Abstract
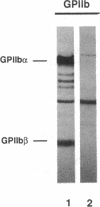
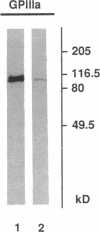
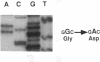
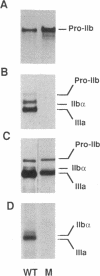
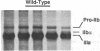
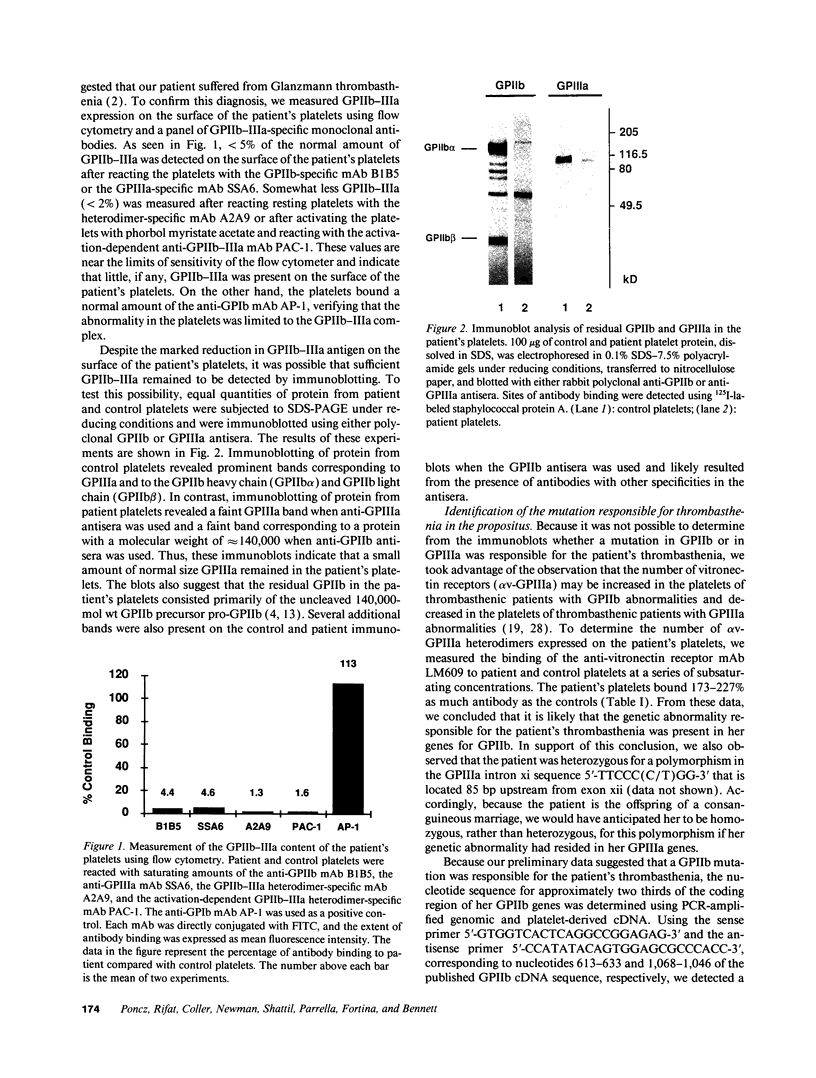
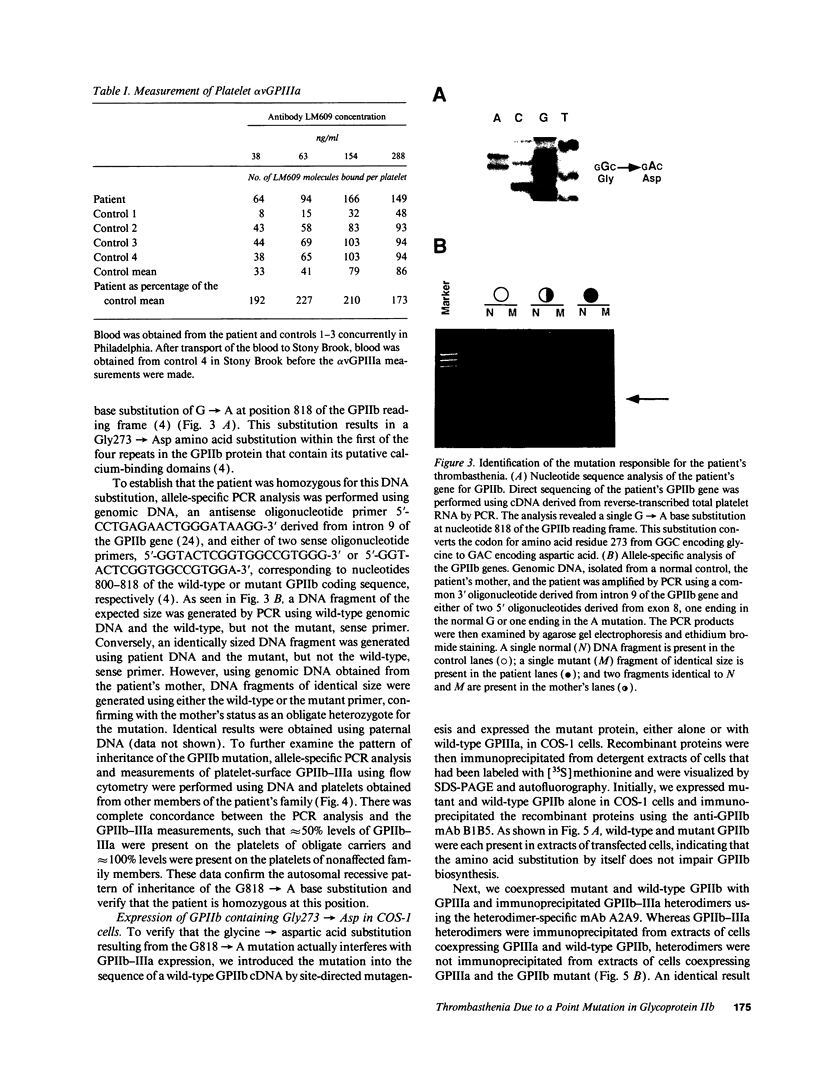
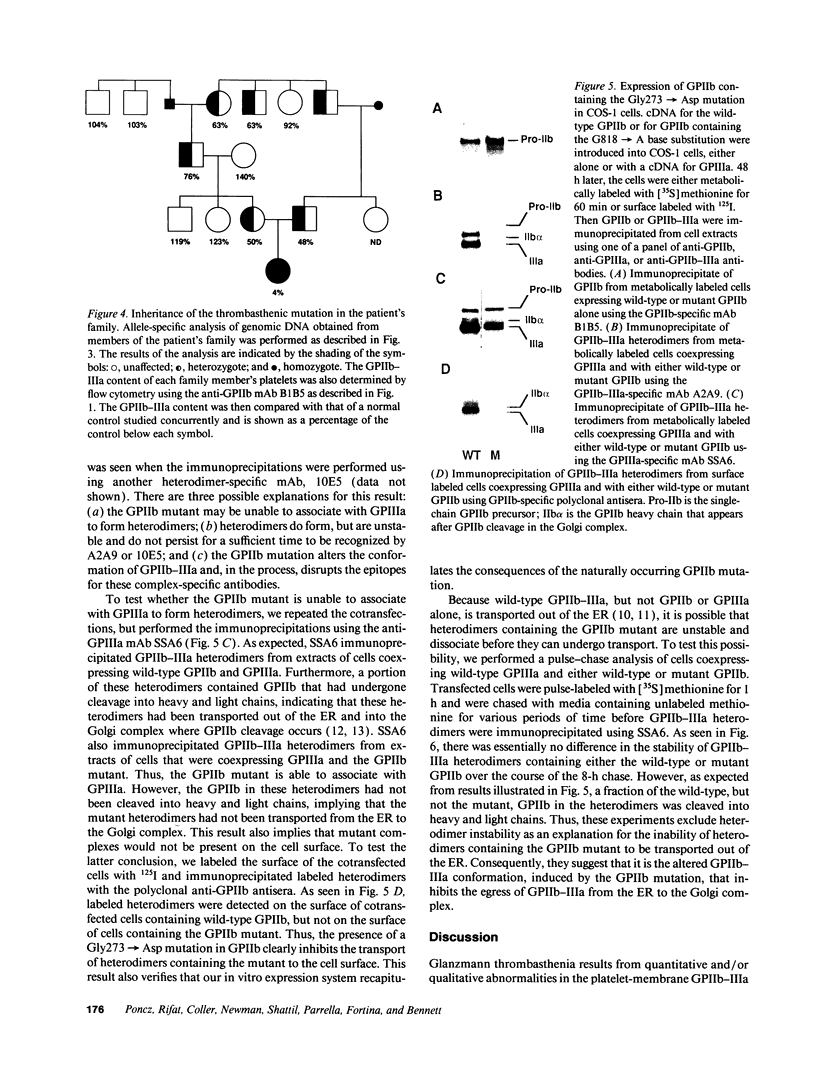
We studied the defect responsible for Glanzmann thrombasthenia in a patient whose platelets expressed < 5% of the normal amount of GPIIb-IIIa. Genetic and biochemical evidence indicated that the patient's GPIIIa genes were normal. However, DNA analysis revealed the patient homozygous for a G818-->A substitution in her GPIIb genes, resulting in a Gly273-->Asp substitution adjacent to the first GPIIb calcium-binding domain. To determine how this mutation impaired GPIIb-IIIa expression, recombinant GPIIb containing the mutation was coexpressed with GPIIIa in COS-1 cells. The GPIIb mutant formed stable GPIIb-IIIa heterodimers that were not immunoprecipitated by either of two heterodimer-specific monoclonal antibodies, indicating that the mutation disrupted the epitopes for these antibodies. Moreover, the GPIIb in the heterodimers was not cleaved into heavy and light chains, indicating that the heterodimers were not transported from the endoplasmic reticulum to the Golgi complex where GPIIb cleavage occurs, nor were the mutant heterodimers expressed on the cell surface. These studies demonstrate that a Gly273-->Asp mutation in GPIIb does not prevent the assembly of GPIIb-IIIa heterodimers, but alters the conformation of these heterodimers sufficiently to impair their intracellular transport. The impaired GPIIb-IIIa transport is responsible for the thrombasthenia in this patient.
Full text
PDF

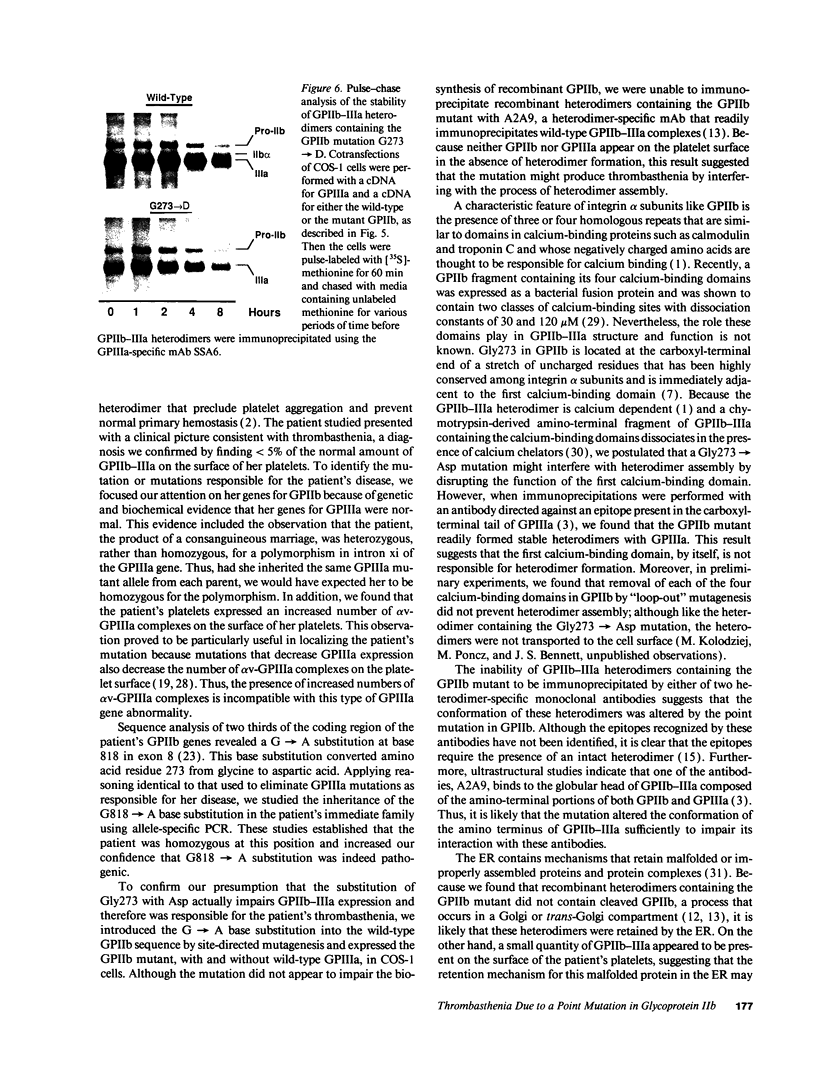


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajt M. L., Ginsberg M. H., Frelinger A. L., 3rd, Berndt M. C., Loftus J. C. A spontaneous mutation of integrin alpha IIb beta 3 (platelet glycoprotein IIb-IIIa) helps define a ligand binding site. J Biol Chem. 1992 Feb 25;267(6):3789–3794. [PubMed] [Google Scholar]
