Abstract
Mycobacterium avium subsp. paratuberculosis, the causative agent of Johne's disease in cattle, was identified in settled-dust samples of Dutch commercial dairy farms, both in the dairy barn and in the young stock housing. Bioaerosols may play a role in within-farm M. avium subsp. paratuberculosis transmission.
Paratuberculosis is an infectious enteric disease caused by Mycobacterium avium subsp. paratuberculosis leading to economic losses in dairy cattle globally (2, 10). The main transmission route is the fecal/oral route from infectious adult cattle to susceptible calves (12).
Preventive calf management was a key point in model studies (7), but 20-year implementation did not lead to farm-level eradication, suggesting uncontrolled routes of transmission (1, 7).
Environmental samples were used to classify commercial dairy herds (3, 9, 11), based on long-term survival of M. avium subsp. paratuberculosis in the environment (16). Recently, bioaerosols containing viable M. avium subsp. paratuberculosis were identified in an experimental setting with 100% M. avium subsp. paratuberculosis prevalence (6) and may thus be a mode of transmission. Dust containing M. avium subsp. paratuberculosis might be ingested or inhaled by calves (4). Experimental M. avium subsp. paratuberculosis challenge studies in sheep successfully used inhalation (8). These transmission routes could hamper current control programs. Our objective was to study whether M. avium subsp. paratuberculosis could be detected in bioaerosols on commercial Dutch dairy farms.
Dairy herds in three Dutch veterinary practices were sampled in 2009. All farms participated in a Dutch M. avium subsp. paratuberculosis monitoring program in 2008, either the Dutch Paratuberculosis Program (PPN; n = 2) or the Dutch Bulk Milk Quality Assurance Program (BMQAP; n = 22) (15). Both PPN herds were certified M. avium subsp. paratuberculosis-free. Herds corresponding to the BMQAP had at least one positive animal identified by enzyme-linked immunosorbent assay (ELISA) (Pourquier ELISA; Institut Pourquier, France). Farms were grouped into three M. avium subsp. paratuberculosis test prevalence levels (control, zero positive animals; group A, one positive animal; group B, two or more positive animals; Table 1).
TABLE 1.
Overview of the results of the questionnaire about relevant M. avium subsp. paratuberculosis management practicesa
| Parameter | Value for groupb |
||
|---|---|---|---|
| Control (n = 2) | A (n = 8) | B (n = 14) | |
| Mean herd size (SD) | 69 (15) | 67 (19) | 102 (26) |
| Median no. of ELISA-positive cows (maximum) | 0 (0) | 1 (1) | 3 (10) |
| No. of farms with: | |||
| Cow brush in barn | 2 | 5 | 13 |
| Cow barn cleaned in summer with high-pressure cleaner | 0 | 6 | 4 |
| Dry cows in young stock housing | 0 | 3 | 3 |
| Young stock housed separately | 1 | 7 | 8 |
| Young stock housing empty in summer | 0 | 0 | 0 |
| Young stock housing cleaned with high-pressure cleaner | 0 | 6 | 1 |
Results of the questionnaire about relevant M. avium subsp. paratuberculosis management practices in 24 Dutch farms enrolled in this study with 0 (control), 1 (group A), or ≥2 (group B) ELISA-positive animals.
n, number of farms.
Farms were visited twice during the housing period. Sampling locations were above the animal level inside the barn. At the first visit (sampling 1 [S1]), settled dust was collected with wipes and a short management questionnaire was taken. At the same time, five to seven electrostatic dust collectors (EDC; Zeeman, Alphen a/d Rhijn, Netherlands) were installed and collected after 4 weeks (sampling 2 [S2]) (6). Settled-dust samples were processed according to a previously described method (6). Results are presented as proportions of positive locations. McNemar's χ2 test was performed to investigate whether S1 differed from S2.
