Abstract
Pantoea stewartii subsp. stewartii is the causative agent of Stewart's wilt, a bacterial disease transmitted by the corn flea beetle mainly to sweet corn (Zea mays). In many countries, it is classified as a quarantine organism and must be differentiated from other yellow enteric bacteria frequently occurring with corn. We have created novel primers from the pstS-glmS region of P. stewartii for use in conventional PCR (cPCR) and quantitative PCR (qPCR). To facilitate rapid diagnosis, we applied matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) analysis. Using whole-cell protein extracts, profiles were generated with a Bruker microflex machine, and the bacteria classified. P. stewartii strains were clearly distinguished from strains of Pantoea agglomerans, Pantoea dispersa, and Pantoea ananatis. Dendrogram analysis of the protein profiles confirmed the score values and showed the formation of separate clades for each species. The identification achieved by MALDI-TOF MS analysis agrees with the diagnosis by specific PCR primers. The combination of both methods allows a rapid and simple identification of the corn pathogen. P. stewartii subsp. stewartii and P. stewartii subsp. indologenes are highly related and can be distinguished not only by virulence assays and indole tests but also by a characteristic pattern in the nucleotide sequence of recA.
Stewart's wilt, caused by Pantoea stewartii subsp. stewartii (synonym Erwinia stewartii) is a serious disease of sweet corn (Zea mays) that was originally described in the United States (17, 18). Its transmission depends on the corn flea beetle (Chaetocnema pulicaria), which ingests the pathogen from infected tissue and transfers the bacteria to healthy plants. The beetle is also the main niche for overwintering of P. stewartii. Direct distribution by seed transmission is also possible (3, 11) but is not considered a major source. Stewart's wilt is also a problem on certain elite inbred maize lines used for producing hybrid field corn seed in the mideastern United States (2). According to data from the European and Mediterranean Plant Protection Organization (EPPO) about its occurrence in Europe, Stewart's wilt was reported from but not established in Austria, Greece, Poland, Romania, and Russia. More than 60 countries place import regulations on maize seed imports from affected areas, and surveillance of traded plant material is required to prevent further distribution of the pathogen (14).
Several detection methods have been described for P. stewartii, including monoclonal antibodies for enzyme-linked immunosorbent assay (ELISA) (8). For the detection of P. stewartii by PCR analysis, primer pairs derived from rRNA genes and chromosomal markers, such as regions coding for the Hrp type III secretion system (hrp) and capsular exopolysaccharide (EPS) synthesis (cps), have been published (4). These primers were derived from chromosomal regions which are also common to other bacteria. A unique DNA area of P. stewartii might therefore be better suited for the design of specific primers. Another approach, the ligase chain reaction, requires radioactively labeled primers (21). Primers complementary to cpsD (wceL) were applied for quantitative PCR (qPCR) (19). A fingerprinting analysis based on miniprimer PCR and utilizing 10-mer short oligonucleotides combined with modified Taq polymerase has been reported (22). The signal intensity of PCRs is often affected by inhibitory plant components in the extracts. Thus, low levels of P. stewartii may not be detected. A collective drawback of PCR-based identification approaches is the detection of DNA from nonviable cells and traces of residual nucleic acids. This could lead to the rejection of safe seed lots. A method involving culturing of bacteria extracted from plants, lysis, and subsequent PCR analysis and named bio-PCR was established to ensure the detection of only viable bacterial populations (16). Screening of individual colonies from a plate with mixed cultures by PCR to verify reisolation of the pathogen is tedious and needs another fast and reliable method. Strains of Pantoea stewartii subsp. indologenes cause leaf spot on foxtail millet (Setaria italica) and pearl millet (Pennisetum americanum) or rot of Ananas comosus, and one strain was isolated from a diseased cluster bean (Cyamopsis tetragonolobus) (10). It is important to distinguish between P. stewartii subsp. stewartii and P. stewartii subsp. indologenes, since only the stewartii subspecies causes Stewart's wilt.
Furthermore, some bacterial isolates might not be unambiguously identified with PCR and with phytopathological methods. The recent successful identification of Erwinia isolates with matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) analysis profiling of protein patterns from whole cells (15) induced us to apply this method for the detection of P. stewartii and its differentiation from Pantoea agglomerans and other Pantoea species.
MATERIALS AND METHODS
Bacterial strains.
The bacterial isolates used in this study are listed in Table 1. The P. stewartii strains were mostly isolated in North America; strain EstA1 was obtained from W. Zeller and is labeled with England as the origin. No further data are available about its isolation.
TABLE 1.
