Abstract
We investigated predator-prey interactions in a model system consisting of the bacterivorous flagellate Poterioochromonas sp. strain DS and the freshwater bacterium Sphingobium sp. strain Z007. This bacterial strain tends to form a subpopulation of grazing-resistant microscopic flocs, presumably by aggregation. Enhanced formation of such flocs could be demonstrated in static batch culture experiments in the presence of the predator. The ratio of aggregates to single cells reached >0.1 after 120 h of incubation in an oligotrophic growth medium. The inoculation of bacteria into supernatants from cocultures of bacteria and flagellates (grown in oligotrophic or in rich media) also resulted in a substantially higher level of floc formation than that in supernatants from bacterial monocultures only. After separation of supernatants on a C18 cartridge, the aggregate-inducing activity could be assigned to the 50% aqueous methanolic fraction, and further separation of this bioactive fraction could be achieved by high-pressure liquid chromatography. These results strongly suggest the involvement of one or several chemical factors in the induction of floc formation by Sphingobium sp. strain Z007 that are possibly released into the surrounding medium by flagellate grazing.
Interactions between free-living aquatic bacteria and predatory flagellates are determined by the balance between bacterial cell growth and mortality rates (1, 8, 23). High levels of grazing mortality have favored the evolution of various bacterial counterstrategies, such as small cell sizes, high-speed motility, and the production of toxins and other growth inhibitors (for a review, see reference 25). The particle uptake abilities of predators set tight constraints on the size of the prey that is preferentially ingested. As a consequence, filamentous cells inedible by protists may accumulate in heavily grazed freshwater bacterial communities, as may cells with other complex morphologies, such as aggregates and microcolonies, that are beyond the prey size spectrum of the predators (14, 18, 27). The formation of such grazing-resistant flocs at high protistan foraging levels is known both from static and continuous culture (13, 26) and from enrichments of natural waters (17).
A shift toward cell aggregates or microcolonies might simply be a result of the elimination of single-celled morphotypes (13) but could also reflect an active response of bacteria to the presence of predators. Two nonexclusive causes can be envisaged for the enhanced growth of cells in aggregated form under such conditions. For one, higher levels of floc formation might be a consequence of higher bacterial growth rates due to the release of additional substrates and nutrients by the predator (5, 8, 14, 36). Second, such growth behavior might be induced by a chemical factor. Inducible morphological defense due to compounds released by the predators is common in other planktonic organisms, e.g., the spine induction in rotifers (12) and daphnids (20, 34) and the formation of grazing-resistant colonies by Scenedesmus (16). The exact nature and molecular action of most of these substances remain unknown, also because of the difficulty of establishing an appropriate bioassay to rapidly detect the effective fraction and to further characterize such compounds (29).
Recently, the formation of grazing-resistant filaments in the presence of a grazer was demonstrated for a Flectobacillus sp. strain in chemostat experiments (7). Since the predators were kept spatially separated from filament-forming cells (inside dialysis bags), this morphological shift was interpreted as an indication for the action of kairomones. Such a continuous culture approach is complex and rather inconvenient for subsequent identification of the chemical that is the inducing factor by bioassay-guided fractionation: depending on the flow rate and vessel size, continuous cultivation will yield relatively large volumes to be processed, and a single experiment in the study described above lasted for 10 to 20 days (7). In contrast, a bioassay based on static batch cultures would be experimentally simple and rapid, and it could be set up in a highly parallel manner in small volumes. However, neither the appropriate organisms nor the conditions for a batch culture model of chemically induced morphological grazing resistance are yet available. So far, the formation of aggregates/microcolonies (15), but not of filaments (7, 14), in the presence of a predator was demonstrated in batch culture. Batch culture experiments with a bacterial strain spatially separated from its predator showed that such cell aggregation may be induced both by the growth state and by conspecific chemical cues (2). Thus, aggregate-forming bacteria seem to be the more promising target in the search for a grazing-related morphogenetic factor in static batch culture.
We sought to establish a batch culture bioassay for the detection and first tentative characterization of (one or several) chemical factors that would affect bacterial floc formation in a model predator-prey system. For this purpose, the growth behavior of a freshwater bacterial isolate was investigated in direct-contact experiments with the flagellate Poterioochromonas sp. strain DS and in supernatants derived from these cocultures.
MATERIALS AND METHODS
Bacterial and flagellate strains.
Sphingobium sp. strain Z007 was originally isolated from the surface water of Lake Zürich (Switzerland) (3). It is a rod-shaped moderately fast growing bacterium with the ability to form flocs (subsequently referred to as aggregates, but potentially also microcolonies; see Discussion). The partial sequence of the 16S rRNA gene of strain Z007 was deposited in the EMBL nucleotide sequence database (accession number FN293045). Sphingobium sp. Z007 was grown in DSMZ 7 medium (yeast extract, 1 g/liter; glucose, 1 g/liter; peptone, 1 g/liter; German Collection of Microorganisms and Cell Cultures [DSMZ], Braunschweig, Germany) in 300-ml Erlenmeyer flasks at 18°C in the dark.
Axenic cultures of the bacterivorous flagellate Poterioochromonas sp. strain DS (3) were grown in undefined Ochromonas medium (yeast extract, 1 g/liter; meat extract, 1 g/liter; glucose, 1 g/liter; peptone, 1 g/liter; Culture Collection of Algae [SAG], University of Göttingen, Göttingen, Germany). The flagellate cultures were kept in the dark at 18°C. They were additionally fed heat-killed cells (1 × 107 cells/ml; preincubated at 70°C for 2 h) of Flectobacillus major DSMZ 103 once per week.