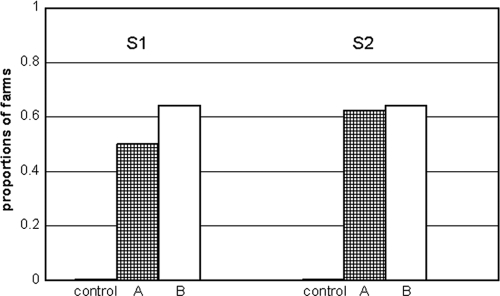
No M. avium subsp. paratuberculosis was detected by real-time PCR in any of the settled-dust samples at control farms (Fig. 1). M. avium subsp. paratuberculosis DNA was detected in dust samples at S1 and S2 in more than 50% of the group A and B farms, with seven farms consistently positive. M. avium subsp. paratuberculosis DNA was detected in the young stock area in 3/6 (S1) and 2/6 (S2) farms of group B with single-barn housing. M. avium subsp. paratuberculosis DNA was also detected in settled-dust samples from separate young stock housings in three farms, of which two cohoused dry cows.
FIG. 1.
Proportions of farms with M. avium subsp. paratuberculosis DNA detected in settled-dust samples collected at samplings 1 and 2. Black bar, control (n = 2); checked bar, group A (n = 8); white bar, group B (n = 14).
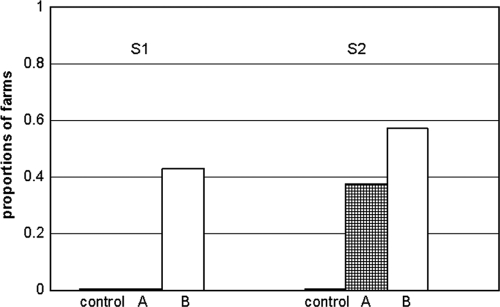
At control farms, no viable M. avium subsp. paratuberculosis was detected in any of the collected dust samples (Fig. 2). Viable M. avium subsp. paratuberculosis was detected in 6 B farms at S1. At S2, viable bacteria were present in 3 A farms and in the majority of B farms (Table 2). On five farms in group B, viable M. avium subsp. paratuberculosis was detected at both samplings.
FIG. 2.
Proportions of farms with viable M. avium subsp. paratuberculosis detected in settled-dust samples collected at samplings 1 and 2. Black bar, control (n = 2); checked bar, group A (n = 8); white bar, group B (n = 14).
TABLE 2.
Detection of M. avium subsp. paratuberculosis DNA or viable M. avium subsp. paratuberculosis in 5 to 7 settled-dust samples collected at sampling 1 or 2
| No. of positive dust samples | No. of farms with: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
M. avium subsp. paratuberculosis DNA |
Viable M. avium subsp. paratuberculosis |
|||||||||||
| Control (n = 2) |
Group A (n = 8) |
Group B (n = 14) |
Control (n = 2) |
Group A (n = 8) |
Group B (n = 14) |
|||||||
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| 0 | 2 | 2 | 4 | 3 | 4 | 5 | 2 | 2 | 8 | 5 | 8 | 6 |
| 1 | 3 | 4 | 4 | 6 | 1 | 2 | 4 | |||||
| 2 | 4 | 3 | 1 | 1 | 2 | |||||||
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | |||||
| 4 | 1 | 2 | ||||||||||
Viable M. avium subsp. paratuberculosis was detected in the young stock housing in 4 and 3 farms of group B with single-barn housing at S1 and S2, respectively. No viable M. avium subsp. paratuberculosis was detected in separate young stock housings.
To our knowledge, this study is the first to confirm the presence of M. avium subsp. paratuberculosis DNA as well as viable M. avium subsp. paratuberculosis in settled-dust samples of commercial dairy farms. M. avium subsp. paratuberculosis dispersion by bioaerosols under experimental conditions was already described (6). These findings support the concept of dust-based environmental dispersion of M. avium subsp. paratuberculosis within farms.
