R2A medium, total cell counts counted by epifluorescence microscopy, and the numbers of colonies visible on the filters used for the isolation of Legionella cells (Table 4). Heterotrophic plate counts at 36°C resulted in moderate CFU counts that were 4 to 6 logs lower than the cell numbers counted by epifluorescence microscopy. At 20°C we observed even fewer CFU per liter of sample volume. On the filters used for the isolation of Legionella sp. strains, we observed only a few gray-white colonies with ground-glass opacity indicating Legionella-like bacteria. By real-time PCR, we obtained numbers ranging from 0.7 to 89.9 Legionella pneumophila genome units (GU) per liter of sample volume. The highest number was detected in the infrequently used men's shower in the D building, and the lowest numbers were detected in the two cold-water samples (<5 GU). Due to a rather small number of Legionella cells in the samples, as detected by real-time PCR, single colonies observed on the agar plates can lead to an overestimation of numbers of Legionella CFU.
TABLE 4.
Microbiological characteristics of sampling sites used in this studya
| Type of drinking water | Site | Total no. of cells/liter | HPC (CFU/liter) on R2A agar at: |
Legionella CFU/liter on BCYE | GU of Legionella pneumophila/liter | |
|---|---|---|---|---|---|---|
| 36°C for 48 h | 22°C for 72 h | |||||
| Hot water | Boiler house | 1.89 × 108 | 3.00 × 102 | 0 | 0 | 5.1 |
| Men's shower, D building | 1.47 × 108 | 6.67 × 103 | 7.00 × 102 | 0 | 89.9 | |
| Scullery, D building | 1.58 × 108 | 2.30 × 103 | 0 | 6.7 | 3.8 | |
| Cold water | Room D0.04, D building | 2.57 × 108 | 0 | 7.00 × 102 | 0 | 1.8 |
| Y building, technical room | 1.95 × 108 | 1.70 × 104 | ND | 6.7 | 0.7 | |
Total bacterial cell numbers in drinking water were determined by epifluorescence microscopy using Sybr green I staining of formaldehyde-fixed samples. Heterotrophic plate counts (HPCs) on R2A agar and Legionella counts on BCYE agar were determined by the spread-plate technique and an appropriate aliquot of sample volume. Genome units of L. pneumophila were detected by real-time PCR by using L. pneumophila-specific primers Lp-16S_246-248F and Lp-16S_246-248R. The detection limit was approximately 0.4 Legionella pneumophila genome units per liter per PCR assay. ND, not determined.
Based on our observations from the genus-specific screening of our drinking water from the tap, we investigated our small-scale drinking water network on the HZI campus regarding the occurrence of Legionella species. We were able to isolate different Legionella-like colonies on BCYE agar and tested them with Legionella genus-specific and Legionella pneumophila-specific primers. These isolations resulted in 15 Legionella sp. strains, which were further characterized by sequencing of the complete 16S rRNA gene (Table 1). Four isolates could be clearly assigned as Legionella pneumophila strain Corby (isolates SK1, SK2, Y7, and Y8), two could be assigned as Legionella anisa (isolates GZ1 and GZ2), and all nine cooling tower isolates could be assigned as Legionella pneumophila. Due to the high sequence similarity of the 16S rRNA genes in the genus Legionella (32), no taxonomic information at the clonal level could be obtained here.
MLVA-8 CE of the reference strain and environmental isolates.
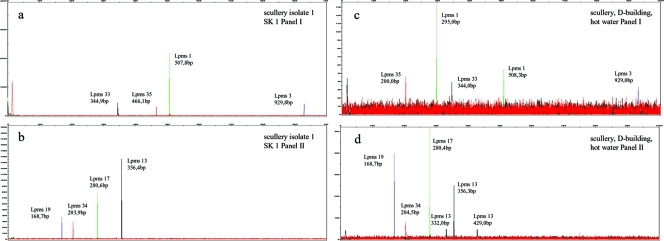
We determined the clonal genotypes of our isolates and reference strain Philadelphia according to the well-established MLVA-8 developed previously by Pourcel et al. (30, 31). These genotypes were determined by using capillary electrophoresis (CE) to assess the PCR fragment size estimation. As shown in Fig. 2a and b (left), the electropherograms of the two panels are shown for isolate SK1, and peaks can be clearly identified for all eight Lpms markers. Based on the PCR product sizes, the numbers of repeats in the alleles were calculated by subtraction of the number of flanking bases and division by the repeat unit length. The observed allele sizes (determined by CE) were also compared with the sizes reported in the help file for the Legionella pneumophila MLVA typing page (http://bacterial-genotyping.igmors.u-psud.fr/Legionella2006/help.htm). This file should assist assignments when calculating allele sizes by gel-based MLVA. However, we observed only minor differences in sizes compared to our CE-based results for the reference strain and our isolates. This did not lead to a false prediction of alleles for all VNTR markers. All four isolates from the rinsing room for laboratory material (scullery) of the D building and the technical room of the Y building showed exactly the same MLVA genotype (Table 5). In addition, all cooling tower isolates showed nearly the same allelic profile, with only a small difference for locus 17, where no PCR product was obtained for isolates KT8 and KT10.
