Abstract
The earthworm gut is an anoxic nitrous oxide (N2O)-emitting microzone in aerated soils. In situ conditions of the gut might stimulate ingested nitrate-reducing soil bacteria linked to this emission. The objective of this study was to determine if dissimilatory nitrate reducers and denitrifiers in the alimentary canal were affected by feeding guilds (epigeic [Lumbricus rubellus], anecic [Lumbricus terrestris], and endogeic [Aporrectodea caliginosa]). Genes and gene transcripts of narG (encodes a subunit of nitrate reductase and targets both dissimilatory nitrate reducers and denitrifiers) and nosZ (encodes a subunit of N2O reductase and targets denitrifiers) were detected in guts and soils. Gut-derived sequences were similar to those of cultured and uncultured soil bacteria and to soil-derived sequences obtained in this study. Gut-derived narG sequences and narG terminal restriction fragments (TRFs) were affiliated mainly with Gram-positive organisms (Actinobacteria). The majority of gut- and uppermost-soil-derived narG transcripts were affiliated with Mycobacterium (Actinobacteria). In contrast, narG sequences indicative of Gram-negative organisms (Proteobacteria) were dominant in mineral soil. Most nosZ sequences and nosZ TRFs were affiliated with Bradyrhizobium (Alphaproteobacteria) and uncultured soil bacteria. TRF profiles indicated that nosZ transcripts were more affected by earthworm feeding guilds than were nosZ genes, whereas narG transcripts were less affected by earthworm feeding guilds than were narG genes. narG and nosZ transcripts were different and less diverse in the earthworm gut than in mineral soil. The collective results indicate that dissimilatory nitrate reducers and denitrifiers in the earthworm gut are soil derived and that ingested narG- and nosZ-containing taxa were not uniformly stimulated in the guts of worms from different feeding guilds.
Earthworms have a profound impact on the structure and fertility of soils and occur in three feeding guilds (6, 17). Epigeic earthworms (e.g., Lumbricus rubellus) feed preferentially on litter, live above the mineral soil, rarely form burrows, and take up minor amounts of soil. Anecic earthworms (e.g., Lumbricus terrestris) feed on organic residues, build deep vertical burrows into the mineral soil, and ingest medium amounts of soil. Endogeic species (e.g., Aporrectodea caliginosa) build predominantly horizontal burrows and ingest large amounts of mineral soil and humified material.
An important feature of the earthworm relative to its ecological function is its alimentary canal, an anatomical structure that constitutes a transient mobile anoxic microzone for ingested soil microbial biomes in aerated soils (16). The abundances of cultivable fermenters and nitrate reducers are up to three orders of magnitude higher in the earthworm gut than in preingested soil (16, 32, 33, 34). The dissimilatory reduction of nitrate (i.e., the reduction of nitrate to nitrite or ammonium) is often facilitated by fermentative organisms (70) and occurs in habitats subject to anoxia and a high level of availability of organic carbon (71), conditions characteristic of the earthworm gut (13, 16, 29, 33, 75). Denitrification (i.e., the reduction of nitrate or nitrite to a nitrogenous gas), the dissimilatory reduction of nitrate, and fermentations appear to occur in the earthworm gut, and nitrous oxide (N2O), dinitrogen (N2), and molecular hydrogen (H2) are emitted in vivo (29, 31, 32, 34, 42, 75, 76). On a dry weight basis, earthworms can emit much larger amounts of N2O than soils (12, 29, 31, 32, 34, 42, 75, 76). The burrowing activities and feeding habits of earthworms, as well as in situ soil conditions, can influence the emission of nitrogenous gases from soils inhabited by earthworms (3, 5, 34, 40, 42, 54).
Denitrification is carried out by several oxidoreductases, i.e., dissimilatory nitrate reductase (encoded by either narGHI or napAB), nitrite reductase (encoded by nirK and nirS), NO reductase (encoded by norBC), and N2O reductase (encoded by nosZ) (50, 77). Dissimilatory nitrate reducers also possess nitrate reductases, most of which are encoded by narGHI (45). Thus, denitrifiers and dissimilatory nitrate reducers can be evaluated by analyzing narG (11, 14, 20, 26, 46, 49), whereas only denitrifiers are targeted by analyzing nosZ (30, 52, 58, 65, 66, 67, 76).
The capacity of earthworms to emit nitrogenous gases is primarily linked to denitrifiers in the alimentary canal (30, 31, 34, 42, 76). However, cultured dissimilatory nitrate reducers outnumber cultured denitrifiers in gut contents (32), suggesting that dissimilatory nitrate reducers might influence in vivo emission of N2O by their capacity to compete with denitrifiers for nitrate. Active nitrate-reducing taxa in the earthworm alimentary canal have not been identified, and although there is evidence indicating that the composition of gut taxa might be influenced by earthworm feeding guilds (1, 39, 69), the potential effect of feeding guilds on the diversity and activities of nitrate-reducing taxa remains largely unknown. Thus, the structural gene markers narG and nosZ were used in this study to elucidate dissimilatory nitrate reducers and denitrifiers in the earthworm alimentary canal of three earthworm feeding guilds and surrounding soils at both gene and gene transcript levels. The two hypotheses addressed were as follows: (i) soil-derived dissimilatory nitrate reducers and denitrifiers are activated in the earthworm alimentary canal; and (ii) earthworm feeding guilds influence this activation.