All microbial strains were transferred into fresh medium 48 h before the start of the experiments. During that period, Poterioochromonas sp. strain DS cultures were no longer fed the heat-killed bacteria. After this period, no cells of F. major DSMZ 103 were detectable by epifluorescence microscopy. When Sphingobium sp. strain Z007 was used a second time during experiments with the supernatants, the culture was again freshly inoculated 48 h prior to use.
Two different media were used for the experiments. Initial cocultivation experiments were carried out in a nutrient-poor oligotrophic medium (artificial lake water [ALW]). The concentrations of macroelements were chosen according to the work of Zotina et al. (39). Additionally, the following ingredients were added: NH4Cl (30 μM), K2HPO4 (322 nM), NH4Fe citrate (110 nM Fe), l-Arg·HCl (5 nM), glucose (50 nM), l-Glu (15 nM), l-His·HCl (7.5 nM), l-Met (7.5 nM), thiaminiumdichloride (7.5 nM), biotin (7.5 nM), and vitamin B12 (7.5 nM). Later experiments with supernatants were carried out both in ALW and in DSMZ 7 medium (described above). On the basis of the outcomes of the assays with whole supernatants, only DSMZ 7 medium was used for the subsequent supernatant fractionation assays.
Direct-contact experiments.
Direct-contact experiments were run in 300-ml Erlenmeyer flasks in a final volume of 50 ml medium at 18°C in the dark. Three batch cultures were inoculated with Sphingobium sp. strain Z007 (1.0 × 106 ml−1) and Poterioochromonas sp. strain DS (1.0 × 103 ml−1). As controls, triplicate batch cultures were inoculated only with Sphingobium sp. strain Z007 (1.0 × 106 ml−1) (Fig. 1, first panel). Subsamples of 1 ml were taken at six different time points (0, 24, 48, 72, 96, and 120 h) and fixed with glutaraldehyde (final concentration, 3%). Samples were stored at 1°C until they were analyzed by flow cytometry (within 24 to 48 h).
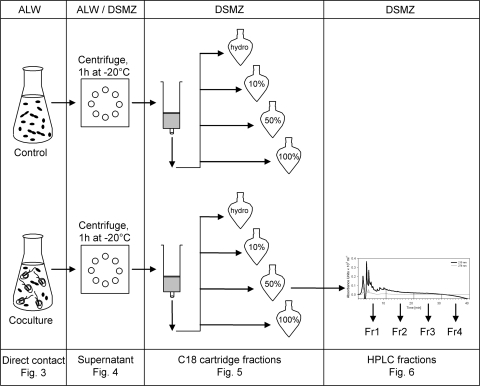
FIG. 1.
Experimental setup: Sphingobium sp. strain Z007 as the control and Sphingobium sp. strain Z007 and Poterioochromonas sp. strain DS in direct-contact experiments (first panel), after centrifugation and freezing in supernatant experiments (second panel), after the first separation step on a C18 cartridge (third panel), and after further separation step on an HPLC C18 ODS-A reverse-phase column (fourth panel). Fr, fraction.
Supernatant experiments.
Additional direct-contact experiments and pure bacterial batch cultures were prepared in ALW and DSMZ 7 medium as described above in order to produce sufficient volumes of supernatants. All treatments were run in triplicate at 18°C in the dark. Supernatants were produced after 72 h, 96 h, and 120 h of incubation in ALW or after 120 h in DSMZ 7 medium. For this, the cultures were first centrifuged at 7,400 × g three times for 15 min each time under sterile conditions. The pellet was subsequently discarded and the supernatants were frozen at −20°C for 1 h (Fig. 1, second panel) in order to destroy any potentially remaining flagellate cells. Immediately after they were thawed, the supernatants were filled in 24-well plates and inoculated with 1.5 × 106 ml−1 Sphingobium sp. strain Z007 (at this time the bacterial culture had an age of 48 h, as described above). The final volume in each treatment was 1.5 ml. The plates were then incubated in the dark. Subsamples (500 μl) were taken after 24 h, fixed with glutaraldehyde (final concentration, 3%), and immediately analyzed by flow cytometry. The pH and dissolved organic carbon (DOC) concentrations of supernatants from the experiments with DSMZ 7 medium were determined both in the control treatments of Sphingobium sp. strain Z007 only and in cocultures of bacteria with Poterioochromonas sp. strain DS (initial abundance, 1.5 × 103 ml−1). For DOC analyses the samples were centrifuged, and the supernatants were transferred into glass vials (prewashed with 2 M HCl and sterile deionized H2O) and analyzed using a TOC-5000 total organic carbon analyzer (Shimadzu, Japan).
Fractionation experiments. (i) C18 cartridge.
Supernatants were produced in triplicate, as described above, from controls (bacteria only) and from cocultures with initial concentrations of 1.5 × 103 ml−1 cells of Poterioochromonas sp. strain DS (DSMZ 7 medium, 120 h of incubation in the dark). After centrifugation and freeze-thawing, the supernatants (20 ml) were loaded onto a preequilibrated C18 ec cartridge (Marchery-Nagel, Düren, Germany). The direct eluate was collected and is subsequently referred to as the “hydrophilic fraction.” Substances bound to the cartridge were first washed out with 10% aqueous (aq.) methanol (MeOH), followed by 50% aq. MeOH and finally by 100% MeOH. After evaporation of the solvents in a rotavapor apparatus (35 mbar; 30°C), the residues were redissolved in fresh DSMZ 7 medium and frozen at −20°C for 1 h (Fig. 1, third panel). Next, 24-well plates were filled with all fractions and the plates were inoculated with 1.5 × 106 ml−1 Sphingobium sp. strain Z007 (final volume, 1.5 ml). The 24-well plates were then incubated in the dark at 18°C. Samples (500 μl) were taken after 48 h, fixed with glutaraldehyde (final concentration, 3%), and immediately analyzed by flow cytometry.