Bacterial strains used in this study
| Species, subspecies, strain | Source of isolation, description | Source or reference |
|---|---|---|
| P. stewartii subsp. indologenes strain CFBP 3614T (synonym: BCCM 2632T) | Setaria italica | BCCM/CFBP |
| P. stewartii subsp. stewartii strains | ||
| DC116 | Zea mays, Missouri, 1976, avirulent | 4 |
| DC117 | Z. mays, Missouri, 1976 | 4 |
| DC119 | Z. mays, Missouri, 1976 | 4 |
| DC122 | Z. mays, Missouri, 1976, avirulent | 4 |
| DC133 | Z. mays, Missouri, 1976, avirulent | 4 |
| DC145 | C. pulicaria, Connecticut, 1976 | 4 |
| DC146 | C. pulicaria, Connecticut, 1976 | 4 |
| DC147 | C. pulicaria, Connecticut, 1976 | 4 |
| DC162 | Z. mays, New York, 1975 | 4 |
| DC172T | Z. mays, Iowa, 1940, avirulent, SS11 | 4 |
| DC283 | Nal mutant of SS104 | 4 |
| DC405 | Virulent, A. Vidaver | 4 |
| DC406 | Virulent, A. Vidaver | 4 |
| DC407 | Virulent, A. Vidaver | 4 |
| DC408 | Virulent, A. Vidaver | 4 |
| EstA1 | England | W. Zeller |
| SS102 | Z. mays, New York | 4 |
| SS104 | Z. mays, Illinois, 1967 | 4 |
| SW2 | Z. mays, Ohio, 1974 | 4 |
| P. agglomerans strains | ||
| 48b/90 | Glycine max | B. Völksch |
| AL807540 | Blood bottle, Heidelberg University | S. Zimmermann |
| AL910982 | Blood bottle, Heidelberg University | S. Zimmermann |
| C9-1 | Malus sylvestris | C. Ishimaru via V. Stockwell |
| Subsp. gypsophilae DC556 | 4 | |
| DSM 3493T | Tanzania, knee laceration | B. Völksch |
| Eh252 | F. Spinelli | |
| Eh2b/89 | A. Burse | |
| Eh583 | M. sylvestris, 1986, Switzerland | 5a |
| Subsp. betae Ehb4188 | I. Barash | |
| Subsp. gypsophilae Ehg102 | I. Barash | |
| Subsp. gypsophilae Ehg3-108 | I. Barash | |
| Subsp. gypsophilae EhgPD713 | I. Barash | |
| EhNZ | Pear fruit, New Zealand | Laboratory collection |
| EhY112 | M. sylvestris, United States, 9/1986 | 5a |
| KasEh1 | Plant surface, Kazakhstan | Laboratory collection |
| EhMB96 | Corn leaf, Ladenburg, Germany | Y. Zhang |
| Pagg81883 | Blood bottle | M. Harvesi |
| Pagg82245 | Blood bottle | M. Harvesi |
| Pagg83873 | Blood bottle | M. Harvesi |
| P. ananatis strains | ||
| CU2093 | Ananas comosus | A. Alvarez |
| DC129 | From Z. mays, Missouri 1976 | 4 |
| DC532 | S. Lindow | |
| P. dispersa strains | ||
| DSM 30073T | B. Völksch | |
| NCBP 2285 | NCPPB | |
| Unidentified species | ||
| DC429 | D. L. Coplin | |
| DC437 | Corn isolate | D. L. Coplin |
| DC443 | D. L. Coplin | |
| DC444 | D. L. Coplin | |
| S. marcescens strains | ||
| Sm B2 | Soil | Laboratory collection |
| Sm B14 | Soil | Laboratory collection |
PCR assays, DNA sequencing, and oligonucleotide primers applied.
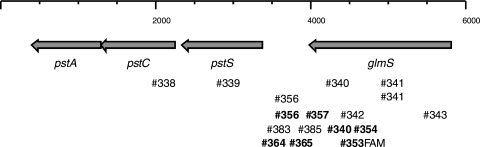
The primer pairs applied in this study are listed in Table 2. They were derived from nucleotide sequences from parts of the P. stewartii genome, accessible via ftp://ftp.hgsc.bcm.tmc.edu/pub/data/Pstewartii, focusing on the pst-glmS region of strain DC283. Their approximate positions are indicated in Fig. 1. The exact sizes of the products can be calculated from the primer names in the second column of Table 2. The assays were done as described before (12). For qPCR with SYBR green, hot start Taq polymerase (Amplicon) was used. With TaqMan probes, we applied normal Taq polymerase. For sequencing of the recA gene, primer set 93/94 or 390/391 (Table 2) was applied and the products commercially sequenced.
TABLE 2.
Primers used in the PCR assaysa
| Assay, primer no. | Primer pair/trio | Sequence |
|---|---|---|
| cPCR | ||
| 338 | PST2026 | GCACCGAATTGTTCGTTAGG |
| 339 | PST2989c | CCGTTGGCGACATCTATCTG |
| 340 | PST4245 | ACCACAATGACCGGCATATC |
| 341 | PST4987c | AATCACGGTGCAGTCGATCT |
| 342 | PST4602 | GCTACACCAATCTCCGTACC |
| 343 | PST5691c | ATCAGCAAGGCCATATCACC |
| 356 | PST3899 | CACTGGAGCAATGCAGTAGC |
| 341 | PST4987c | AATCACGGTGCAGTCGATCT |
| 383 | PST3581 | GCAATGTCGTCCTGCTCTGT |
| 385 | PST3909c | TTGCTCCAGTGCACGCTGTT |
| qPCR | ||
| 340 | PST4245 | ACCACAATGACCGGCATATC |
| 353 | PST4267FAM | FAM-CATCGATCAACGCCAGCGGA-BH1 |
| 354 | PST4354 | GCGCTCAAGCTGAAGGAGAT |
| 356 | PST3899 | CACTGGAGCAATGCAGTAGC |
| 357 | PST3991c | TCGGTAACGGTCGAGTAATG |
| 364 | PST3587 | TCGTCCTGCTCTGTACGCTT |
| 365 | PST3685c | TATAGCCAGAGCGCCTCTGT |
| recA sequencing | ||
| 93 | RECA81 | CATGCGCCTGGGTGAAGACC |
| 94 | RECA779c | TCAGCCTGCTTGAACGGCGC |
| 390b | PAGRECA21 | GGTGAAGACCGCTCAATGGA |
| 391b | PAGRECA621c | CACCGATACGGCGGATATCA |
FIG. 1.