FIG. 2.
Representative electropherograms of the MLVA-8 PCR products separated by CE and identified according to their sizes and colors. The electropherograms correspond to the PCR products from Legionella pneumophila scullery isolate SK1 (a and b, left) and in situ MLVA of DNA from the scullery sample (c and d, right). Panel I, Lpms1 (VIC [green]), Lpms3 (FAM [blue]), Lpms33 (NED [black]), and Lpms35 (PET [red]); panel II, Lpms13 (NED), Lpms17 (VIC), Lpms19 (FAM), and Lpms34 (PET).
TABLE 5.
MLVA-8 profiles for L. pneumophila isolates and in situ samples using CEa
| Isolate or in situ analysis | Sampling site or isolate source | MLVA-8 type at locus: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Lpms1 | Lpms3 | Lpms13 | Lpms17 | Lpms19 | Lpms33 | Lpms34 | Lpms35 | ||
| Isolates | |||||||||
| L. pneumophila Philadelphia | 8 | 8 | 11 | 2 | 4 | 1 | 1 | 3 | |
| SK1 | Scullery, D building | 7 | 8 | 8 | 2 | 4 | 2 | 1 | 18 |
| SK2 | Scullery, D building | 7 | 8 | 8 | 2 | 4 | 2 | 1 | 18 |
| Y7 | Y building, technical room | 7 | 8 | 8 | 2 | 4 | 2 | 1 | 18 |
| Y8 | Y building, technical room | 7 | 8 | 8 | 2 | 4 | 2 | 1 | 18 |
| KT2 | Cooling tower | 7 | 7 | 10 | 2 | 4 | 3 | 1 | 17 |
| KT3 | Cooling tower | 7 | 7 | 10 | 2 | 4 | 3 | 1 | 17 |
| KT1 | Cooling tower | 7 | 7 | 12 | 2 | 4 | 3 | 1 | 17 |
| KT7 | Cooling tower | 7 | 7 | 12 | 2 | 4 | 3 | 1 | 17 |
| KT8 | Cooling tower | 7 | 7 | 12 | −1 | 4 | 3 | 1 | 17 |
| KT9 | Cooling tower | 7 | 7 | 12 | 2 | 4 | 3 | 1 | 17 |
| KT10 | Cooling tower | 7 | 7 | 12 | −1 | 4 | 3 | 1 | 17 |
| KT11 | Cooling tower | 7 | 7 | 12 | 2 | 4 | 3 | 1 | 17 |
| KT12 | Cooling tower | 7 | 7 | 12 | 2 | 4 | 3 | 1 | 17 |
| In situ analyses | |||||||||
| MD | Men's shower | 7 | 8 | 8 | 2 | 4 | 2 | 1 | 3 |
| D0.04 | D0.04, D building | 7 | −1 | 11 | 2 | 4 | 1 | 1 | −1 |
| KH | Boiler house | 7 | 8 | 8 | 2 | 4 | 2 | 1 | 3 |
| KH | Boiler house | 7 | 8 | 11 | 2 | 4 | 2 | 1 | 3 |
| KH | Boiler house | 7 | 8 | 8 | 2 | 4 | 2 | 2 | 3 |
| KH | Boiler house | 7 | 8 | 11 | 2 | 4 | 2 | 2 | 3 |
| KH | Boiler house | 4 | 8 | 8 | 2 | 4 | 2 | 1 | 3 |
| KH | Boiler house | 4 | 8 | 11 | 2 | 4 | 2 | 1 | 3 |
| KH | Boiler house | 4 | 8 | 8 | 2 | 4 | 2 | 2 | 3 |
| KH | Boiler house | 4 | 8 | 11 | 2 | 4 | 2 | 2 | 3 |
| SK | Scullery, D building | 7 | 8 | 8 | 2 | 4 | 2 | 1 | 3 |
| SK | Scullery, D building | 7 | 8 | 11 | 2 | 4 | 2 | 1 | 3 |
| SK | Scullery, D building | 7 | 8 | 3 | 2 | 4 | 2 | 1 | 3 |
| SK | Scullery, D building | 2 | 8 | 8 | 2 | 4 | 2 | 1 | 3 |
| SK | Scullery, D building | 2 | 8 | 11 | 2 | 4 | 2 | 1 | 3 |
| SK | Scullery, D building | 2 | 8 | 3 | 2 | 4 | 2 | 1 | 3 |
Data for in situ analyses show all possible MLVA-8 profiles of the in situ samples that can be calculated from the detected VNTR alleles. −1, no PCR product. Multiple sample designations reflect the various MLVA-8 profiles possible.