In one experiment, fresh DSMZ 7 medium was loaded onto the C18 cartridge as an additional control. Bacterial aggregate formation did not differ between treatments amended with such fractionated fresh medium or with fractionated supernatants of the bacterial cultures (data not shown).
(ii) HPLC.
Supernatants were produced in triplicate, as described above, from controls (bacteria only) and from cocultures with initial concentrations of 1.5 × 103 ml−1 cells of Poterioochromonas sp. strain DS (120 h of incubation in the dark). The supernatants were separated by the C18 cartridge and again tested for their ability to enhance aggregate formation by Sphingobium sp. strain Z007 in order to verify the results of the experiments described above. The aggregate-inducing fractions derived from the supernatants of triplicate cocultures (50% aq. MeOH) were then further separated (Fig. 1, fourth panel). Fractionation by high-pressure liquid chromatography (HPLC) was performed on a Shimadzu 10AVP system with a photodiode array detector and a C18 ODS-A reversed-phase column (250 by 4.6 mm, 5-μm particle size; YMC Europe GmbH, Dinslaken, Germany). The flow rate was 1 ml/min. Solvent A was composed of UV-treated deionized H2O and 0.05% trifluoroacetic acid (TFA; Fluka, Switzerland), and solvent B was acetonitrile and 0.05% TFA. A linear increase was applied (solvent B from 20% to 100% in 41 min). The injection volume was 500 μl. Four subfractions were collected according to their retention times (1 to 11 min, 11 to 21 min, 21 to 31 min, 31 to 41 min). The subfractions of five runs were combined for further analyses. The solvents were evaporated using a vacuum evaporator at 30°C, the residues were redissolved in 2.5 ml of fresh DSMZ 7 medium, and the pH was adjusted to 7.2. Subsequently, the subfractions were frozen at −20°C for 1 h. After they were thawed, 24-well plates were filled with the subfractions and the plated were inoculated with 1.5 × 106 ml−1 Sphingobium sp. strain Z007 (final volume, 1.5 ml). The 24-well plates were then incubated in the dark at 18°C. Samples (500 μl) were taken after 48 h, fixed with glutaraldehyde (final concentration, 3%), and immediately analyzed by flow cytometry.
Flow cytometric enumeration.
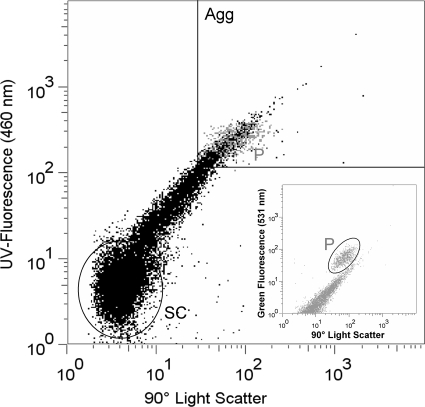
Samples for flow cytometry were stained with 4′,6-diamidino-2-phenylindole (DAPI; final concentration, 1 μg ml−1) for 10 min in the dark and analyzed using an Influx V-GS cell sorter (Becton Dickinson Inc., San Jose, CA). A blue laser (200 mW, 488 nm; Sapphire; Coherent Inc.) was used for detection of light scatter and the autofluorescence of flagellates, and a UV laser (60 mW, 355 nm; CY-PS; Lightwave Electronics) was used for detection of DAPI fluorescence. If required, samples were diluted with deionized water prefiltered with a 0.2-μm-pore-size filter to avoid particle coincidence. To convert the flow cytometry counts to cell numbers, a defined amount of microspheres (latex beads; diameter, 1.0 μm; FlowCheck High Intensity Green Alignment; Polyscience) was added to the samples. Bead working solutions were freshly prepared daily. Poterioochromonas sp. strain DS was identified using 90° light scatter versus green fluorescence (531 nm). Single cells of Sphingobium sp. strain Z007 were identified using 90° light scatter versus DAPI fluorescence (Fig. 2, SC). Due to the markedly larger size and a lower abundance by up to three orders of magnitude, the aggregates were counted separately by applying different cytometric settings. Large cell aggregates (Fig. 2, Agg) were operationally defined by their fluorescence and scatter properties, i.e., signals with scatter/fluorescence equal to or higher than that of Poterioochromonas sp. strain DS (Fig. 2, P) at comparable instrument settings (as determined by fluorescent microspheres). This approach allowed the reproducible quantification of aggregates above a fixed size threshold and the reliable distinction of aggregates from single cells, the results of which were also verified by cell sorting and microscopic inspection.
FIG. 2.
Cytogram (90° light scatter versus UV fluorescence) of Sphingobium sp. strain 007 after staining of the cells with DAPI. The gate in the upper right corner of the graph comprises events that were counted as bacterial cell aggregates. Gray dots depict cells of Poterioochromonas sp. strain DS in the same sample. (Inset) Cytogram used for the counting of Poterioochromonas sp. strain DS (90° light scatter versus green fluorescence). Agg, aggregates; SC, single cells; P, Poterioochromonas cells.
Statistical analyses.