MATERIALS AND METHODS
Field sites and sampling.
Soils and specimens of A. caliginosa, L. terrestris, and L. rubellus were collected in spring 2007 (narG libraries), late summer 2008 (nosZ libraries), and early winter 2008 (narG and nosZ transcript libraries; terminal restriction fragment length polymorphism [TRFLP] analyses) from the meadow Trafo Wiese near Bayreuth, Germany, that is described elsewhere (29). Worms were collected and identified by standard protocols (7, 33, 42, 55). Soil samples were taken from the surface (uppermost soil, including decaying organic material) and from a depth of 15 cm (mineral soil). Worms and soils were stored in the dark for 1 h at 5°C before use.
Extraction of nucleic acids, reverse transcription of RNA, and amplification of narG and nosZ.
Earthworms (6 to 18 specimens per worm species) were washed, sedated with CO2, sacrificed by brief immersion in 70% ethanol, and dried under oxic conditions (33, 34). Alimentary canals were dissected, and RNA and DNA were coextracted from an approximately 0.5-g sample by bead-beating lysis, organic solvent extraction, and precipitation (27). The separation of RNA and DNA was performed with a Qiagen RNA/DNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. The absence of DNA in RNA fractions was indicated by the inability to gain a narG or nosZ PCR product under the conditions described below. Reverse transcription of RNA into cDNA was performed with a SuperScript Vilo cDNA synthesis kit (Invitrogen, Carlsbad, CA) using approximately 50 ng of RNA per 20 μl of reaction mixture and random hexamers for 10 min at 25°C, 120 min at 42°C, and 5 min at 85°C with a TGradient cycler (Biometra, Göttingen, Germany). PCR amplification was performed with primers narG1960f/narG2650r (5′-TAY GTS GGS CAR GAR AA-3′; 5′-TTY TCR TAC CAB GTB GC-3′ [49]) and nosZF/nosZR (5′-CGC TGT TCI TCG ACA GYC AG-3′; 5′-ATG TGC AKI GCR TGG CAG AA-3′ [52]) (Biomers GmbH, Ulm, Germany). Each 25-μl PCR mixture contained 10 μl of 5 Prime MasterMix (×2.5) (5 Prime, Hamburg, Germany), 2 μl of bovine serum albumin (30 μg μl−1), 1 μl of additional magnesium chloride (25 mM), 1.5 μl of each primer (100 pmol μl−1 for narG and 10 pmol μl−1 for nosZ), 8 ml of double-distilled H2O, and 1 μl each of DNA and cDNA, respectively. Each PCR was carried out with an initial denaturation (95°C, 8 min). Terminal elongation was for 10 min at 72°C; denaturation and elongation of each PCR and each cycle were performed at 95°C (1 min) and 72°C (2 min), respectively. For narG, the annealing temperature was lowered stepwise from 56°C to 52°C in 8 precycles, followed by 35 cycles with the annealing temperature at 52°C. narG PCR products were not obtained with cDNA samples, and conditions for cDNA were switched to 45 cycles with the annealing temperature at 58°C. For nosZ, the annealing temperature was lowered stepwise from 58°C to 52°C in 12 precycles, followed by 30 cycles with the annealing temperature at 52°C.
Cloning, sequencing, and sequence analysis.