In situ MLVA-8 CE of environmental samples.
We also performed the MLVA-8 capillary electrophoresis analysis directly on the DNA samples from our sampling and were able to detect most of the markers in every sample. We did not get any PCR product in the room D0.04 sample for Lpms3 and Lpms35. The electropherograms of these “in situ” MLVAs also had very high levels of background fluorescence and were difficult to analyze due to low peak signals and an inability of the software to automatically allocate the peaks to the different VNTR markers (Fig. 2c and d, right). Nevertheless, we identified more than one single peak for some samples by the manual analysis of the electropherograms. For the scullery in situ sample, for example, we detected one (Lpms1) or two (Lpms13) additional peaks corresponding to other repeat sizes (Fig. 2c and d) in the sample. Due to the difficulties with direct MLVA-8 CE on environmental DNA (e.g., very high background, low peak signals, and no possibility for direct sequencing), we applied single-strand conformation polymorphism (SSCP) gel electrophoresis for the analysis of these complex samples. For this method, high resolution for the separation of PCR amplicons has been demonstrated (9), and it allows the sequencing of the separated amplicons to identify single VNTR loci and determine precisely the MLVA profile.
SSCP gel electrophoresis of strain-specific MLVA profiles.
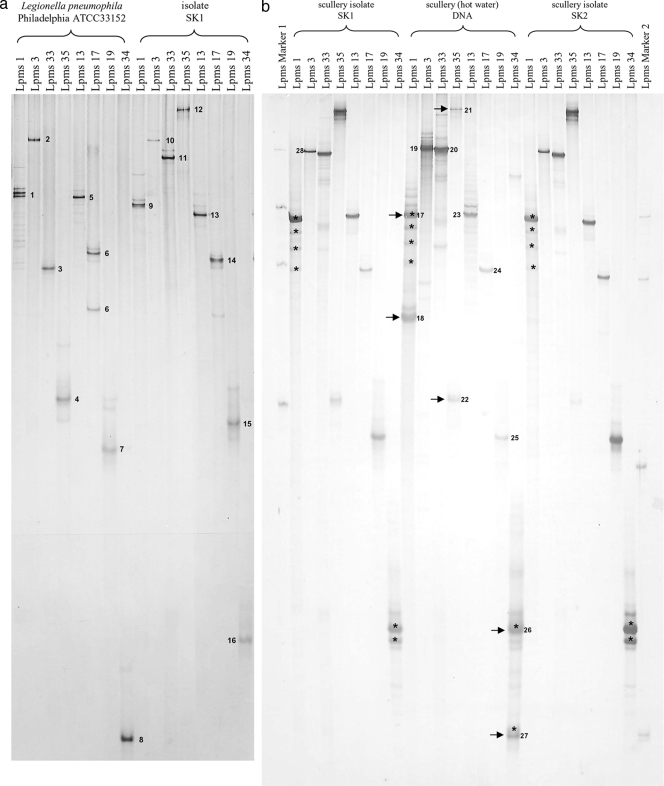
We applied the MLVA-8 analysis directly to environmental DNA for the specific identification of different L. pneumophila genotypes in a single sample without prior isolation. For the testing of this approach, we amplified all eight loci and prepared the single-stranded amplicons for SSCP analysis for a reference strain and one isolate (SK1) (Fig. 3a). In every lane one major band was dominating, with only a few exceptions (e.g., Lpms1). These double bands occurred probably due to the binding of a primer to the inner repeat region of the locus, as assumed by Nederbragt et al. (26). To confirm the high resolution of the developed approach, we cut out all major bands from the gel, reamplified them with the respective primer pairs, and sequenced the bands as indicated in Fig. 3. For some additional bands we were not able to reamplify the single-stranded PCR product, supporting the assumption mentioned above (data not shown). All other products resulted in the same repeat sizes as those obtained by capillary electrophoresis. A minor limitation of the direct sequencing technique that we used was the maximum read length (about 800 bp). Therefore, we were not able to sequence the complete product of the Lpms3 locus because it was too long (∼930 bp) to be read completely with our sequencing approach.