The relatively small number of farms and the convenience sampling are limitations of this study that could have introduced bias. However, this study is a proof of principle that viable M. avium subsp. paratuberculosis can be detected in settled-dust samples on farms with a low M. avium subsp. paratuberculosis prevalence. The environmental method also seems specific for M. avium subsp. paratuberculosis, since no M. avium subsp. paratuberculosis could be detected in any samples of known M. avium subsp. paratuberculosis-free herds.
Paratuberculosis control measures aim to prevent fecal-oral contact between infectious shedding adults and susceptible calves as the main transmission route of M. avium subsp. paratuberculosis. Several studies showed that “calf hygiene improvement” decreased prevalence but did not eliminate the disease (1, 7, 14), suggesting the existence of other transmission routes. In utero transmission, transmission via milk, and calf-to-calf transmission have been described previously (1, 12, 13). Additionally, infection via ingestion and/or inhalation of bioaerosols may be possible (4, 8).
Twenty-three of 24 herds were housed in free stalls with one tie-stall herd. Most farmers (n = 15) separated young stock from adult cattle as standard procedure. However, six of these farmers cohoused dry cows in the young stock housing occasionally, indicating the difficulties of consequently implementing management advice. Three farmers did not raise young stock on their farms. In almost all barns, cow brushes were present, as they were recommended to enhance cow well-being in group housings (5), but at the same time they contribute to aerosolization of dust. Animal movement on slatted floors also contributes to dust formation, especially during the winter housing period.
Most farmers from group A farms, compared to only a few from group B farms, intended to clean their barns yearly, but only 50% met this aim. Young stock housings were never totally empty, but high-pressure cleaning was occasionally performed at 6/8 farms of group A and at 1 of group B. The numbers of farms in this study precluded statistical testing, but the difference in cleaning attitude seemed remarkable.
Comparison of the two methods of dust collection showed no statistical difference. No M. avium subsp. paratuberculosis, neither DNA nor viable M. avium subsp. paratuberculosis, could be detected on known negative farms, whereas on farms of groups A and B, M. avium subsp. paratuberculosis DNA was present in comparable numbers of locations. Viable M. avium subsp. paratuberculosis was present only in group B farms at S1 and in both group A and B farms at S2. It seems that M. avium subsp. paratuberculosis can survive in dust for some time. Besides having a possible role in M. avium subsp. paratuberculosis transmission, dust might also be a useful predictor of M. avium subsp. paratuberculosis presence or M. avium subsp. paratuberculosis introduction on dairy farms, even on farms with low M. avium subsp. paratuberculosis prevalence.
In conclusion, this study showed that dust on farms with a low M. avium subsp. paratuberculosis seroprevalence contained viable M. avium subsp. paratuberculosis, which indicated a role in M. avium subsp. paratuberculosis transmission. Further research is needed to study if and how infection with M. avium subsp. paratuberculosis-contaminated dust is possible. Additionally, dust sampling may be an alternative tool to monitor M. avium subsp. paratuberculosis status in control programs.
Footnotes
Published ahead of print on 23 July 2010.