Genomic region of strain DC283 with part of the pst operon and glmS used for design of the PCR primers, indicated below by the numbers listed in Table 2. Numbers in boldface indicate primers for qPCR.
MALDI-TOF MS.
Sample preparation was performed as described previously (15). Briefly, bacteria were grown on LB agar plates for 24 h at 28°C or in 1 ml of LB broth with 1% glycerol in 2-ml reaction tubes. Cells were suspended in water or the medium removed by centrifugation, and the pellet was washed with 1 ml water to remove residual components of the growth medium, pelleted again, and resuspended in 0.3 ml water and 0.8 ml ethanol. For lysis, cells were pelleted, air dried to remove ethanol, and resuspended thoroughly in 40 μl 70% formic acid and 40 μl acetonitrile. The cell debris was removed by centrifugation, and the clear lysates were stored at −20°C. One- to two-microliter amounts of the extracts were placed on an MSP 96 polished steel target and cocrystallized with the same amount of matrix (saturated alpha-cyano-4-hydroxy cinnamic acid in 50% acetonitrile-2.5% trifluoroacetic acid) for analysis with a Bruker microflex machine.
Biotyper analysis.
Protein profiles were derived as the averages of 250 spectra and analyzed with Biotyper software in the automatic mode (version 2.0; Bruker Daltonics, Bremen, Germany). Pattern analysis was performed against the reference library, version 3.0. The results were interpreted according to a log score scheme (15). Values of 2.0 or above represent a high likelihood of a positive identification. Each strain identification was repeated at least 3 times.
Plant assays.
One-week-old seedlings of sweet corn (‘Golden Bantam') were cut approximately 1 cm above the soil (4). The bacterial inocula were derived from overnight cultures in LB broth. The cell density was adjusted to 5 × 105 CFU/ml. Five-microliter amounts of the bacterial suspensions in water were placed on each plant stump. The negative control was the addition of water. The plants were further incubated for 7 to 10 days in a growth chamber (16 h light at 26°C/8 h dark at 22°C). Leaves and stem sections (approximately 50 mg) were sliced and extracted in 1.5 ml water for 15 min. Aliquots of the suspensions were diluted 100-fold, 0.2-ml amounts plated on LB agar with cycloheximide, and the plates incubated at 28°C for 2 days. The colonies were washed from the agar surface, suspended in 3 ml water, diluted to an optical density of 2, and processed for MALDI-TOF MS analysis, or Tween lysates (suspension diluted 1:100) were used for PCR analysis.
RESULTS
Detection of P. stewartii by PCR with primers from the pstS-glmS region.
The pst operon of bacteria participates in phosphate metabolism, and the adjacent glmS gene encodes glutamine synthase. The DNA region in between is quite heterogenous among bacterial species. For P. stewartii, the distance between pstS and glmS is only 600 bp (Fig. 1), relatively small in comparison to the distance in Erwinia amylovora, which is 3,822 bp (GenBank accession number AJ831832). The stretches of homology within this 600-bp sequence are shorter than 20 nucleotides in BLAST analysis against the genome sequences of Erwinia species (E. amylovora, E. billingiae, E. pyrifoliae, and E. tasmaniensis) and other bacteria. Accordingly, this region seems to be specific for P. stewartii. On the other hand, the design of PCR primers within this sequence, especially for qPCR with TaqMan probes, is difficult due to its low GC content. We therefore designed primers that include the flanking pst and glmS sequences (Fig. 1).
Five primer pairs were used in conventional PCR (cPCR) assays (Table 3). All produced positive signals with DNA from strains of P. stewartii subsp. stewartii and P. stewartii subsp. indologenes. When the band patterns obtained were compared, primers 356 and 341, as well as primers 383 and 385, were found to have produced signals only from P. stewartii strains and not with any of the other species shown in Table 3. For cPCR, the primers derived from the intergenic region were highly specific. Other primer combinations occasionally resulted in product formation for other species, but the weak PCR signals were of deviant sizes. Of all the strains analyzed, only Pantoea dispersa strain NCPPB 2285 showed a positive signal of intermediate strength with three primer pairs (338/339, 340/341, and 342/343). No signals or only weak to intermediate signals of deviant product size were obtained for P. agglomerans and Pantoea ananatis (data not shown).
TABLE 3.