FIG. 3.
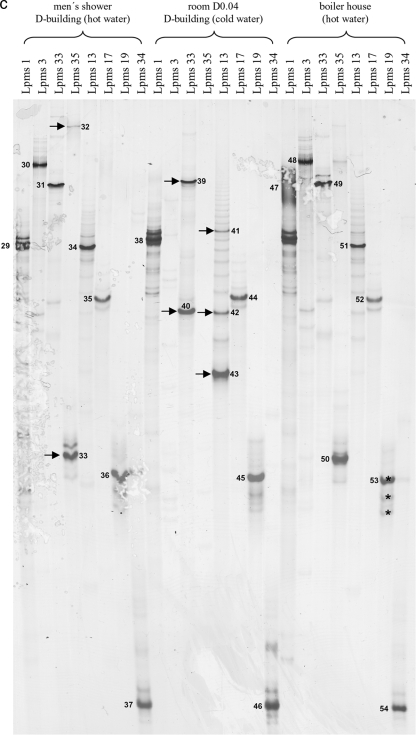
(a) SSCP gel electrophoresis of strain-specific MLVA profiles of Legionella pneumophila strain Philadelphia and isolate SK1. Single-stranded amplicons of all eight MLVA loci for the reference strain and SK1 were applied. (b) SSCP gel electrophoresis of MLVA profiles of isolates SK1 and SK2 and the corresponding environmental DNA. (c) SSCP gel electrophoresis of MLVA-8 profiles of different environmental DNA samples. Arrows indicate major bands. Asterisks indicate identical sequences verified by sequence alignment. Band numbers represent sequences deposited in the GenBank database with the accession numbers given in Materials and Methods.
SSCP gel electrophoresis of MLVA-8 PCR products from environmental DNA.
We performed the same SSCP analysis of the single loci for two isolates (here SK1 and SK2) and the corresponding DNA sample extracted from the source of the isolates, i.e., the scullery bulk water, to test if the SSCP fingerprinting was working directly on our environmental DNA (Fig. 3b). On this gel, we used the amplicons of panel I and panel II MLVA-PCR from strain Philadelphia as a single-strand marker on the outside (marker I, panel I; marker 2, panel II). The two isolates showed distinct major bands for all loci corresponding to the gel shown in Fig. 3a (SK1). For the environmental DNA from the scullery, we were able to detect more than one major band per locus for the Lpms1, Lpms35, and Lpms34 loci. When calculating the repeats from the sequences (Table 5), we observed that only the two additional bands from the markers Lpms1 and Lpms35 were also corresponding to other repeat sizes. For Lpms1, the detected repeat size was exactly the same as that detected by in situ capillary electrophoresis. The two different major bands of Lpms34 had almost the same sequence.
We analyzed additional environmental samples with our SSCP fingerprinting approach. As shown in Fig. 3c, the Lpms products for two hot-water samples (men's shower and boiler house) and a cold-water sample (room D0.04) are shown. Except for the markers Lpms3 and Lpms35, which we also could not detect by CE, we were able to get amplicons and ssDNA for all other markers and samples. Again, we sequenced the major bands from the gel resulting in repeat information similar to that gathered by capillary electrophoresis. Except for Lpms1, where we expected several other bands due to the additional binding of primers, we observed several additional bands only for the room D0.04 sample. Through the sequencing of the three major Lpms13 amplicons, we found that these amplicons corresponded to three different alleles for this marker (Fig. 3c and Table 6), consisting of 3, 5, and 11 repeats. For all other loci, we could not observe additional alleles by the sequencing of additional bands. For example, for Lpms19 in the boiler house sample, all three major bands exhibited the same sequence. Therefore, we decided to sequence only the major bands of the SSCP MLVA-8 fingerprints. For the in situ samples, all possible genotypes were calculated based on the different results from CE and SSCP sequencing (Table 5).
TABLE 6.