narG and nosZ PCR products were electrophoresed on agarose gel (1%), excised, purified with a Montage gel extraction kit (Millipore Corp., Billerica, MA) according to the manufacturer's protocol, and ligated into pGEM-T vectors (Promega, Mannheim, Germany). Competent cells of Escherichia coli JM109 were transformed with the vector (protocol per manufacturer's instructions; Promega, Mannheim, Germany). narG and nosZ inserts were amplified with primers M13uni (5′-GTA AAA CGA CGG CCA G-3′) and M13rev (5′-CAG GAA ACA GCT ATG ACC-3′) (43). The conditions were 10 min for denaturation at 95°C and 35 cycles with 1 min at 95°C, 45 s at 50°C, and 90 s at 72°C. The final elongation step was 10 min at 72°C. Aliquots of the PCR product were electrophoresed on agarose gel (1%) to check for inserts of the right length. Clones with the correct insert were chosen randomly and selected for sequencing at Macrogen (Seoul, South Korea). Analyses of narG and nosZ sequences were performed with MEGA4 (68) and BLAST (http://blast.ncbi.nlm.nih.gov/). DOTUR 1.53 was used for defining species level genotypes (i.e., operational taxonomic units [OTUs]) (59). Except for TRFLP analysis, all phylogenic and statistical analyzes were performed with amino acid sequences obtained by translation of narG and nosZ sequences. Threshold dissimilarity values of 41% and 14% of amino acid sequences were used for defining species level OTUs for narG and nosZ, respectively (48). These threshold dissimilarity values were chosen because comparative sequence analyses of narG and nosZ with 16S rRNA genes indicated that these values correspond with 90% probability to a 16S rRNA sequence similarity of ≥97% (48), a conservative estimate for species level differentiation (64). DOTUR 1.53 was used for assessing coverage, richness (average of the richness estimators Chao1, ACE, jackknife, and bootstrap with a standard deviation), and diversity (Shannon-Weaver diversity index, Simpson's diversity index, Simpson's reciprocal index). Evenness was calculated as the Shannon-Weaver diversity index divided by the natural logarithm of the calculated genotype number (51) at species level OTU threshold dissimilarity values of 41% and 14% for narG and nosZ, respectively.
Phylogenetic analysis.
Phylogenetic trees were calculated from aligned amino acid sequences (ClustalW; alignment was manually refined) with MEGA4 (68) using the neighbor-joining method (56) with a bootstrap test (10,000 replicates) (22). All positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons (pairwise deletion option).
TRFLP analysis.
narG and nosZ gene fragments were amplified with the fluorescently labeled primers narG1960f-DY681 and nosZF-DY681 (Biomers GmbH, Ulm, Germany), respectively. Triplicate PCRs were carried out for each sample. PCR products were run on an agarose gel (1%). Gel-purified DNA (Montage gel extraction kit; Millipore Corp., Billerica, MA) was digested with mung bean nuclease (New England Biolabs, Frankfurt/Main, Germany) according to the manufacturer's protocol to reduce the probability of single-stranded DNA causing pseudoterminal restriction fragments (18). PCR products were purified (Montage gel extraction kit) and digested with the restriction enzyme BanI (New England Biolabs, Frankfurt/Main, Germany) or MaeIII (Roche Diagnostics GmbH, Mannheim, Germany) for narG and with HhaI (New England Biolabs, Frankfurt/Main, Germany) for nosZ according to the manufacturers' protocol but with 3 units and a 16-h digestion step in a reaction volume of 10 μl. TRFLP analysis was performed on a Nen 4300 DNA analyzer (Li-Cor, Lincoln, NE). The polyacrylamide gel consisted of 15 g of urea (Roche Diagnostics GmbH, Mannheim, Germany), 3.75 ml of 40% acrylamide-bis solution (37.5:1; Bio-Rad, Hercules, CA), 6 ml of Tris-borate-EDTA buffer (54 g of Tris, 27.5 g of boric acid, 20 ml of 0.5 M EDTA [pH 8.0], and double-distilled H2O to 1,000 ml), and 3.25 ml of double-distilled H2O. A bind-silane solution (1:1 ratio of bind-silane [PlusOne; GE Healthcare, Piscataway, NJ] and 10% acetic acid) was applied to the glass plates for stabilization of the comb region of the gel. Electrophoresis was performed for 3 h at 1,500 V and 45°C. Gels were analyzed with GelQuest (version 2.6.3; SequentiX, Klein Raden, Germany). Mean values and standard deviations were calculated from triplicate analyses of each sample. All terminal restriction fragments (TRFs) with a relative fluorescence of >3% for at least one sample were used for analysis, and their collective relative fluorescences were set as 100%. TRFs were assigned to genotypes by in silico analysis (MEGA4 [68]). TRFLP analysis of the narG transcript with BanI mainly yielded TRFs that were too small to evaluate and were dissimilar to narG TRFs (data not shown). Principal-component analyses of narG and nosZ TRFs were performed with RapidMiner.
Nucleotide sequence accession numbers.
The sequences obtained in this study are available from the EMBL nucleotide sequence database under accession numbers FN859458 to FN859704 (narG), FN859705 to FN859774 (nosZ), and FN859874 to FN859960 (nosZ).
RESULTS
Diversity and phylogeny of narG and narG transcripts.