Detection and identification of P. stewartii and other Pantoea species by PCR and MALDI-TOF MS analysis
| Species, strain (other datuma) | cPCR resultb with primers: |
qPCR resultc withd: |
Log score in MALDI-TOF MS for speciesd | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 338/339 | 340/341 | 356/341 | 342/343 | 383/385 | TM and primer set 340/354 | SG and primer set 356/357 | SG and primer set 364/365 | ||
| P. stewartii | |||||||||
| CFBP 3614T (i+) | + | + | + | + | + | 20.6 | 21.1 | 20.9 | 2.0 |
| DC116 (i−) | + | + | + | + | + | 20.1 | 19.8 | 17.3 | 2.1 |
| DC117 (Ps) | + | + | + | + | + | 20.2 | 19.2 | 18.9 | 2.2 |
| DC119 (Ps) | + | + | + | + | + | 20.1 | 19.1 | 19.2 | 2.1 |
| DC122 (i−) | + | + | + | + | + | 20.0 | 19.6 | 20.1 | 2.1 |
| DC133 (i−) | + | + | + | + | + | 20.3 | 20.0 | 20.6 | 2.2 |
| DC145 (Ps) | + | + | + | + | + | 20.2 | 18.3 | 18.1 | 2.2 |
| DC146 | + | + | + | + | + | 20.7 | 20.3 | 20.4 | 2.2 |
| DC147 | + | + | + | + | + | 20.8 | 19.8 | 19.0 | 2.2 |
| DC162 | + | + | + | + | + | 20.0 | 19.2 | 17.8 | 2.2 |
| DC172T (Ps, i−) | + | + | + | + | + | 20.5 | 18.7 | 19.1 | 2.0 |
| DC283 (Ps, i−) | + | + | + | + | + | 21.6 | 20.3 | 20.2 | 2.1 |
| DC405 | + | + | + | + | + | 21.3 | 18.7 | 19.7 | 2.3 |
| DC406 | + | + | + | + | + | 21.1 | 19.0 | 19.8 | 2.2 |
| DC407 (Ps) | + | + | + | + | + | 20.9 | 19.9 | 17.7 | 2.4 |
| DC408 | + | + | + | + | + | 20.7 | 17.9 | 18.8 | 2.2 |
| EstA1 (i−) | + | + | + | + | + | 18.3 | 20.4 | 17.7 | 2.2 |
| SS102 (Ps) | + | + | + | + | + | 19.9 | 20.1 | 17.3 | 2.2 |
| SS104 (Ps) | + | + | + | + | + | 19.8 | 18.7 | 20.0 | 2.0 |
| SW2 (Ps) | + | + | + | + | + | 20.6 | 19.5 | 19.2 | 2.1 |
| P. agglomerans | |||||||||
| 48b/90 (i−) | − | − | − | − | − | 32.1 | 35.1 | 31e | 2.4 |
| AL807540 | ND | ND | − | ND | − | >40 | 36.6 | 35.6 | 2.2 |
| AL910982 | ND | ND | − | ND | − | 32.6 | 37.6 | 33e | 2.0 |
| C9-1 | − | − | − | − | − | 35.0 | >40 | >40 | 2.2 |
| DC556 | − | − | − | − | − | 37.9 | 38.3 | >40 | 2.3 |
| DSM 3493T | − | − | − | − | − | 36.0 | 39.3 | ND | 2.3 |
| Eh252 | − | − | − | − | − | 38.6 | 38.2 | 33.7 | 2.2 |
| Eh2b/89 | − | − | − | − | − | 36.9 | 34.1 | 36.4 | 2.1 |
| Eh583 | − | − | − | − | − | 37.8 | >40 | >40 | 2.4 |
| Ehb4188 | − | − | − | − | − | 36.8 | 36.0 | >40 | 2.4 |
| Ehg102 | − | − | − | − | − | 37.6 | 35.6 | >40 | 2.3 |
| Ehg3-108 | − | − | − | − | − | >40 | 38.1 | 36.3 | 2.4 |
| EhgPD713 | − | − | − | − | − | >40 | 37.6 | 37.8 | 2.3 |
| EhNZ | − | − | − | − | − | 33.1 | 38.3 | >40 | 2.4 |
| EhY112 (i−) | − | − | − | − | − | 34.8 | >40 | >40 | 2.3 |
| KasEh1 | − | − | − | − | − | >40 | ND | ND | 2.3 |
| EhMB96 (i−) | − | − | − | − | − | 36.0 | >40 | 35.8 | 2.3 |
| Pa81883 | − | − | − | − | − | 35.7 | 36.8 | >40 | 2.3 |
| Pa82245 | − | − | − | − | − | 34.9 | 34.6 | >40 | 2.3 |
| Pa83873 | − | − | − | − | − | 33.3 | 36.0 | >40 | 2.3 |
| P. ananatis | |||||||||
| CU2093 | − | − | − | − | − | >40 | >40 | 36.9 | No ID |
| DC129 (i+) | − | SB | − | − | − | >40 | >40 | >40 | 2.1 |
| DC532 | − | SB | − | − | − | 38.2 | >40 | >40 | 2.1 |
| P. dispersa | |||||||||
| DSM 30073T (i−) | − | − | − | − | − | 31.9 | 39.5 | 37.9 | 2.0 |
| NCPPB 2285 | + | + | − | (+) | − | 36.6 | >40 | >40 | 2.3 |
| Others | |||||||||
| DC429 (i−) | − | − | − | − | − | >40 | 37.0 | >40 | No ID |
| DC437 (i−) | − | − | − | − | − | 38.2 | 35.3 | 36.1 | No ID |
| DC443 (G+) | − | − | − | − | − | >40 | 33.9 | 34.7 | No ID |
| DC444 (G+) | − | − | − | − | − | 35.5 | 32.6 | 35.6 | No ID |
| S. marcescens | |||||||||
| Sm B2 | − | − | − | − | − | >40 | >40 | 35.8 | 2.2 |
| Sm B14 | − | − | − | − | − | 36.1 | >40 | 35.2 | 2.2 |
i+ or i−, result of indole assay; Ps, previously identified as P. stewartii; G+, Gram positive (KOH test).