MLVA-8 profiles determined by SSCP analysis for L. pneumophila type strain Philadelphia, isolate SK1, and the in situ samples
| Strain or sample | No. of repeats at locusa: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Lpms1 | Lpms3 | Lpms13 | Lpms17 | Lpms19 | Lpms33 | Lpms34 | Lpms35 | |
| L. pneumophila Philadelphia | 8 | # | 11 | 2 | 4 | 1 | 1 | 3 |
| L. pneumophila SK1 | 7 | # | 8 | 2 | 4 | 2 | 1 | 18 |
| Scullery in situ | 7 | # | 8 | 2 | 4 | 2 | 1 | 3 |
| Additional VNTR alleles | 2 | >16 | ||||||
| Men's shower in situ | 7 | # | 8 | 2 | 4 | 2 | 1 | 3 |
| Additional VNTR alleles | 18 | |||||||
| D0.04 in situ | 7 | ND | 11 | 2 | 4 | 1 | 1 | ND |
| Additional VNTR alleles | 3 and 5 | 2 | ||||||
| Boiler house in situ | 7 | # | 8 | 2 | 4 | 2 | 1 | 3 |
| Additional VNTR alleles | 4 | |||||||
ND, not detected (no PCR product); #, sequence information too short for analysis.
DISCUSSION
Legionella pneumophila can pose a significant health threat to humans, most notably to immunocompromised persons, if present in human-made aquatic environments (37). To understand infections by this pathogen, especially regarding its epidemiological aspects, an identification of strains at the subspecies level is necessary. Molecular tools based on the analysis of bacterial DNA, such as MLST or MLVA, have become widely accepted in molecular typing studies of pathogenic bacteria (15, 17, 18, 24). The MLVA analysis is based on polymorphic minisatellites (VNTRs) on different loci, where recombination and DNA polymerase slipping often happen. If occurring with certain frequencies, these events can result in changes of the repeat sizes between different strains at a given locus (38). Currently, MLVA data for L. pneumophila and several other pathogens, such as Mycobacterium tuberculosis and Pseudomonas aeruginosa, can be obtained from the central GPMS website (http://minisatellites.u-psud.fr/), and the amount of data is increasing steadily (19). Recently, Pourcel et al. (30, 31) developed an MLVA-8 gel-based typing profile for L. pneumophila. Nederbragt et al. (26) were able to transfer this method to capillary electrophoresis (CE) for an improved typing of L. pneumophila isolates from patients or the environment. Nevertheless, partly due to the VBNC state of Legionella, the isolation of L. pneumophila from environmental samples (bulk water and biofilms, etc.) or clinical samples (sputum and lung biopsy specimens, etc.) poses a great challenge (28, 36).
We monitored our drinking water for members of the genus Legionella using cultivation-independent molecular techniques during 1 years. Most of the detected species were uncultured members of the family Legionellaceae, as identified by the sequencing of SSCP gel bands (data not shown). By the use of an additional L. pneumophila-specific PCR, we were able to detect L. pneumophila in some of the samples. As the minimum infectious dose for a severe infection with L. pneumophila is not known exactly (27) and depends greatly on the susceptibility of the exposed person (34), we chose to quantify the detected L. pneumophila population in a culture-independent way using specific real-time PCR. We detected only low numbers (0.7 to 1.8 L. pneumophila genome units/liter of bulk water) in the cold-water samples but relatively high numbers in the hot-water samples (up to 90 genome units/liter), especially in the seldom-used men's shower. This is in accordance with several previously reported studies where Legionella was isolated predominantly from hot-water sources such as dead-end tubes of the mains or infrequently used taps (23). In a German study of hot-water samples from 452 households, water temperature was observed to be probably the most important factor for the multiplication of legionellae (25). Furthermore, it has been observed for many outbreaks of Legionnaires' disease that hot water was the most frequent source of infection (5, 11, 20, 29).
To bridge the gap to cultivation, we isolated and characterized 15 Legionella sp. strains that we obtained by the sampling of different points on the HZI campus. By 16S rRNA gene sequencing, 13 strains could be identified as being L. pneumophila strains. The rather small number of isolates could be explained by the VBNC state of the bacteria, their intracellular occurrence in their natural protozoan host, and the accompanying microflora complicating the acquisition of isolates. We genotyped the 13 L. pneumophila isolates with the MLVA method described previously by Nederbragt et al. (26) using CE. VNTR analysis of the eight loci resulted in three different MLVA-8 genotypes. When analyzing our environmental DNA with CE, we had to face several problems, such as PCR inhibition resulting in low amplification rates as well as high background signals complicating the analysis of these complex samples. Therefore, a calculation of the number repeats for the VNTR loci from CE data was not always feasible. To overcome these analytical problems, we chose single-strand conformation polymorphism (SSCP) gel electrophoresis. We were able to detect fragments in a size range from 90 to 1,000 bp using a long glass plate for the separation of single-stranded PCR amplicons. This range was suitable for the separation of all single-stranded tandem-repeat locus amplicons from MLVA-8. We demonstrated that the method gave clear and informative results for every one of the eight MLVA loci described previously by Nederbragt et al. (26) by using a reference strain and some of our L. pneumophila isolates. Through sequencing of the single bands of the SSCP gel, we demonstrated that not only the length of the PCR product but also the complete underlying sequence information could be determined from environmental DNA by this method.