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Kolodziej M. A., Vilaire G., Poncz M. Determinants of the intracellular fate of truncated forms of the platelet glycoproteins IIb and IIIa. J Biol Chem. 1993 Feb 15;268(5):3580–3585. [PubMed] [Google Scholar]
- Bodary S. C., Napier M. A., McLean J. W. Expression of recombinant platelet glycoprotein IIbIIIa results in a functional fibrinogen-binding complex. J Biol Chem. 1989 Nov 15;264(32):18859–18862. [PubMed] [Google Scholar]
- Bonthron D. T., Handin R. I., Kaufman R. J., Wasley L. C., Orr E. C., Mitsock L. M., Ewenstein B., Loscalzo J., Ginsburg D., Orkin S. H. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986 Nov 20;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- Bray P. F., Shuman M. A. Identification of an abnormal gene for the GPIIIa subunit of the platelet fibrinogen receptor resulting in Glanzmann's thrombasthenia. Blood. 1990 Feb 15;75(4):881–888. [PubMed] [Google Scholar]
- Burk C. D., Newman P. J., Lyman S., Gill J., Coller B. S., Poncz M. A deletion in the gene for glycoprotein IIb associated with Glanzmann's thrombasthenia. J Clin Invest. 1991 Jan;87(1):270–276. doi: 10.1172/JCI114982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. P., Djaffar I., Pidard D., Steiner B., Cieutat A. M., Caen J. P., Rosa J. P. Ser-752-->Pro mutation in the cytoplasmic domain of integrin beta 3 subunit and defective activation of platelet integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10169–10173. doi: 10.1073/pnas.89.21.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Coller B. S., Cheresh D. A., Asch E., Seligsohn U. Platelet vitronectin receptor expression differentiates Iraqi-Jewish from Arab patients with Glanzmann thrombasthenia in Israel. Blood. 