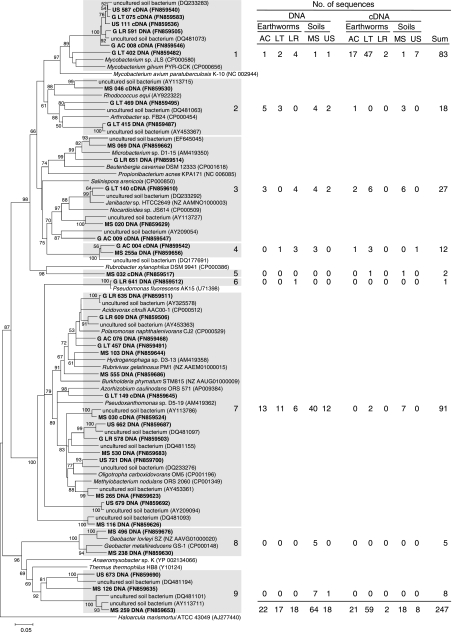
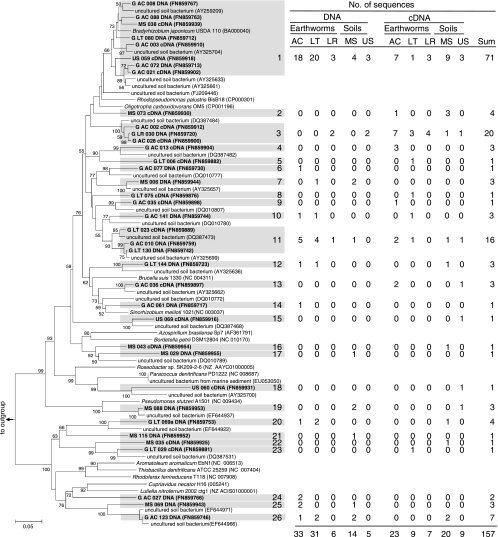
A collective total of 247 narG fragments and narG transcript fragments were retrieved from alimentary canal and soil samples, forming 9 species level OTUs at an amino acid sequence dissimilarity of 41% (48) (Fig. 1). This species level cutoff value is very conservative, and the real number of species is assumed to be higher, as evidenced by the large number of known species affiliated with certain OTUs (e.g., OTUs 3 and 7 [Fig. 1]). The 139 narG sequences (82 from soil, 57 from gut) were distributed in 8 OTUs. Soil- and gut-derived gene sequences were each distributed in 5 OTUs. The 108 narG transcripts (26 from soil, 82 from gut) were distributed in 4 OTUs. Soil- and gut-derived transcripts were each distributed in 3 OTUs.
FIG. 1.
Phylogenetic tree of representative narG sequences obtained from earthworms and soil and the related narG sequences. Sequences obtained in this study are in bold, and accession numbers are in parentheses. The tree is based on 215 translated amino acids. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (10,000 replicates) are shown next to the branches (values below 50% are masked) (22). The table shows the origin of sequences in each cluster (shaded text) as calculated with DOTUR 1.53. Abbreviations: G, gut; AC, A. caliginosa; LT, L. terrestris; LR, L. rubellus; MS, mineral soil; US, uppermost soil.
Coverage for all narG libraries approximated 100% (Table 1). The estimated richness of all narG sequences (i.e., 10 OTUs) was only slightly higher than that of the 9 species level OTUs (Table 1 and Fig. 1). The predicted numbers of OTUs for gene and transcript sequences were 8 and 4, respectively. Diversity indices (e.g., Shannon-Weaver) for narG differed minimally between gut-derived sequences and soil-derived sequences (Table 1). In contrast, diversity indices for narG transcripts were higher for soil-derived sequences than for gut-derived sequences.
TABLE 1.
Estimated genotypes, coverage percentages, and diversity indices of narG and nosZ amino acid sequences from the alimentary canals of earthworms and from soilsa
| Gene | Library | No. of sequences | No. of OTUs | Coverage (%) | Diversity indices |
|||
|---|---|---|---|---|---|---|---|---|
| Richness | H′b | Evenness | 1/Dc | |||||
| narGd | DNA, guts | 57 | 5 | 100 | 5 ± 0 | 1.30 | 0.81 | 2.95 |
| DNA, soils | 82 | 5 | 100 | 5 ± 0 | 1.16 | 0.72 | 2.33 | |
| cDNA, guts | 82 | 3 | 100 | 3 ± 0 | 0.55 | 0.50 | 1.45 | |
| cDNA, soils | 26 | 3 | 100 | 3 ± 0 | 1.06 | 0.97 | 3.00 | |
| DNA, total | 139 | 8 | 99.3 | 8 ± 0 | 1.41 | 0.68 | 2.67 | |
| cDNA, total | 108 | 4 | 100 | 4 ± 0 | 0.82 | 0.59 | 1.80 | |
| All narG sequences | 247 | 9 | 99.6 | 10 ± 0 | 1.57 | 0.71 | 3.75 | |
| nosZe | DNA, guts | 70 | 11 | 98.6 | 12 ± 1 | 1.53 | 0.64 | 2.75 |
| DNA, soils | 19 | 8 | 84.2 | 10 ± 1 | 1.84 | 0.88 | 6.33 | |
| cDNA, guts | 39 | 9 | 92.3 | 11 ± 1 | 1.74 | 0.79 | 4.72 | |
| cDNA, soils | 29 | 13 | 72.4 | 20 ± 3 | 2.08 | 0.81 | 5.64 | |
| DNA, total | 89 | 15 | 95.5 | 17 ± 1 | 1.78 | 0.66 | 3.23 | |
| cDNA, total | 68 | 18 | 83.8 | 41 ± 13 | 2.15 | 0.74 | 5.68 | |
| All nosZ sequences | 157 | 26 | 92.4 | 66 ± 48 | 2.15 | 0.66 | 4.30 | |
All calculations were carried out with DOTUR 1.53 and are based on amino acid sequences as described in Materials and Methods.