Statistical analyses were performed using the software SPSS 16 (SPSS Inc., Chicago, IL). The following hypotheses were tested: (i) the sizes of the fractions of bacterial aggregates in direct-contact experiments after 72 h of incubation were greater in cocultures of bacteria and flagellates than in bacterial cultures (one-sided paired t test on arcsine-transformed pooled data from time points of 72, 96, and 120 h); (ii) the numbers of bacterial aggregates after 120 h of incubation in the supernatants of the coculture were higher than those in the supernatants of pure bacterial cultures (one-sided Mann-Whitney U test, performed separately for supernatants from DSMZ medium and ALW); and (iii) the fractions separated by the C18 cartridge and the subfractions separated by HPLC differed in their potential to induce aggregate formations (two-sided Kruskal-Wallis analyses of variance, followed by Scheffé post hoc tests on arcsine-transformed data).
RESULTS
Direct-contact experiments.
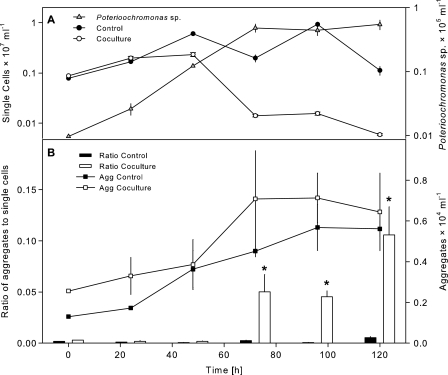
The number of single cells of Sphingobium sp. strain Z007 in the controls increased over time from 0.8 × 106 ml−1 to 6.0 × 106 ml−1 after 48 h of incubation. The increase in bacterial single-cell abundance in the cocultures with Poterioochromonas sp. strain DS diminished in parallel with the rise of the flagellate population (0.14 × 106 ml−1 after 72 h) (Fig. 3A). At 48 h, the abundance of aggregates was similar for both treatments. However, concomitant with the increasing concentrations of flagellates, the number of aggregates almost doubled during the following 24 h and thereafter remained stable until the end of the experiment. Comparable ratios of aggregates to single cells were present in the controls and in the cocultures during the first 24 h of the experiments. Minor differences could already be observed after 48 h and became increasingly more pronounced between 72 h and the end of the experiment (Fig. 3B). During the latter period, the ratio of aggregates to single cells in treatments with direct contact between bacteria and flagellates was significantly (P < 0.01, up to 20 times) higher than that for the controls. After 120 h of incubation, the ratio of aggregates to single cells in the coculture reached more than 0.1. It should be noted that this value represents the value only for the fraction of aggregates with at least the same size (i.e., side scatter value) as the flagellates (Fig. 2). Taking into account all aggregates that were clearly distinguishable from single cells in the cytograms, this ratio reached a value of over one-third for single cells in the coculture treatment after 120 h but less than 0.04 for the control treatment.
FIG. 3.
(A) Time course of changes in abundance of Poterioochromonas sp. strain DS and of single cells of Sphingobium sp. strain Z007 in controls (bacteria only) and in coculture with the flagellate during batch culture incubation in artificial lake water medium. (B) Ratios of aggregates to single cells of Sphingobium sp. strain Z007 in controls and in coculture with the flagellate and abundance of aggregates of Sphingobium sp. strain Z007 in controls and in coculture with the flagellate. Error bars indicate the standard errors of three replicates. Asterisks, significantly higher than control treatments at P < 0.01.
Supernatant experiments.
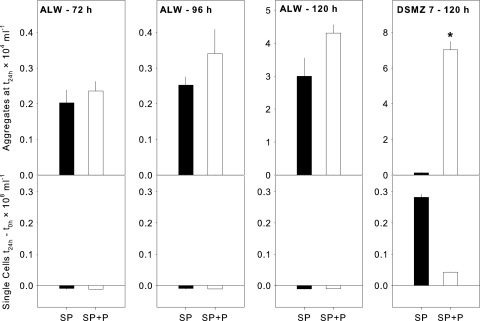
The numbers of aggregates formed after 24 h of incubation in supernatants from cocultures of bacteria and flagellates in ALW were always slightly (but not significantly) higher than the numbers in supernatants from the controls (supernatants from bacteria only in ALW) (Fig. 4). This difference was the most pronounced between supernatants from cultures or cocultures that had been incubated for 120 h. At the same time, the numbers of single cells in these supernatants did not increase or even slightly decreased within 24 h.
FIG. 4.
Abundance of aggregates at 24 h (t24 h; upper panels) and differences in single-cell numbers between time zero (t0 h) and 24 h (lower panels) of Sphingobium sp. strain Z007 in the supernatants of pure bacterial cultures (black bars; SP) and in supernatants of cocultures with Poterioochromonas sp. strain DS (white bars; SP+P). Supernatants were derived from experiments that were performed using ALW and rich medium (DSMZ 7) and from direct-contact experiments at different time points (72 h, 96 h, 120 h). Error bars indicate the standard errors of three replicates. Asterisk, significantly higher than other fractions at P < 0.01.
In contrast, a clear increase in single-cell abundance was observed in supernatants that were derived either from bacteria or from cocultures of bacteria and flagellates grown in DSMZ 7 medium (Fig. 4). This rise in cell numbers was roughly 7 times higher in the supernatants from the control treatments (bacteria only) than in those from the cocultures. At the same time, the number of aggregates in the supernatants from the cocultures was significantly (P < 0.01, approximately 60 times) higher than the number in those from the pure bacterial cultures, resulting in a >300 times higher ratio of aggregates to single cells (Fig. 4). The pH values were 7.5 in the supernatants from the control treatments (bacteria only, grown in DSMZ 7 medium) and 7.1 in supernatants from the coculture with Poterioochromonas sp. strain DS. The DOC concentrations were 703 mg liter−1 in the supernatants from the control treatments and only slightly higher in those from the cocultures (841 mg liter−1).
Fractionation experiments.