1991 Jan 1;77(1):75–83. [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Poncz M., Steiner B., Rall S. C., Jr, Bennett J. S., Phillips D. R. Comparison of cDNA-derived protein sequences of the human fibronectin and vitronectin receptor alpha-subunits and platelet glycoprotein IIb. Biochemistry. 1987 Dec 15;26(25):8158–8165. doi: 10.1021/bi00399a021. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Steiner B., Rall S. C., Jr, Lo S. S., Phillips D. R. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to "integrin". J Biol Chem. 1987 Mar 25;262(9):3936–3939. [PubMed] [Google Scholar]
- George J. N., Caen J. P., Nurden A. T. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990 Apr 1;75(7):1383–1395. [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gu J. M., Xu W. F., Wang X. D., Wu Q. Y., Chi C. W., Ruan C. G. Identification of a nonsense mutation at amino acid 584-arginine of platelet glycoprotein IIb in patients with type I Glanzmann thrombasthenia. Br J Haematol. 1993 Mar;83(3):442–449. doi: 10.1111/j.1365-2141.1993.tb04669.x. [DOI] [PubMed] [Google Scholar]
- Gulino D., Boudignon C., Zhang L. Y., Concord E., Rabiet M. J., Marguerie G. Ca(2+)-binding properties of the platelet glycoprotein IIb ligand-interacting domain. J Biol Chem. 1992 Jan 15;267(2):1001–1007. [PubMed] [Google Scholar]
- Heidenreich R., Eisman R., Surrey S., Delgrosso K., Bennett J. S., Schwartz E., Poncz M. Organization of the gene for platelet glycoprotein IIb. Biochemistry. 1990 Feb 6;29(5):1232–1244. doi: 10.1021/bi00457a020. [DOI] [PubMed] [Google Scholar]
- Kolodziej M. A., Vilaire G., Gonder D., Poncz M., Bennett J. S. Study of the endoproteolytic cleavage of platelet glycoprotein IIb using oligonucleotide-mediated mutagenesis. J Biol Chem. 1991 Dec 5;266(34):23499–23504. [PubMed] [Google Scholar]
- Kolodziej M. A., Vilaire G., Rifat S., Poncz M., Bennett J. S. Effect of deletion of glycoprotein IIb exon 28 on the expression of the platelet glycoprotein IIb/IIIa complex. Blood. 1991 Nov 1;78(9):2344–2353. [PubMed] [Google Scholar]