+, positive PCR result; −, negative PCR result; SB, shadow band in wrong position; (+), weak product formation.
TM, TaqMan primer 353; SG, SYBR green.
ND, not done; ID, identification.
Atypical baseline increase.
qPCR.
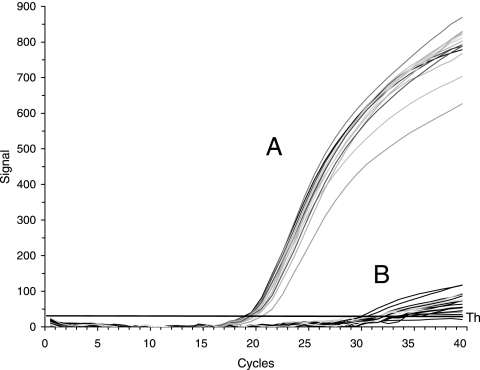
For the detection of P. stewartii in large-scale screenings and for quantitative assays, we designed several primer pairs to amplify the pst-glmS intergenic region in order to produce short fragments for visualization with SYBR green and, in addition, a TaqMan probe with three oligonucleotides (Table 2). The DNA of the strains listed was isolated from lysates with an equivalent of 105 cells. The signals that were positive for P. stewartii strains were in the range of 18 to 21 cycle thresholds (CT) (Fig. 2 and Table 3). Also, P. agglomerans subsp. gypsophilae (9) and subsp. betae did not produce a signal in quantitative and conventional PCR analyses (Table 3). The signals obtained for P. stewartii subsp. stewartii and subsp. indologenes were species specific and allowed the detection of small amounts of cells down to 10 CFU. Some strains of Pantoea spp. produced a signal of around 32 CT, i.e., at least 10 more cycles than for P. stewartii. It should be noted that DNA from several Erwinia species, such as E. amylovora, E. pyrifoliae, E. billingiae, E. tasmaniensis, E. persicina and E. rhapontici, likewise did not produce a signal with primers 340/352FAM/354 in qPCR (data not shown).
FIG. 2.
qPCR profiles for quantitative detection of P. stewartii isolates. Cell lysates of strains listed in Table 1 were amplified with primers 340, 353FAM, and 354. Area A comprises only P. stewartii strains, with cycle thresholds of 18 to 21, and area B comprises other bacteria, with cycle thresholds above 32.
Identification of P. stewartii by MALDI-TOF MS analysis.
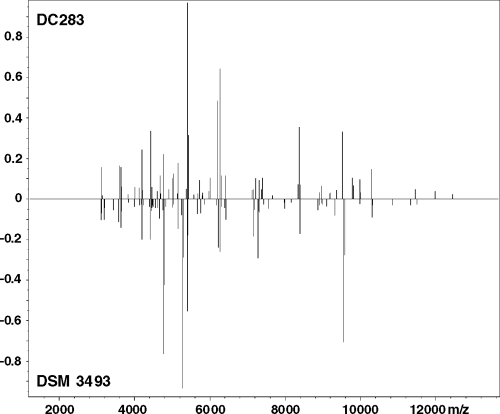
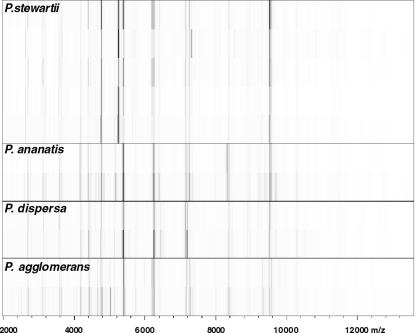
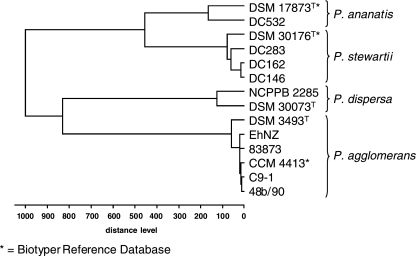
Protein profiles were derived for the whole-cell lysates of more than 50 bacterial strains and analyzed against the reference database. With log score values greater than or equal to the cutoff value of 2.0, positive species identification was obtained for 20 P. stewartii, 20 P. agglomerans, two P. ananatis, and two Serratia marcescens strains (Table 3). Direct comparison of spectra by peak list comparison (identification view in Fig. 3 and gel view in Fig. 4) showed discriminatory patterns for the different species. P. dispersa was not included in version 3.0 of the Biotyper database, which was reflected in the low log scores obtained for DSM 30073T and NCPPB 2285. However, the spectra obtained for both isolates clustered together tightly in a dendrogram (Fig. 5). This documentation complements identification by score values. The strain relatedness documented in the dendrogram can also be used for the identification of bacteria without score values and without known references. The incorporation of a new reference spectrum also resulted in clear reidentification of P. dispersa (Table 3). The distance levels for the Pantoea species analyzed were below the threshold of 500. P. agglomerans strains of medical and environmental origin were also highly matched to each other. The protein patterns for Pantoea stewartii subsp. stewartii and subsp. indologenes did not differentiate them sufficiently to allow subspecies identification. Most other species in the genus Pantoea were identified by MALDI-TOF MS analysis. No positive identification was obtained for CU2093, described previously as P. ananatis, and other “yellow” bacteria (DC429 and DC437).