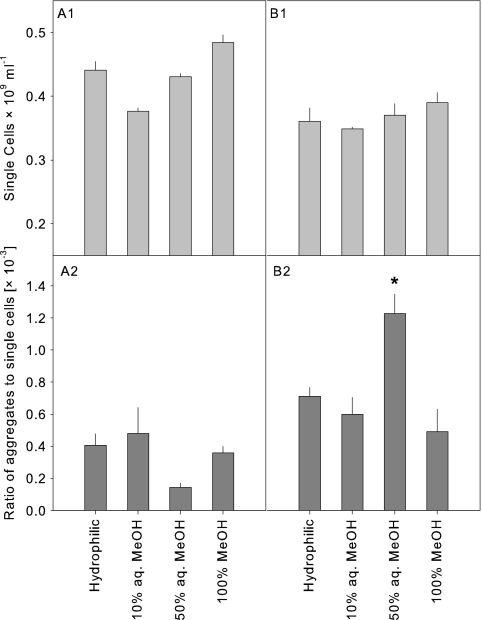
In the next step, several fractions derived from the separation of supernatants on a C18 cartridge were tested for their potential to induce aggregate formation by Sphingobium sp. strain Z007 (Fig. 5). The abundance of single cells was generally slightly but not significantly higher in treatments supplemented with fractions from the control supernatants than in those with added fractions from the coculture supernatants (4.3 × 108 ± 0.43 × 108 ml−1 and 3.7 × 108 ± 0.28 × 108 ml−1, respectively). At the same time, the total abundance of aggregates was typically higher in the treatments supplemented with supernatant fractions from the cocultures (data not shown). However, only the 50% aq. MeOH fraction from the coculture supernatant induced a significant increase in aggregates, resulting in a significantly (P < 0.05) higher ratio of aggregates to single cells compared to the ratio for the controls.
FIG. 5.
Abundance of single cells and ratios of aggregates to single cells of Sphingobium sp. strain Z007 after 48 h of incubation in DSMZ 7 medium amended with fractions obtained by C18 cartridge separation of supernatants from pure bacterial cultures (A1 and A2) and in supernatants from cocultures (B1 and B2). Error bars indicate the standard errors of three replicates. Asterisk, significantly higher than the other fractions at P < 0.05.
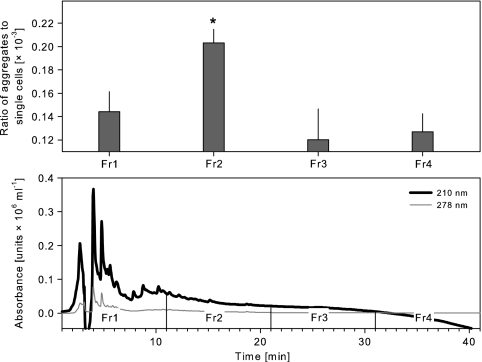
In the final experiment, this 50% aq. MeOH fraction derived from the supernatants of cocultures was further separated by HPLC (Fig. 6). All together, four subfractions from this HPLC separation were tested in triplicate experiments for their potential to trigger aggregate formation by Sphingobium sp. strain Z007. Only one subfraction (eluted between 11 and 21 min under the conditions applied) induced a significant (P < 0.05) increase in the proportions of aggregates formed in comparison with the proportions for the other subfractions. This subfraction showed no absorption in the visible light spectrum (data not shown) and only low absorption in the UV spectrum at 210 nm (Fig. 6, lower panel).
FIG. 6.
(Upper panel) Ratios of aggregates to single cells of Sphingobium sp. strain Z007 after 48 h of incubation in DSMZ 7 medium amended with four subfractions obtained by HPLC separation of the 50% aq. MeOH fraction of the supernatant of the bacteria and flagellate coculture. Error bars indicate the standard errors of three replicates. Asterisk, significantly higher than the other fractions at P < 0.05. (Lower panel) HPLC chromatograms (at 210 nm and 278 nm) of the 50% aq. MeOH fraction with the marked collected fractions (Fr1 to Fr4).
DISCUSSION
Bacterial floc formation during flagellate grazing.
Members of the Sphingomonadaceae family have repeatedly been isolated from freshwater habitats (11, 24) and could be enriched after addition of low concentrations of natural dissolved organic matter (9). These bacteria are widely distributed across freshwater habitats, where they may produce densities up to >106 cells ml−1 (28). Sphingomonas spp. and related bacteria have also been found in newly formed and aged freshwater organic aggregates (lake snow) (19, 31), and the planktonic Sphingomonas natatoria may even coaggregate with other species in order to establish and expand its populations within freshwater biofilms (22). Such findings suggest that the Sphingobium sp. strain Z007 used in our study may represent an appropriate model organism with which to investigate the induction of aggregate/microcolony formation. It has the tendency to spontaneously form flocs even in pure cultures during particular phases of batch growth in rich media (2) and also, to a small extent, at the comparatively oligotrophic growth conditions in artificial lake water (Fig. 3).
The inoculation of flagellates into the batch cultures resulted in reduced abundances of single bacterial cells, and the rise in grazing pressure at higher flagellate densities was paralleled by a proportional increase in the aggregated bacterial subpopulation (Fig. 3). This clearly demonstrates a protection of this size class of aggregates against grazing by Poterioochromonas sp. strain DS. Moreover, the high ratio of aggregates with a larger size than the flagellates to single cells in the oligotrophic artificial lake water medium (Fig. 3B) points to the ecological relevance of aggregate formation in the presence of predators under close-to-natural growth conditions. Batch culture systems have successfully been used before in predator-prey interactions with freshwater bacterial isolates and axenic flagellates. Increased formation of suspended microcolonies by Pseudomonas sp. strain MWH1 (15) and by two strains of the Betaproteobacteria class (13) were found in static batch cultures in the presence of Ochromonas sp. strain DS (now Poterioochromonas sp. strain DS [4]).