H′, Shannon-Weaver diversity index.
1/D, reciprocal Simpson's diversity index.
narG at a species level cutoff value of 41% dissimilarity of amino acid sequences.
nosZ at a species level cutoff value of 14% dissimilarity of amino acid sequences.
narG sequences were affiliated with sequences of Gram-negative genera (e.g., Acidovorax, Azorhizobium, Oligotropha, Methylobacterium, Geobacter, and Thermus [OTUs 6 to 9]) and Gram-positive genera (i.e., Actinobacteria; e.g., Mycobacterium, Rhodococcus, Arthrobacter, Microbacterium, Beutenbergia, Janibacter, and Rubrobacter [OTUs 1 to 5]) that are common to soils (Fig. 1). Most (i.e., 233 out of 247) narG sequences had the highest levels of similarity to those of uncultured soil bacteria and occurred in shared OTUs (i.e., OTUs that contained both soil- and gut-derived sequences), suggesting that narG sequences retrieved from the earthworm gut originated primarily from ingested soil bacteria.
Most narG sequences from soil were affiliated with OTUs of Gram-negative bacteria (mainly OTU 7), whereas narG sequences from the gut occurred more evenly in OTUs of both Gram-negative bacteria and Actinobacteria (Fig. 1). OTU 1 (Mycobacterium) contained the majority of narG transcripts, and sequences obtained from the gut and uppermost soil were dominant. Mineral soil-derived narG transcripts clustered mainly with OTUs 2, 3, and 7.
TRF patterns of narG and narG transcripts.
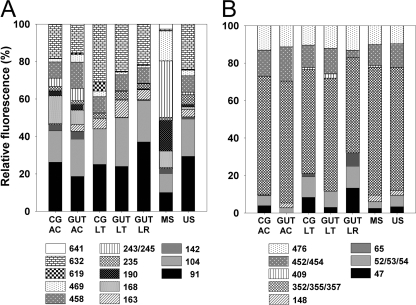
A total of 13 narG TRFs were obtained (Fig. 2 A). TRFs of the three earthworm feeding guilds were highly overlapping (i.e., were more similar than dissimilar), with the bulk of the sequences occurring in TRFs 91 bp (primarily indicative of OTUs 2 and 3), 104 bp (primarily indicative of OTU 3), 458 bp (OTU 7), and 632 bp (could not be assigned to an OTU) (Fig. 2A; see also Table S1 in the supplemental material). narG TRFs from mineral and uppermost soils were dissimilar (Fig. 2A and 3 A). Certain dominant TRFs in mineral soil (i.e., TRFs 168 bp, 190 bp, and 243/245 bp, all indicative of OTU 7) were apparent only in A. caliginosa. Significant differences between narG TRFs from crop/gizzard and those from gut were not apparent. Of the assignable narG sequences, those of Actinobacteria were dominant in all three feeding guilds and moist, uppermost soils, whereas Gram-negative bacteria were dominant in drier mineral soils and were also detected in the gut of the endogeic earthworm A. caliginosa.
FIG. 2.
TRFLP analysis of earthworm alimentary canal- and soil-derived narG and nosZ sequences. (A) narG (digestion was with BanI). (B) nosZ (digestion was with HhaI). Abbreviations: CG, crop/gizzard; AC, A. caliginosa; LT, L. terrestris; LR, L. rubellus; MS, mineral soil; US, uppermost soil. The legend shows the lengths of the TRFs. Shown are mean values (n = 3).
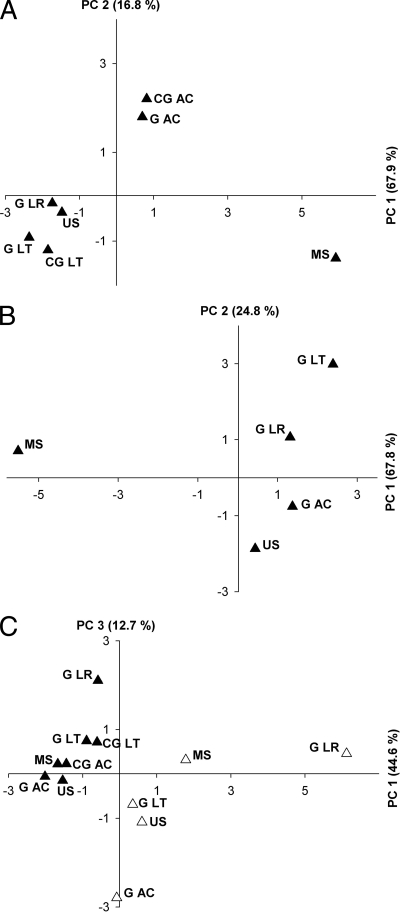
FIG. 3.