FIG. 3.
Protein profiles for P. stewartii and P. agglomerans. Typical protein profiles for P. stewartii DC283 and P. agglomerans DSM 3493T were selected and analyzed in identification view. The x axis represents the molecular mass, and the y axis represents the relative signal strength.
FIG. 4.
Bar code representation of protein profiles. Protein spectra of various Pantoea isolates were visualized in a pseudo-gel representation with peak heights converted to color intensity. Lines are as follows (top to bottom): P. stewartii strains DC283, DC116, SW2, DC145, and DC405; P. ananatis strains DC129 and DC532; P. dispersa strains NCPPB 2285 and DSM 30073; and P. agglomerans strains DSM 3493T and C9-1.
FIG. 5.
MALDI-TOF MS log score-oriented dendrogram of Pantoea species. Reference spectra (*) were clustered with protein profiles from laboratory isolates. The critical cluster distance level is assumed to be 500.
Phylogeny with partial recA sequences.
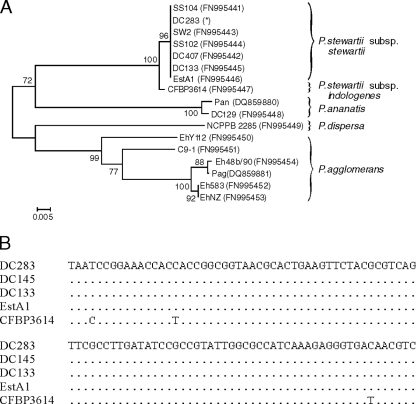
To distinguish P. stewartii subsp. stewartii and P. stewartii subsp. indologenes from each other and to confirm the dendrogram obtained from MALDI-TOF MS analysis, we sequenced part of the recA genes from strains of several Pantoea species. A dendrogram created from the alignment (Fig. 6 A) allows several conclusions. The corn pathogen P. stewartii subsp. stewartii is a homogeneous species, whereas P. agglomerans is relatively divergent. In particular, P. stewartii subsp. stewartii strains are very closely related to each other yet distinct from P. stewartii subsp. indologenes. P. ananatis, which is also positive in the indole assay (7), is distantly related to P. stewartii, but P. dispersa is closer to P. agglomerans. Since P. stewartii subsp. stewartii and P. stewartii subsp. indologenes cannot be distinguished by PCR or MALDI-TOF MS analysis (Table 3), the difference in the indole assay was confirmed in the nucleotide sequence alignment of recA, which shows typical base changes for the subspecies indologenes (Fig. 6B). Three base changes in the depicted part of the recA gene separate them into different subspecies. The alignment comprises strain DC283, isolated from corn with Stewart's wilt and used for the genome-sequencing project, strain DC145, isolated from a corn flea beetle, and EstA1, of undefined origin. Our limited access to strains of subspecies indologenes provided sequence information for the type strain CFBP 3614T from India that was identical with BCCM 2632T from the Belgian strain collection.
FIG. 6.
A dendrogram was created from the alignment of part of the recA gene of Pantoea species. For the dendrogram, primers 390 and 391 (P. agglomerans) or 93 and 94 were applied for PCR amplification; for sequencing, primer 390 or 93 was used. (A) The dendrogram was done with the program MEGA, version 4, by neighbor-joining and bootstrap analysis (1,000 replications). The scale bar represents nucleotide substitutions per site. GenBank accession numbers are listed beside the strain names. *, recA sequence from contigs downloaded via the Internet address ftp://ftp.hgsc.bcm.tmc.edu/pub/data/Pstewartii. (B) The alignment shows part of the recA genes of P. stewartii subsp. stewartii strains DC283 (isolated from Z. mays), DC145 (isolated from C. pulicaria), and EstA1 (unknown history) and P. stewartii subsp. indologenes strain CFBP 3614T, which is identical with BCCM 2632T. The sequences start at position 615 of the recA from strain DC283.
Plant assays.
Several strains were subjected to virulence assays on corn seedlings to confirm their identification as P. stewartii subsp. stewartii and to demonstrate recovery of the pathogen from plant material. We reisolated P. stewartii strain DC283 and SW2 after inoculation of young corn seedlings for the cut stump assay. We only recovered high bacterial populations from plants inoculated with P. stewartii, which exhibited characteristic Stewart's wilt symptoms, and not from plants inoculated with P. agglomerans or P. stewartii subsp. indologenes. In the negative control and after inoculation with P. stewartii subsp. indologenes CFBP 3614T and P. agglomerans strain MB96, only a few bacterial colonies were detected. MALDI-TOF MS analysis of the reisolated bacteria after DC283 and SW2 inoculation identified them as P. stewartii with a high score value (2.1 to 2.2), whereas inoculation with the other bacteria did not produce sufficient cell numbers for analysis.