After adapting our approach to environmental DNA, we could identify MLVA genotypes in samples without isolation success (e.g., for the room D0.04 sample, we were not able to isolate legionellae) and detect additional MLVA profiles based on different alleles for one marker (e.g., Lpms13 for the room D0.04 sample) (Fig. 3c). By sequencing, we confirmed that additional SSCP bands for one VNTR locus were derived from different alleles. We tested our approach with additional DNA extracts from hot and cold water and could obtain clear bands for every MLVA locus. We assume that problems in the detection of one locus can arise if the concentration of L. pneumophila cells per liter would decrease under a detection limit of 2 genome units/liter because we had problems in obtaining PCR products for one or two of the eight loci in the room D0.04 sample, where we detected only 1.8 genome units/liter of finished drinking water. We were not able to obtain in situ MLVA results when the concentration was even lower (Y building) (data not shown). Other studies dealing with the quantification of a few pathogens in environmental samples are facing the same problem. Nevertheless, we think that the approach is very sensitive and can detect additional genotypes even if cells are in low concentrations, as they are with certainty in finished drinking water samples, where these kinds of pathogens are normally rare. Optionally, specific VNTR loci could be quantified by locus-specific real-time PCR. A subsequent melting-curve analysis would then provide detailed information about the abundance of single genotypes in a given environmental sample.
To our knowledge, this is the first study applying the MLVA approach directly to environmental DNA samples such as drinking water. We think that the developed approach could help identify outbreak strains long after the outbreak has occurred if the DNA or the water samples have been preserved for later analysis. In addition, this approach could also be applied to clinical samples without the cultivation of the infective strains and thereby contributes to an improved surveillance of Legionnaires' disease.
Acknowledgments
This work was supported by funds from the European Commission for the Healthy Water project (FOOD-CT-2006-036306).
The authors are solely responsible for the content of this publication. It does not represent the opinion of the European Commission. The European Commission is not responsible for any use that might be made of data appearing therein.
We thank Manuela Hölscher and Julia Strömpl for outstanding technical support and Katherine Garcia and Erika Harth-Chu for help with the CE and the data analysis.
Footnotes
Published ahead of print on 23 July 2010.
REFERENCES
- 1.Adeleke, A. A., B. S. Fields, R. F. Benson, M. I. Daneshvar, J. M. Pruckler, R. M. Ratcliff, T. G. Harrison, R. S. Weyant, R. J. Birtles, D. Raoult, and M. A. Halablab. 2001. Legionella drozanskii sp. nov., Legionella rowbothamii sp. nov. and Legionella fallonii sp. nov.: three unusual new Legionella species. Int. J. Syst. Evol. Microbiol. 51:1151-1160. [DOI] [PubMed] [Google Scholar]
- 2.Atlas, R. M. 1999. Legionella: from environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1:283-293. [DOI] [PubMed] [Google Scholar]
- 3.Bassam, B. J., G. Caetano-Anoll, and P. M. Gresshoff. 1991. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal. Biochem. 196:80-83. [DOI] [PubMed] [Google Scholar]
- 4.Benson, R. F., and B. S. Fields. 1998. Classification of the genus Legionella. Semin. Respir. Infect. 13:90-99. [PubMed] [Google Scholar]
- 5.Berthelot, P., F. Grattard, A. Ros, F. Lucht, and B. Pozzetto. 1998. Nosocomial legionellosis outbreak over a three-year period: investigation and control. Clin. Microbiol. Infect. 4:385-391. [DOI] [PubMed] [Google Scholar]
- 6.Colbourne, J. S., and P. J. Dennis. 1985. Distribution and persistence of Legionella in water systems. Microbiol. Sci. 2:40-43. [PubMed] [Google Scholar]
- 7.Colbourne, J. S., P. J. Dennis, R. M. Trew, C. Berry, and G. Vesey. 1988. Legionella and public water supplies. Water Sci. Technol. 4(20):5-10. [Google Scholar]
- 8.Eichler, S., M. G. Weinbauer, D. Dominik, and M. Höfle. 2004. Extraction of total RNA and DNA from bacterioplankton, p. 103-120. In G. A. Kowalchuk, F. J. D. Bruijn, I. M. Head, A. D. L. Akkermans, and J. D. van Elsas (ed.), Molecular microbial ecology manual, 2nd ed. Kluwer Academic Publishers, Dordrecht, Netherlands.