Various grazing-resistant bacterial morphologies have been observed in single strains and bacterial assemblages, such as microcolonies, aggregates, and filaments (13, 30, 32). While filamentous morphotypes are easily definable, it is more difficult to distinguish between bacterial aggregates and microcolonies. Microcolonies originate from cells that do not separate after division, whereas aggregates result from the encounter of freely dispersed single cells (15). Thus, both, aggregates and microcolonies might quickly increase in number during the early phase of batch growth. However, as soon as the abundance of single cells declines due to grazing, there should be little further increase in aggregate numbers (15), whereas no such limitation would apply for microcolonies. In our cocultures, the number of bacterial flocs doubled within 24 h after the flagellates had reached high densities (between 48 and 72 h) (Fig. 3) but remained stable thereafter, when the numbers of single cell were low. This seems to suggest that aggregation is the major mechanism of floc formation. Moreover, the growth rate of single cells of Sphingobium sp. strain Z007 in the oligotrophic medium was low (0.04 h−1), which also speaks against the formation of bacterial flocs (typically consisting of 20 cells or more) by cell division only. Finally, microscopic inspections suggested that single cells of Sphingobium sp. strain Z007 were motile and would thus be able to actively assemble. Nevertheless, we cannot exclude the possibility that the bacterial flocs—tentatively classified as aggregates—were in part also formed by cell division processes.
Technical aspects of bioassay development.
An important step in the development of the bioassay was to produce supernatants that were entirely free of flagellates. In the past, filtration through a 0.2-μm-pore-size filter has been applied for this purpose (6, 15). However, extractable substances from different membrane filters may by themselves induce morphological changes, as was shown for the green alga Scenedesmus obliquus (21). Therefore, the use of filtration might lead to false-positive or -negative results. A combination of centrifugation and freezing of the supernatant seems to be more appropriate to avoid introduction of any additional chemical components into the supernatants. Dilution of the supernatants—as was done in previous studies (6, 7, 15)—might, moreover, decrease the concentration of the trigger compounds. Since the treatment-specific responses of bacteria were most clearly observed in supernatants from the rich medium than in those from the oligotrophic one (Fig. 4), the former were chosen for use in all subsequent fractionation experiments. However, it should be noted that the substantially higher numbers of single cells in these supernatants also led to relative proportions of aggregates generally lower than those observed when artificial lake water was used.
In order to assess the relationship between aggregation and growth state, it was, moreover, important to accurately determine the numbers of both single cells and aggregates over a large range of total cell concentrations (Fig. 2). Our flow cytometric assay provided a fast and precise means of simultaneously quantifying free single cells and an operationally well-defined population of aggregates in the same sample, e.g., those that exceed the approximate size of the predators, as estimated by their respective light scatter properties (Fig. 2). The sorting and microscopic inspection of different regions from cytograms, moreover, allow verification of the counting gates (and additional analyses of subpopulations).
Effects of supernatants on cell aggregation.
Chemical factors have previously been suggested to be agents inducing bacterial cell aggregation during predation. For example, the feeding of Poterioochromonas sp. strain DS on Sphingobium sp. strain Z007 outside dialysis bags led to significantly higher proportions of bacterial aggregates inside the bags (2). In our experiments, the addition of supernatants from a 120-h-old coculture of bacteria and flagellates incubated in oligotrophic artificial lake water medium to a pure culture of Sphingobium sp. strain Z007 resulted in the enhanced formation of aggregates compared to that achieved by treatments with supernatants from bacterial monocultures (Fig. 4). The treatment-specific enrichment of aggregates in supernatants from cocultures was particularly evident in the experiments using the rich DSMZ 7 growth medium (Fig. 4). In view of the comparable concentrations of DOC, the enhanced cell aggregation in the supernatants from the cocultures represents first evidence for the presence of one or several chemical factors that are involved in the stimulation of cell aggregation of Sphingobium sp. strain Z007 and that are possibly secreted by Poterioochromonas sp. strain DS as a consequence of bacterivory. Moreover, the formation of considerably higher numbers of single cells in the supernatants from pure bacterial cultures (Fig. 4) suggests that this cell aggregation was not merely due to higher growth rates in supernatants from cocultures.
Several investigations have explored if morphological changes of bacteria can be induced by supernatants from batch cocultures of flagellates and bacteria (6, 7, 13, 15). So far, no comparable observation of chemical-induced bacterial floc formation has been reported. This may indicate that the findings from our model system cannot be generalized, and it is possible that aggregate or microcolony formation in other experimental predator-prey systems might not have been triggered by chemical factors (7, 13, 15). Moreover, the visible development of a grazing-resistant bacterial subpopulation might depend on the balance between bacterial growth and grazing losses in the cocultures from which the supernatants were produced. The generation of active compounds is arguably linked to flagellate digestion. Thus, low initial concentrations of flagellates might produce only a relatively small amount of the aggregate-forming substance(s). At predator-prey ratios that are too high, bacteria might be consumed too quickly and flagellates might be starved at the time point when the cocultures are processed for supernatant production. We tested various initial predator-prey ratios prior to our study (data not shown). A ratio of 1:1,000 cells resulted in the most significant increase in the level of cell aggregation in the supernatant experiments and was therefore chosen for use in all successive experiments. Finally, chemical interactions between bacteria and flagellates might also be obscured by choosing different bacteria as the food source for the predators (for the production of supernatants) and as test organisms (for subsequent examination of the effects). Various aquatic organisms exhibit a morphological defense against predators that is triggered only by conspecific chemical cues (10, 33, 38). Enhanced aggregate formation by our tested strain was induced only by flagellate grazing on cells from the same species (2).