- Lam S. C. Isolation and characterization of a chymotryptic fragment of platelet glycoprotein IIb-IIIa retaining Arg-Gly-Asp binding activity. J Biol Chem. 1992 Mar 15;267(8):5649–5655. [PubMed] [Google Scholar]
- Lanza F., Stierlé A., Fournier D., Morales M., André G., Nurden A. T., Cazenave J. P. A new variant of Glanzmann's thrombasthenia (Strasbourg I). Platelets with functionally defective glycoprotein IIb-IIIa complexes and a glycoprotein IIIa 214Arg----214Trp mutation. J Clin Invest. 1992 Jun;89(6):1995–2004. doi: 10.1172/JCI115808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus J. C., O'Toole T. E., Plow E. F., Glass A., Frelinger A. L., 3rd, Ginsberg M. H. A beta 3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science. 1990 Aug 24;249(4971):915–918. doi: 10.1126/science.2392682. [DOI] [PubMed] [Google Scholar]
- Montgomery R. R., Kunicki T. J., Taves C., Pidard D., Corcoran M. Diagnosis of Bernard-Soulier syndrome and Glanzmann's thrombasthenia with a monoclonal assay on whole blood. J Clin Invest. 1983 Feb;71(2):385–389. doi: 10.1172/JCI110780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Seligsohn U., Lyman S., Coller B. S. The molecular genetic basis of Glanzmann thrombasthenia in the Iraqi-Jewish and Arab populations in Israel. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3160–3164. doi: 10.1073/pnas.88.8.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiarowski S., Norton K. J., Eckardt A., Lukasiewicz H., Holt J. C., Kornecki E. Structural and functional characterization of major platelet membrane components derived by limited proteolysis of glycoprotein IIIa. Biochim Biophys Acta. 1989 Jul 24;983(1):91–99. doi: 10.1016/0005-2736(89)90384-2. [DOI] [PubMed] [Google Scholar]