- 9.Eichler, S., R. Christen, C. Höltje, P. Westphal, J. Bötel, I. Brettar, A. Mehling, and M. G. Höfle. 2006. Composition and dynamics of bacterial communities of a drinking water supply system as assessed by RNA- and DNA-based 16S rRNA gene fingerprinting. Appl. Environ. Microbiol. 72:1858-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exner, M., and T. Kistemann. 2004. Bedeutung der Verordnung über die Qualität von Wasser für den menschlichen Gebrauch (Trinkwasserverordnung 2001) für die Krankenhaushygiene. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 47:384-391. [DOI] [PubMed] [Google Scholar]
- 11.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry, N. K., B. Afshar, P. Visca, D. Jonas, J. Duncan, E. Nebuloso, A. Underwood, and T. G. Harrison. 2005. Assessment of fluorescent amplified fragment length polymorphism analysis for epidemiological genotyping of Legionella pneumophila serogroup 1. Clin. Microbiol. Rev. 11:704-712. [DOI] [PubMed] [Google Scholar]
- 13.Gaia, V., N. K. Fry, B. Afshar, P. C. Luck, H. Meugnier, J. Etienne, R. Peduzzi, and T. G. Harrison. 2005. Consensus sequence-based scheme for epidemiological typing of clinical and environmental isolates of Legionella pneumophila. J. Clin. Microbiol. 43:2047-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glick, T. H., M. B. Gregg, B. Berman, G. Mallison, W. W. Rhodes, and I. Kassanoff. 1978. Pontiac fever. An epidemic of unknown etiology in a health department. I. Clinical and epidemiologic aspects. Am. J. Epidemiol. 107:149-160. [DOI] [PubMed] [Google Scholar]
- 15.Harth-Chu, E., R. T. Espejo, R. Christen, C. A. Guzman, and M. G. Höfle. 2009. Multiple-locus variable-number tandem-repeat analysis for clonal identification of Vibrio parahaemolyticus isolates by using capillary electrophoresis. Appl. Environ. Microbiol. 75:4079-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.International Organization for Standardization. 2004. Water quality—detection and enumeration of Legionella. ISO 11731-2. International Organization for Standardization, Geneva, Switzerland.
- 16.Joseph, C. A., and the European Working Group for Legionella Infections. 2004. Legionnaires' disease in Europe 2000-2002. Epidemiol. Infect. 132:417-424.15188711 [Google Scholar]
- 17.Keim, P., L. B. Price, A. M. Klevytska, K. L. Smith, J. M. Schupp, R. Okinaka, P. J. Jackson, and M. E. Hugh-Jones. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J. Bacteriol. 182:2928-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klevytska, A. M., L. B. Price, J. M. Schupp, P. L. Worsham, J. Wong, and P. Keim. 2001. Identification and characterization of variable-number tandem repeats in the Yersinia pestis genome. J. Clin. Microbiol. 39:3179-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Flèche, P., Y. Hauck, L. Onteniente, A. Prieur, F. Denoeud, V. Ramisse, P. Sylvestre, G. Benson, F. Ramisse, and G. Vergnaud. 2001. A tandem repeats database for bacterial genomes: application to the genotyping of Yersinia pestis and Bacillus anthracis. BMC Microbiol. 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leoni, E., G. De Luca, P. P. Legnani, R. Sacchetti, S. Stampi, and F. Zanetti. 2005. Legionella waterline colonization: detection of Legionella species in domestic, hotel and hospital hot water systems. J. Appl. Microbiol. 98:373-379. [DOI] [PubMed] [Google Scholar]
- 21.Lo Presti, F., S. Riffard, H. Meugnier, M. Reyrolle, Y. Lasne, P. A. Grimont, F. Grimont, R. F. Benson, D. J. Brenner, A. G. Steigerwalt, J. Etienne, and J. Freney. 2001. Legionella gresilensis sp. nov. and Legionella beliardensis sp. nov., isolated from water in France. Int. J. Syst. Evol. Microbiol. 51:1949-1957. [DOI] [PubMed] [Google Scholar]
- 22.Lo Presti, F., S. Riffard, H. Meugnier, M. Reyrolle, Y. Lasne, P. A. Grimont, F. Grimont, F. Vandenesch, J. Etienne, J. Fleurette, and J. Freney. 1999. Legionella taurinensis sp. nov., a new species antigenically similar to Legionella spiritensis. Int. J. Syst. Bacteriol. 49:397-403. [DOI] [PubMed] [Google Scholar]