Principal-component analyses of TRFs. (A) narG TRFs (digestion was with BanI). A variance of 84.7% is covered by the x (67.9%) and y (16.8%) axes. (B) narG transcript TRFs (digestion was with MaeIII). A variance of 92.6% is covered by the x (67.8%) and y (24.8%) axes. (C) nosZ TRFs (filled symbols) and nosZ transcript TRFs (open symbols). Digestion was with HhaI. A variance of 57.3% is covered by the x (44.6%) and y (12.7%) axes. Abbreviations: G, gut; CG, crop/gizzard; AC, A. caliginosa; LT, L. terrestris; LR, L. rubellus; MS, mineral soil; US, uppermost soil.
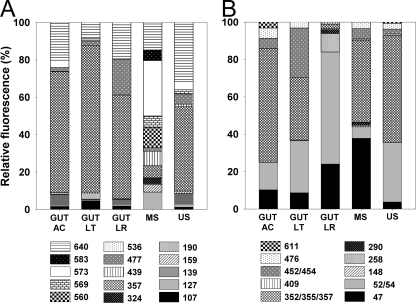
A total of 15 narG transcript TRFs were obtained (Fig. 4 A). narG transcript TRFs from all three feeding guilds were similar, whereas narG transcript TRFs from mineral and uppermost soils were dissimilar (Fig. 3B and 4A). TRF 357 bp (OTU 1; see Table S2 in the supplemental material) was dominant in all three feeding guilds and in uppermost soils. In contrast, the most dominant TRFs in mineral soils were 573 bp (OTU 7), 560 bp (could not be assigned), and 127 bp (OTU 7). TRF patterns corroborated the sequence data and revealed a high level of prevalence of OTU 1 narG transcripts in gut and uppermost-soil libraries, whereas sequences from mineral soil were mostly affiliated with OTUs 2, 3, and 7. Gut samples from A. caliginosa and L. terrestris showed highly similar narG transcript TRF patterns, whereas TRF 477 bp (OTU 4) was significant only in the gut of L. rubellus (Fig. 4A).
FIG. 4.
TRFLP analysis of earthworm alimentary canal- and soil-derived narG transcripts and nosZ transcripts. (A) narG (digestion was with MaeIII). (B) nosZ (digestion was with HhaI). Abbreviations: CG, crop/gizzard; AC, A. caliginosa; LT, L. terrestris; LR, L. rubellus; MS, mineral soil; US, uppermost soil. The legend shows the lengths of the TRFs. Shown are mean values (n = 3).
Diversity and phylogeny of nosZ and nosZ transcripts.
A collective total of 157 nosZ fragments and nosZ transcript fragments were retrieved from alimentary canal and soil samples, forming 26 species level OTUs at an amino acid sequence dissimilarity of 14% (48) (Fig. 5). The 89 nosZ sequences (19 from soil, 70 from gut) were distributed among 15 OTUs. Soil- and gut-derived gene sequences were distributed among 8 and 11 OTUs, respectively. The 68 nosZ transcripts (29 from soil, 39 from gut) were distributed among 18 OTUs. Soil- and gut-derived transcripts were distributed among 13 and 9 OTUs, respectively.
FIG. 5.
Phylogenetic tree of representative nosZ sequences obtained from earthworms and soil and the related nosZ sequences. Sequences obtained in this study are in bold, and accession numbers are in parentheses. The tree is based on 212 translated amino acids. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (10,000 replicates) are shown next to the branches (values below 50% are masked) (22). The table shows the origin of sequences in each cluster (shaded text) as calculated with DOTUR 1.53. The outgroup was Haloarcula marismortui ATCC 43049 (AY5962197). Abbreviations: G, gut; AC, A. caliginosa; LT, L. terrestris; LR, L. rubellus; MS, mineral soil; US, uppermost soil.