- O'Toole T. E., Loftus J. C., Plow E. F., Glass A. A., Harper J. R., Ginsberg M. H. Efficient surface expression of platelet GPIIb-IIIa requires both subunits. Blood. 1989 Jul;74(1):14–18. [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Poncz M., Eisman R., Heidenreich R., Silver S. M., Vilaire G., Surrey S., Schwartz E., Bennett J. S. Structure of the platelet membrane glycoprotein IIb. Homology to the alpha subunits of the vitronectin and fibronectin membrane receptors. J Biol Chem. 1987 Jun 25;262(18):8476–8482. [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Cunningham M., Hoxie J. A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987 Jul;70(1):307–315. [PubMed] [Google Scholar]
- Shattil S. J., Hoxie J. A., Cunningham M., Brass L. F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985 Sep 15;260(20):11107–11114. [PubMed] [Google Scholar]
- Simsek S., Heyboer H., de Bruijne-Admiraal L. G., Goldschmeding R., Cuijpers H. T., von dem Borne A. E. Glanzmann's thrombasthenia caused by homozygosity for a splice defect that leads to deletion of the first coding exon of the glycoprotein IIIa mRNA. Blood. 1993 Apr 15;81(8):2044–2049. [PubMed] [Google Scholar]
- Sosnoski D. M., Emanuel B. S., Hawkins A. L., van Tuinen P., Ledbetter D. H., Nussbaum R. L., Kaos F. T., Schwartz E., Phillips D., Bennett J. S. Chromosomal localization of the genes for the vitronectin and fibronectin receptors alpha subunits and for platelet glycoproteins IIb and IIIa. J Clin Invest. 1988 Jun;81(6):1993–1998. doi: 10.1172/JCI113548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel J. W., Nagaswami C., Vilaire G., Bennett J. S. Examination of the platelet membrane glycoprotein IIb-IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J Biol Chem. 1992 Aug 15;267(23):16637–16643. [PubMed] [Google Scholar]
- Zimrin A. B., Eisman R., Vilaire G., Schwartz E., Bennett J. S., Poncz M. Structure of platelet glycoprotein IIIa. A common subunit for two different membrane receptors. J Clin Invest. 1988 May;81(5):1470–1475. doi: 10.1172/JCI113478. [DOI] [PMC free article] [PubMed] [Google Scholar]