- 23.Lück, P. C., I. Leupold, M. Hlawitschka, J. H. Helbig, I. Carmienke, L. Jatzwauk, and T. Guderitz. 1993. Prevalence of Legionella species, serogroups, and monoclonal subgroups in hot water systems in south-eastern Germany. Zentralbl. Hyg. Umweltmed. 193:450-460. [PubMed] [Google Scholar]
- 24.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, et al. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathys, W., J. Stanke, M. Harmuth, and E. Junge-Mathys. 2008. Occurrence of Legionella in hot water systems of single-family residences in suburbs of two German cities with special reference to solar and district heating. Int. J. Hyg. Environ. Health 211:179-185. [DOI] [PubMed] [Google Scholar]
- 26.Nederbragt, A. J., A. Balasingham, R. Sirevåg, H. Utkilen, K. S. Jakobsen, and M. J. Anderson-Glenna. 2008. Multiple-locus variable-number tandem repeat analysis of Legionella pneumophila using multi-colored capillary electrophoresis. J. Microbiol. Methods 73:111-117. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien, S. J., and R. S. Bhopal. 1993. Legionnaires' disease: the infective dose paradox. Lancet 342:5-6. [DOI] [PubMed] [Google Scholar]
- 28.Paszko-Kolva, C., M. Shahamat, and R. R. Colwell. 1992. Long-term survival of Legionella pneumophila serogroup 1 under low-nutrient conditions and associated morphological changes. FEMS Microbiol. Lett. 102:45-55. [Google Scholar]
- 29.Perola, O., J. Kauppinen, J. Kusnetsov, J. Heikkinen, C. Jokinen, and M. L. Katila. 2002. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 110:863-868. [DOI] [PubMed] [Google Scholar]
- 30.Pourcel, C., Y. Vidgop, F. Ramisse, G. Vergnaud, and C. Tram. 2003. Characterization of a tandem repeat polymorphism in Legionella pneumophila and its use for genotyping. J. Clin. Microbiol. 41:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pourcel, C., P. Visca, B. Afshar, S. D'Arezzo, G. Vergnaud, and N. K. Fry. 2007. Identification of variable-number tandem-repeat (VNTR) sequences in Legionella pneumophila and development of an optimized multiple-locus VNTR analysis typing scheme. J. Clin. Microbiol. 45:1190-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratcliff, R. M., J. A. Lanser, P. A. Manning, and M. W. Heuzenroeder. 1998. Sequence-based classification scheme for the genus Legionella targeting the mip gene. J. Clin. Microbiol. 36:1560-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratzow, S., V. Gaia, J. H. Helbig, N. K. Fry, and P. C. Luck. 2007. Addition of neuA, the gene encoding N-acylneuraminate cytidylyl transferase, increases the discriminatory ability of the consensus sequence-based scheme for typing Legionella pneumophila serogroup 1 strains. J. Clin. Microbiol. 45:1965-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roig, J. 2003. Legionnaires' disease: a rational approach to therapy. J. Antimicrob. Chemother. 51:1119-1129. [DOI] [PubMed] [Google Scholar]
- 35.Samrakandi, M. M., S. L. G. Cirillo, D. A. Ridenour, L. E. Bermudez, and J. D. Cirillo. 2002. Genetic and phenotypic differences between Legionella pneumophila strains. J. Clin. Microbiol. 40:1352-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinert, M., L. Emödy, R. Amann, and J. Hacker. 1997. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl. Environ. Microbiol. 63:2047-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinert, M., U. Hentschel, and J. Hacker. 2002. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol. Rev. 26:149-162. [DOI] [PubMed] [Google Scholar]
- 38.Tuntiwechapikul, W., and M. Salazar. 2002. Mechanism of in vitro expansion of long DNA repeats: effect of temperature, repeat length, repeat sequence, and DNA polymerases. Biochemistry 41:854-860. [DOI] [PubMed] [Google Scholar]
- 39.Weinbauer, M. G., C. Beckmann, and M. G. Höfle. 1998. Utility of green fluorescent nucleic acid dyes and aluminum oxide membrane filters for rapid epifluorescence enumeration of soil and sediment bacteria. Appl. Environ. Microbiol. 64:5000-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu, V. L., J. F. Plouffe, M. C. Pastoris, J. E. Stout, M. Schousboe, A. Widmer, J. Summersgill, T. File, C. M. Heath, D. L. Paterson, and A. Chereshsky. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186:127-128. [DOI] [PubMed] [Google Scholar]