Chemical nature of aggregate-inducing factor.
Supernatants of bacterial monocultures and of cocultures of bacteria and flagellates could be successfully fractionated using a C18 cartridge (Fig. 5). Since the fractionated supernatants were redissolved into fresh medium for these experiments, it is not possible to directly compare the level of aggregate formation in these assays with the level observed after addition of whole supernatants (which were likely depleted in substrates). Instead, we focused on the differences between the treatments amended with the individual fractions. Upon addition of the 50% aq. MeOH fraction from the coculture, the level of bacterial aggregate formation was significantly higher than that in all other fractions from this treatment type or from the supernatants of the pure bacterial cultures (Fig. 5). Subsequently, the 50% aq. MeOH fraction was further separated using common reversed-phase HPLC methods, and one of these subfractions could again be shown to disproportionally induce cell aggregation (Fig. 6).
Under the conditions applied, the aggregate-inducing compound was of a moderate lipophilic nature. As corresponding absorption characteristics are missing, this compound most likely does not posses a chromophore or protein/peptide substructures. However, the absorption of very low concentrations of a highly potent active compound might be underestimated with our specific setup. Our findings are in line with what is known about the nature of comparable chemical factors from aquatic organisms. For example, the putative kairomones from fish and Chaoborus larvae are moderate lipophilic substances of low molecular weight and high thermal and pH stability (35, 37), but their exact chemical nature remains unknown. While the results of our fractionation experiments are strong evidence for the involvement of a predation-related chemical factor in induced floc formation of Sphingobium sp. strain Z007, the elucidation of the chemical nature of this factor would go beyond the scope of this study. Since the bioassay presented here is relatively fast and can be performed in small volumes (1.5 ml) compared to the conditions required for models that involve chemostats (7) or larger metazoan predators, it could provide a means for the future characterization of a factor that apparently modifies the growth behavior of an aquatic bacterium.
Acknowledgments
We thank F. Jüttner for valuable discussions during the studies and preparation of the manuscript and T. Posch for providing cultures of Poterioochromonas sp. strain DS.
The Swiss National Science Foundation is acknowledged for financial support (grants SNF 3100A0-112106 and 31003A_124786).
Footnotes
Published ahead of print on 23 July 2010.
REFERENCES
- 1.Billen, G., P. Servais, and S. Becquevort. 1990. Dynamics of bacterioplankton in oligotrophic and eutrophic aquatic environments: bottom-up or top-down control? Hydrobiologia 207:37-42. [Google Scholar]
- 2.Blom, J. F., K. Horňák, K. Šimek, and J. Pernthaler. 19 April 2010. Aggregate formation in a freshwater bacterial strain induced by growth state and conspecific chemical cues. Environ. Microbiol. [Epub ahead of print.] [DOI] [PubMed]
- 3.Blom, J. F., and J. Pernthaler. 2010. Antibiotic effects of three strains of chrysophytes (Ochromonas, Poterioochromonas) on freshwater bacterial isolates. FEMS Microbiol. Ecol. 71:281-290. [DOI] [PubMed] [Google Scholar]
- 4.Boenigk, J., K. Pfandl, and P. J. Hansen. 2006. Exploring strategies for nanoflagellates living in a ‘wet desert.’ Aquat. Microb. Ecol. 44:71-83. [Google Scholar]
- 5.Caron, D. A., J. C. Goldman, and M. R. Dennett. 1988. Experimental demonstration of the roles of bacteria and bacterivorous protozoa in plankton nutrient cycles. Hydrobiologia 159:27-40. [Google Scholar]
- 6.Corno, G. 2006. Are grazer-induced adaptations of bacterial abundance and morphology time dependent? J. Limnol. 65:35-40. [Google Scholar]
- 7.Corno, G., and K. Jürgens. 2006. Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity. Appl. Environ. Microbiol. 72:78-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corno, G., and K. Jürgens. 2008. Structural and functional patterns of bacterial communities in response to protist predation along an experimental productivity gradient. Environ. Microbiol. 10:2857-2871. [DOI] [PubMed] [Google Scholar]
- 9.Eiler, A., S. Langenheder, S. Bertilsson, and L. J. Tranvik. 2003. Heterotrophic bacterial growth efficiency and community structure at different natural organic carbon concentrations. Appl. Environ. Microbiol. 69:3701-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelowitz, C. M., A. Mathis, and R. J. F. Smith. 1993. Chemosensory recognition of northern pike (Esox lucius) by brook stickleback (Culaea inconstans)—population differences and the influence of predator diet. Behaviour 127:105-118. [Google Scholar]
- 11.Gich, F., K. Schubert, A. Bruns, H. Hoffelner, and J. Overmann. 2005. Specific detection, isolation, and characterization of selected, previously uncultured members of the freshwater bacterioplankton community. Appl. Environ. Microbiol. 71:5908-5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, J. J. 1999. Kairomone-induced morphological defenses in rotifers, p. 127-141. In R. Tollrian and C. D. Harvell (ed.), The ecology and evolution of inducible defenses. University Press, Princeton, NJ.