Coverages for soil and earthworm nosZ libraries ranged from approximately 72.4% to 84.2% and 92.3% to 98.6%, respectively, with the collective coverage of all sequences approximating 92% (Table 1). The estimated richness of all nosZ sequences yielded 66 OTUs, a much larger number than the 26 species level OTUs (Table 1 and Fig. 5). The predicted numbers of OTUs for gene and transcript sequences were 17 and 41, respectively. Diversity indices of nosZ and nosZ transcripts were lower for the earthworm gut than for soil (Table 1), suggesting that a restricted rather than a general stimulation of soil-derived denitrifiers occurred in the earthworm alimentary canal.
nosZ sequences were affiliated with Alphaproteobacteria (e.g., genera Bradyrhizobium, Oligotropha, Sinorhizobium, and Paracoccus), Betaproteobacteria (e.g., genera Bordetella, Cupriavidus, and “Lutiella”), and Pseudomonas stutzeri A1501, with most sequences having the highest similarity to nosZ sequences from uncultured soil bacteria. OTUs 1 and 11 were dominant in nosZ sequences, whereas OTUs 1 and 3 were dominant in nosZ transcripts. The closest relatives of OTUs 1, 3, and 11 were Bradyrhizobium japonicum USDA 110 and two uncultured soil bacteria, respectively. Most (i.e., 134 out of 157) nosZ sequences from gut and soil occurred in shared OTUs, suggesting that nosZ sequences retrieved from the earthworm gut originated primarily from ingested soil bacteria.
TRF patterns of nosZ and nosZ transcripts.
A total of 8 nosZ TRFs (Fig. 2B) and 10 nosZ transcript TRFs (Fig. 4B) were obtained. For gut-derived TRFs, gene level TRF 352/355/357 bp (of which OTUs 1, 3, and 11 contained the highest numbers of sequences [see Table S3 in the supplemental material]) was dominant (51 to 65% relative fluorescences) in all worm species (Fig. 2B), whereas this TRF at the transcript level was variable (61%, 33%, and 2% relative fluorescences for A. caliginosa, L. terrestris, and L. rubellus, respectively) (Fig. 4B). In contrast, gut-derived TRF 52/54 bp (of which OTUs 1, 3, and 11 contained the highest numbers of sequences [see Table S3]) was less significant at the gene level (3 to 12% relative fluorescences) than at the transcript level (15%, 28%, and 60% relative fluorescences for A. caliginosa, L. terrestris, and L. rubellus, respectively). Gut-derived TRF 47 bp (of which OTU 3 contained the highest number of sequences [see Table S3]) yielded higher relative fluorescences at the transcript level (10 to 24% relative fluorescences) than at the gene level (0 to 13% relative fluorescences). TRF 258 bp (OTU 23) was detected only in gut-derived transcripts from L. rubellus (10% relative fluorescence) and L. terrestris (1% relative fluorescence). Although minimal differences occurred between crop/gizzard- and gut-derived gene level TRFs obtained from L. terrestris and A. caliginosa, the relative fluorescence of TRF 47 bp was higher for that derived from crop/gizzard (Fig. 2B and 3C). The collective data indicate that TRF patterns of nosZ and nosZ transcripts are distinct and that gut-derived TRF patterns from L. rubellus are different from those from the other earthworm species (Fig. 3C).
The two soil samples were similar at the gene level but differed at the transcript level, especially in the case of TRF 47 bp, which yielded a high relative fluorescence (37%) in mineral soil, and TRF 52/54 bp, which yielded a high relative fluorescence (31%) in the uppermost soil (Fig. 2B and 4B). nosZ transcript profiles of both soils were more similar to those from the guts of A. caliginosa and L. terrestris than to the nosZ transcript profile from the gut of L. rubellus (Fig. 3C).
DISCUSSION
Guts of the earthworm species evaluated in this study have high capacities to reduce nitrate to nitrite and nitrogenous gases (29, 31, 32, 34, 42, 75, 76). The detection of narG and nosZ transcripts in earthworm alimentary canals (Fig. 1 and 5) corroborate these capacities, and the phylogenic affiliations of the detected transcripts suggest that dissimilatory nitrate reducers and denitrifiers are active in the earthworm gut.
Selective stimulation.
narG and nosZ transcripts were different and less diverse in the earthworm gut than in mineral soil (Fig. 3, 4), suggesting that ingested narG- and nosZ-containing taxa were not uniformly stimulated by the in situ conditions of the earthworm gut. Detected community compositions can differ between the earthworm gut and preingested soil (19, 36), and bacteria isolated from earthworm casts can be more capable of reducing nitrate than bacteria isolated from bulk soil (24). Based on these collective observations together with the assumed soil-derived origin of narG- and nosZ-containing taxa detected in the present and previous studies (30, 76), one might speculate that a selective activation of ingested microbiota rather than a substantial in situ growth of ingested microbiota occurs during gut passage and that this activation might enhance the cultivability of certain microorganisms (16, 32, 33, 34). Although this speculation is reinforced by the fact that total cell counts increase only marginally if at all during gut passage (60, 61, 74), this speculation must be qualified, as certain ingested taxa (e.g., large pseudomonads [60, 61, 74]) are subject to degradation during gut passage. Thus, since certain ingested taxa decrease in number due to degradation, it seems likely that other ingested taxa are subject to at least a minimum amount of replication during gut passage. Otherwise, cell numbers of ingested material would decrease during gut passage. Anoxia and nitrate induce denitrification by model denitrifiers (e.g., P. stutzeri, Ralstonia [renamed Wautersia] eutropha, and B. japonicum [2, 49, 72]) and also regulate the dissimilatory reduction of nitrate to nitrite by E. coli (4). Thus, a potential stimulation of microbes capable of reducing nitrate is likely linked to the in situ conditions of the earthworm alimentary canal, conditions that include anoxia, large amounts of nitrate and nitrite, high moisture content, a nearly neutral pH, and high-quality organic compounds such as sugars and amino acids (13, 15, 16, 29, 33, 75).