DISCUSSION
P. stewartii subsp. stewartii is a pathogen affecting some corn varieties. Its possible distribution by corn seeds can be minimized by field inspections and monitoring of plant material for the presence of the pathogenic bacteria. ELISAs allow large sampling numbers (8, 11), but the detection limit is often quite high, so small pathogen populations might be missed. PCR assays are by far more sensitive but can sometimes produce ambiguous signals resulting in false identification as P. stewartii. Several primers for the detection of P. stewartii have been described (4, 19, 22). It would be risky to rely on a simple assay using a design from common regions of plant-pathogenic bacteria, such as rRNA genes, the hrp region, or EPS-encoding gene clusters, to identify Stewart's wilt. We have therefore selected the genes of the pst operon and glmS, including the intergenic region in between, which seems to be highly specific for P. stewartii. Some primer combinations can occasionally produce a false-positive band, so we propose the use of primers 356 and 341 for unambiguous signals in cPCR and the use of a TaqMan probe with primers 340 and 354 for qPCR.
The detection of P. stewartii by PCR assays was confirmed or, alternatively, done by MALDI-TOF MS analysis of protein profiles. This procedure, widely used for characterization of individual proteins, can be applied to obtain protein patterns of whole-cell lysates. Like the previously described miniprimer PCR (22), this method is only applicable to single isolates. For identification of bacteria, protein patterns in the size range of 2,000 to 14,000 Da are compared. A previous study of strains belonging to the genus Erwinia demonstrated that the placement of isolates into several Erwinia species by MALDI-TOF MS was in agreement with other deterministic and molecular characteristics (15). We have extended this approach to the genus Pantoea to identify the corn pathogen P. stewartii and discriminate it from phenotypically similar species and subspecies, such as P. agglomerans, P. dispersa, and P. ananatis (1). Single isolates could be clearly distinguished by Biotyper analysis at the species level. This is especially true for strains of P. agglomerans, which are widely distributed on plant surfaces and need to be discerned from the quarantine organism P. stewartii. In addition, P. agglomerans is also a potential human pathogen, and its isolation from wounds has been reported. However, our comparison of field and clinical isolates of P. agglomerans for their protein patterns in MALDI-TOF MS analysis showed no significant differences associated with their origin. This observation is in line with other data showing that this species is very diverse on the basis of biochemical and sequence analysis (5, 20). Recently, Pasquer and coworkers have published a microarray setup for fingerprinting analysis of various Pantoea and other bacteria (13). This method is based on the hybridization of genomic DNA against 95,000 oligonucleotide probes. Array techniques enable the generation of profiles based on large probe sets, implying a higher resolution than with MALDI-TOF MS patterns. Yet, the direct profile generation in MALDI-TOF MS circumvents labor-intensive sample preparation and contingent hybridization steps, enabling its application for large sample sizes.
For the identification of P. stewartii, the MALDI-TOF MS data compared favorably with identification by cPCR and qPCR, and there was good agreement between these methods. MALDI-TOF MS analysis and PCR may be complementary to each other. The advantage of cPCR is its sensitivity for traces of P. stewartii in mixed bacterial populations of plant extracts. qPCR allows quantitative determination of the pathogen and large-scale screening. Nevertheless, small amounts of P. stewartii may be difficult to identify. Melting curve analysis of products detected with SYBR green can narrow down the specificity of signal analysis. In contrast, MALDI-TOF MS analysis can only detect proteins extracted from propagated cells. Its advantage is based on the robustness of pattern analysis compared to that of methods relying on individual markers that might be lost or modified. Sample preparation for MALDI-TOF MS analysis is fast and cost efficient and, thus, applicable for large-scale screenings. Still, mixed cultures are not suited for this method unless the bacteria to be identified are in the majority (15).
For reliable monitoring of P. stewartii, we propose a procedure similar to that used for the detection of E. amylovora (6). Plant tissue from corn plants with symptoms of Stewart's wilt is extracted with water and plated on LB agar, and yellow colonies are screened as possible P. stewartii isolates, starting with PCR. At least two primer pairs should be applied to confirm the presence of the pathogen. qPCR determines its relative amount, and MALDI-TOF MS analysis identifies P. stewartii in single colonies. MALDI-TOF MS analysis can only be applied if the proportion of the pathogen is not too low in a bacterial population. Analysis of the recA gene and indole assays, together with virulence assays on corn seedlings, distinguish the subspecies stewartii and indologenes. The procedure can be adapted for seeds and allows efficient monitoring for the presence of the pathogen.
Acknowledgments
This study was financially supported by the Julius Kuehn Institute and Jacobs University Bremen.
We thank Marina Gernold for excellent support in establishing the novel methods for the identification of P. stewartii and David Coplin for valuable comments on the manuscript.
Footnotes
Published ahead of print on 23 July 2010.
REFERENCES
- 1.Brady, C. L., S. N. Venter, I. Cleenwerck, K. Engelbeen, M. Vancanneyt, J. Swings, and T. A. Coutinho. 2009. Pantoea vagans sp. nov., Pantoea eucalypti sp. nov., Pantoea deleyi sp. nov. and Pantoea anthophila sp. nov. Int. J. Syst. Evol. Microbiol. 59:2339-2345. [DOI] [PubMed] [Google Scholar]
- 2.CAB International. 2009. Pantoea stewartii. Distribution maps of plant diseases no. 41, 5th ed. CAB International, Wallingford, United Kingdom.