- 13.Hahn, M. W., H. Lunsdorf, and L. Janke. 2004. Exopolymer production and microcolony formation by planktonic freshwater bacteria: defence against protistan grazing. Aquat. Microb. Ecol. 35:297-308. [Google Scholar]
- 14.Hahn, M. W., E. R. B. Moore, and M. G. Höfle. 1999. Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla. Appl. Environ. Microbiol. 65:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn, M. W., E. R. B. Moore, and M. G. Höfle. 2000. Role of microcolony formation in the protistan grazing defense of the aquatic bacterium Pseudomonas sp. MWH1. Microb. Ecol. 39:175-185. [DOI] [PubMed] [Google Scholar]
- 16.Hessen, D. O., and E. Van Donk. 1993. Morphological changes in Scenedesmus induced by substances released from Daphnia. Arch. Hydrobiol. 127:129-140. [Google Scholar]
- 17.Jürgens, K., H. Arndt, and H. Zimmermann. 1997. Impact of metazoan and protozoan grazers on bacterial biomass distribution in microcosm experiments. Aquat. Microb. Ecol. 12:131-138. [Google Scholar]
- 18.Jürgens, K., and C. Matz. 2002. Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Van Leeuwenhoek 81:413-434. [DOI] [PubMed] [Google Scholar]
- 19.Knoll, S., W. Zwisler, and M. Simon. 2001. Bacterial colonization of early stages of limnetic diatom microaggregates. Aquat. Microb. Ecol. 25:141-150. [Google Scholar]
- 20.Lass, S., and P. Spaak. 2003. Chemically induced anti-predator defences in plankton: a review. Hydrobiologia 491:221-239. [Google Scholar]
- 21.Lürling, M., and W. Beekman. 2002. Extractable substances (anionic surfactants) from membrane filters induce morphological changes in the green alga Scenedesmus obliquus (Chlorophyceae). Environ. Toxicol. Chem. 21:1213-1218. [PubMed] [Google Scholar]
- 22.Min, K. R., and A. H. Rickard. 2009. Coaggregation by the freshwater bacterium Sphingomonas natatoria alters dual-species biofilm formation. Appl. Environ. Microbiol. 75:3987-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pace, M. L., and J. J. Cole. 1994. Comparative and experimental approaches to top-down and bottom-up regulation of bacteria. Microb. Ecol. 28:181-193. [DOI] [PubMed] [Google Scholar]
- 24.Page, K. A., S. A. Connon, and S. J. Giovannoni. 2004. Representative freshwater bacterioplankton isolated from Crater Lake, Oregon. Appl. Environ. Microbiol. 70:6542-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pernthaler, J. 2005. Predation on prokaryotes in the water column and its ecological implications. Nat. Rev. Microbiol. 3:537-546. [DOI] [PubMed] [Google Scholar]
- 26.Pernthaler, J., T. Posch, K. Šimek, J. Vrba, R. Amann, and R. Psenner. 1997. Contrasting bacterial strategies to coexist with a flagellate predator in an experimental microbial assemblage. Appl. Environ. Microbiol. 63:596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pernthaler, J., B. Sattler, K. Šimek, A. Schwarzenbacher, and R. Psenner. 1996. Top-down effects on the size-biomass distribution of a freshwater bacterioplankton community. Aquat. Microb. Ecol. 10:255-263. [Google Scholar]
- 28.Piccini, C., D. Conde, C. Alonso, R. Sommaruga, and J. Pernthaler. 2006. Blooms of single bacterial species in a coastal lagoon of the southwestern Atlantic Ocean. Appl. Environ. Microbiol. 72:6560-6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pohnert, G., M. Steinke, and R. Tollrian. 2007. Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends Ecol. Evol. 22:198-204. [DOI] [PubMed] [Google Scholar]
- 30.Salcher, M. M., J. Pernthaler, R. Psenner, and T. Posch. 2005. Succession of bacterial grazing defense mechanisms against protistan predators in an experimental microbial community. Aquat. Microb. Ecol. 38:215-229. [Google Scholar]
- 31.Schweitzer, B., I. Huber, R. Amann, W. Ludwig, and M. Simon. 2001. α- and β-Proteobacteria control the consumption and release of amino acids on lake snow aggregates. Appl. Environ. Microbiol. 67:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Šimek, K., J. Vrba, J. Pernthaler, T. Posch, P. Hartman, J. Nedoma, and R. Psenner. 1997. Morphological and compositional shifts in an experimental bacterial community influenced by protists with contrasting feeding modes. Appl. Environ. Microbiol. 63:587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stabell, O. B., F. Ogbebo, and R. Primicerio. 2003. Inducible defences in Daphnia depend on latent alarm signals from conspecific prey activated in predators. Chem. Senses 28:141-153. [DOI] [PubMed] [Google Scholar]
- 34.Tollrian, R., and S. I. Dodson. 1999. Inducible defenses in Cladocera, p. 177-202. In R. Tollrian and C. D. Harvell (ed.), The ecology and evolution of inducible defenses. University Press, Princeton, NJ.
- 35.Tollrian, R., and E. von Elert. 1994. Enrichment and purification of Chaoborus kairomone from water—further steps toward its chemical characterization. Limnol. Oceanogr. 39:788-796. [Google Scholar]
- 36.Verhagen, F. J. M., and H. J. Laanbroek. 1992. Effects of grazing by flagellates on competition for ammonium between nitrifying and heterotrophic bacteria in chemostats. Appl. Environ. Microbiol. 58:1962-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Elert, E., and G. Pohnert. 2000. Predator specificity of kairomones in diel vertical migration of Daphnia: a chemical approach. Oikos 88:119-128. [Google Scholar]
- 38.Wilson, D. J., and H. Lefcort. 1993. The effect of predator diet on the alarm response of red-legged frog, Rana aurora, tadpoles. Anim. Behav. 46:1017-1019. [Google Scholar]
- 39.Zotina, T., O. Köster, and F. Jüttner. 2003. Photoheterotrophy and light-dependent uptake of organic and organic nitrogenous compounds by Planktothrix rubescens under low irradiance. Freshwater Biol. 48:1859-1872. [Google Scholar]