REFERENCES
- 1.Benedictus, A., R. M. Mitchell, M. Linde-Widmann, R. Sweeney, T. Fyock, Y. H. Schukken, and R. H. Whitlock. 2008. Transmission parameters of Mycobacterium avium subspecies paratuberculosis infections in a dairy herd going through a control program. Prev. Vet. Med. 83:215-227. [DOI] [PubMed] [Google Scholar]
- 2.Benedictus, G., A. A. Dijkhuizen, and J. Stelwagen. 1987. Economic losses due to paratuberculosis in dairy cattle. Vet. Rec. 121:142-146. [DOI] [PubMed] [Google Scholar]
- 3.Berghaus, R. D., T. B. Farver, R. J. Anderson, C. C. Jaravata, and I. A. Gardner. 2006. Environmental sampling for detection of Mycobacterium avium ssp. paratuberculosis on large California dairies. J. Dairy Sci. 89:963-970. [DOI] [PubMed] [Google Scholar]
- 4.Corner, L. A., D. U. Pfeiffer, and K. A. Abbott. 2004. The respiratory tract as a hypothetical route of infection of cattle with Mycobacterium avium subspecies paratuberculosis. Aust. Vet. J. 82:170-173. [DOI] [PubMed] [Google Scholar]
- 5.DeVries, T. J., M. Vankova, D. M. Veira, and M. A. von Keyserlingk. 2007. Short communication: usage of mechanical brushes by lactating dairy cows. J. Dairy Sci. 90:2241-2245. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg, S. W., M. Nielen, W. Santema, D. J. Houwers, D. Heederik, and A. P. Koets. 2010. Detection of spatial and temporal spread of Mycobacterium avium subsp. paratuberculosis in the environment of a cattle farm through bio-aerosols. Vet. Microbiol. 143:284-292. [DOI] [PubMed] [Google Scholar]
- 7.Groenendaal, H., M. Nielen, A. W. Jalvingh, S. H. Horst, D. T. Galligan, and J. W. Hesselink. 2002. A simulation of Johne's disease control. Prev. Vet. Med. 54:225-245. [DOI] [PubMed] [Google Scholar]
- 8.Kluge, J. P., R. S. Merkal, W. S. Monlux, A. B. Larsen, K. E. Kopecky, F. K. Ramsey, and R. P. Lehmann. 1968. Experimental paratuberculosis in sheep after oral, intratracheal, or intravenous inoculation lesions and demonstration of etiologic agent. Am. J. Vet. Res. 29:953-962. [PubMed] [Google Scholar]
- 9.Lombard, J. E., B. A. Wagner, R. L. Smith, B. J. McCluskey, B. N. Harris, J. B. Payeur, F. B. Garry, and M. D. Salman. 2006. Evaluation of environmental sampling and culture to determine Mycobacterium avium subspecies paratuberculosis distribution and herd infection status on US dairy operations. J. Dairy Sci. 89:4163-4171. [DOI] [PubMed] [Google Scholar]
- 10.Ott, S. L., S. J. Wells, and B. A. Wagner. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40:179-192. [DOI] [PubMed] [Google Scholar]
- 11.Raizman, E. A., S. J. Wells, S. M. Godden, R. F. Bey, M. J. Oakes, D. C. Bentley, and K. E. Olsen. 2004. The distribution of Mycobacterium avium ssp. paratuberculosis in the environment surrounding Minnesota dairy farms. J. Dairy Sci. 87:2959-2966. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney, R. W. 1996. Transmission of paratuberculosis. Vet. Clin. North Am. Food Anim. Pract. 12:305-312. [DOI] [PubMed] [Google Scholar]
- 13.van Roermund, H. J., D. Bakker, P. T. Willemsen, and M. C. de Jong. 2007. Horizontal transmission of Mycobacterium avium subsp. paratuberculosis in cattle in an experimental setting: calves can transmit the infection to other calves. Vet. Microbiol. 122:270-279. [DOI] [PubMed] [Google Scholar]
- 14.van Roermund, H. J. W., A. M. van Vos, and M. C. M. de Jong. 2002. Within-herd transmission of paratuberculosis and the possible role of infectious calves, p. 368-370. Proc. 7th Int. Colloq. Paratuberculosis. International Association for Paratuberculosis, Kennett Square, PA.
- 15.Weber, M. F., M. Nielen, A. G. Velthuis, and H. J. van Roermund. 2008. Milk quality assurance for paratuberculosis: simulation of within-herd infection dynamics and economics. Vet. Res. 39:12. [DOI] [PubMed] [Google Scholar]
- 16.Whittington, R. J., D. J. Marshall, P. J. Nicholls, I. B. Marsh, and L. A. Reddacliff. 2004. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 70:2989-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]