- 3.Cook, K. A., R. A. Weinzierl, J. K. Pataky, P. D. Esker, and F. W. Nutter, Jr. 2005. Population densities of corn flea beetle (Coleoptera: Chrysomelidae) and incidence of Stewart's wilt in sweet corn. J. Econ. Entomol. 98:673-682. [DOI] [PubMed] [Google Scholar]
- 4.Coplin, D. L., D. R. Majerczak, Y. Zhang, W.-S. Kim, S. Jock, and K. Geider. 2002. Identification of Pantoea stewartii subsp. stewartii by PCR and strain differentiation by PFGE. Plant Dis. 86:304-311. [DOI] [PubMed] [Google Scholar]
- 5.Deletóile, A., D. Decre, S. Courant, V. Passet, J. Audo, P. Grimont, G. Arlet, and S. Brisse. 2009. Phylogeny and identification of Pantoea species and typing of Pantoea agglomerans strains by multilocus gene sequencing. J. Clin. Microbiol. 47:300-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Falkenstein, H., P. Bellemann, S. Walter, W. Zeller, and K. Geider. 1988. Identification of Erwinia amylovora, the fireblight pathogen, by colony hybridization with DNA from plasmid pEA29. Appl. Environ. Microbiol. 54:2798-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geider, K. 2005. Molecular detection of fire blight and differentiation of Erwinia amylovora strains. Phytopathol. Pol. 35:57-68. [Google Scholar]
- 7.Kovacs, N. 1956. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature 178:703. [DOI] [PubMed] [Google Scholar]
- 8.Lamka, G. L., J. H. Hill, D. C. Mcgee, and E. J. Braun. 1991. Development of an immunosorbent assay for seed borne Erwinia stewartii in corn seeds. Phytopathology 81:839-846. [Google Scholar]
- 9.Manulis, S., N. Kogan, L. Valinsky, O. Dror, and F. Kleitman. 1998. Detection of Erwinia herbicola pv. gypsophilae in gypsophila plants by PCR. Eur. J. Plant Pathol. 104:85-91. [Google Scholar]
- 10.Mergaert, J., L. Verdonck, and K. Kersters. 1993. Transfer of Erwinia ananas (Synonym, Erwinia uredovora) and Erwinia stewartii to the genus Pantoea emend. as Pantoea ananas (Serrano 1928) comb. nov. and Pantoea stewartii (Smith 1898) comb. nov., respectively, and description of Pantoea stewartii subsp. indologenes subsp. nov. Int. J. Syst. Bacteriol. 43:162-173. [Google Scholar]
- 11.Michener, P. M., J. K. Pataky, and D. G. White. 2002. Transmission of Erwinia stewartii from plants to kernels and reactions of corn hybrids to Stewart's wilt. Plant Dis. 86:167-172. [DOI] [PubMed] [Google Scholar]
- 12.Mohammadi, M., E. Moltmann, W. Zeller, and K. Geider. 2009. Characterisation of naturally occurring Erwinia amylovora strains lacking the common plasmid pEA29 and their detection with real-time PCR. Eur. J. Plant Path. 124:293-302. [Google Scholar]
- 13.Pasquer, F., C. Pelludat, B. Duffy, and J. E. Frey. 2010. Broad spectrum microarray for fingerprint-based bacterial species identification. BMC Biotechnol. 10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pataky, J., and R. Ikin. 2003. Pest risk analysis: the risk of introducing Erwinia stewartii in maize seed. The International Seed Federation, Nyon, Switzerland.
- 15.Sauer, S., A. Freiwald, T. Maier, M. Kube, R. Reinhardt, M. Kostrzewa, and K. Geider. 2008. Classification and identification of bacteria by mass spectrometry and computational analysis. PLoS One 3:e2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaad, N. W., Y. Berthier-Schaad, A. Sechler, and D. Knorr. 1999. Detection of Clavibacter michiganensis subsp. sepedonicus in potato tubers by BIO-PCR and an automated real-time fluorescence detection system. Plant Dis. 83:1095-1100. [DOI] [PubMed] [Google Scholar]
- 17.Smith, E. F. 1898. Note on Stewart's sweet-corn germ, Pseudomonas stewarti, n. sp. Proc. Am. Assoc. Adv. Sci. 47:422-426. [Google Scholar]
- 18.Stewart, F. C. 1897. A bacterial disease of sweet corn. N.Y. Agric. Exp. Stn. Bull. 130:422-439. [Google Scholar]
- 19.Tambong, J. T., K. N. Mwange, M. Bergeron, T. Ding, F. Mandy, L. M. Reid, and X. Zhu. 2008. Rapid detection and identification of the bacterium Pantoea stewartii in maize by TaqMan real-time PCR assay targeting the cpsD gene. J. Appl. Microbiol. 104:1525-1537. [DOI] [PubMed] [Google Scholar]
- 20.Völksch, B., S. Thon, I. D. Jacobsen, and M. Gube. 2009. Polyphasic study of plant- and clinic-associated Pantoea agglomerans strains reveals indistinguishable virulence potential. Infect. Genet. Evol. 9:1381-1391. [DOI] [PubMed] [Google Scholar]
- 21.Wilson, W. J., M. Wiedmann, H. R. Dillard, and C. A. Batt. 1994. Identification of Erwinia stewartii by a ligase chain reaction assay. Appl. Environ. Microbiol. 60:278-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu, R., Q. Chen, Z. Robleh Djama, and J. T. Tambong. 2010. Miniprimer PCR assay targeting multiple genes: a new rapid and reliable tool for genotyping Pantoea stewartii subsp. stewartii. Lett. Appl. Microbiol. 50:216-222. [DOI] [PubMed] [Google Scholar]