Phylogeny of narG.
narG and narG transcripts are related to those of actinobacterial and proteobacterial taxa common to soils (Fig. 1, 2A, and 4A). Indeed, narG sequences related to those detected in this study have been retrieved from soil (11, 14, 20, 49). Species of detected genera have been cultured from earthworm gut contents and casts (9, 24, 28, 32, 37, 69). Most narG transcripts retrieved from earthworm guts were affiliated with Mycobacterium (Fig. 1 and 4A). Mycobacterium-related narG sequences have been retrieved from soil (14, 49), species of Mycobacterium can reduce nitrate to nitrite (73), and mycobacterial species and other Actinobacteria occur in earthworm guts and casts and might be associated with gut walls (9, 23, 24, 37, 69). Furthermore, the percentages of Actinobacteria-related 16S rRNA genes can be higher in earthworm gut and cast than in soil (24, 47), accentuating the likelihood that certain taxa are stimulated during gut passage. Mycobacterium tuberculosis reduces nitrate to nitrite via a narG-containing nitrate reductase that is constitutive, i.e., expressed during aerobic growth in the absence of nitrate or nitrite (63, 73). The narGHJI operon of M. tuberculosis mediates the assimilatory reduction of nitrate (41), but the nitrate reductase encoded by this operon might also dissimilate nitrate (63, 73). The detection of Mycobacterium-related narG transcripts in the earthworm gut and wet, uppermost soil suggests that the encoded nitrate reductase is important for the dissimilation and/or assimilation of nitrate. narG transcripts retrieved from N2O-emitting Chironomus plumosus larvae are affiliated with Gram-negative bacteria rather than Mycobacterium (65).
Actinobacterial narGs and narG transcripts were dominant in earthworm guts (Fig. 1, 2, and 4; see also Tables S1 and S2 in the supplemental material). Many Actinobacteria reduce nitrate, but very few known actinobacterial species are capable of denitrification (21, 25, 38, 62). Thus, Actinobacteria likely compete with gammaproteobacterial denitrifiers for nitrate in the earthworm gut and might thereby affect in vivo emission of nitrogenous gases.
Phylogeny of nosZ.
nosZ and nosZ transcripts affiliated mostly with denitrifying proteobacterial genera common to soil (e.g., Bradyrhizobium and Pseudomonas) (Fig. 5) (77). nosZ sequences related to these genera have been retrieved from soils (20, 30, 52, 66, 76) and earthworm guts (30, 76). Most nosZ sequences and nosZ TRFs from the earthworm gut were closely affiliated with B. japonicum USDA 110 (Fig. 2B, 4B, and 5), a facultative soil denitrifier that can form symbiotic dinitrogen-fixing associations with soybean roots (2). B. japonicum lacks narG and reduces nitrate via a less oxygen-sensitive nap-encoded nitrate reductase (2, 44). B. japonicum-related nosZ sequences have been retrieved from soil (20, 30, 52, 66, 76) and the earthworm gut (30, 76). In addition, Bradyrhizobium-related 16S rRNA gene sequences have been retrieved from the earthworm gut wall (69). Thus, Bradyrhizobium-related species might be important members of the transient denitrifying community in the earthworm gut. The capacity of B. japonicum to utilize atmospheric N2O (i.e., 0.34 ppm of N2O) (57) demonstrates that related species might be capable of not only the production but also the efficient consumption of N2O.
Effect of earthworm feeding guilds.
A. caliginosa consumes large amounts of mineral soil (17). That some narG TRFs in the gut of A. caliginosa were also present in mineral soil but not apparent in the gut of L. terrestris or L. rubellus is consistent with the tendency of the latter two species to not consume large amounts of mineral soil. nosZ-related community composition also varied among the three feeding guilds (Fig. 2B, 3C, and 4B). Feeding guilds of earthworms can influence 16S rRNA gene diversity of gut biota (19, 36, 37, 69), underscoring the importance that feeding guilds might have on the composition of narG- and nosZ-containing taxa in the earthworm gut. As noted above, the disruption of large bacteria during gut passage might contribute to the selective occurrence of smaller ingested bacteria in the gut (8, 60, 61, 74,). Fluids that are toxic to certain bacteria are released into the gut lumen of A. caliginosa (10, 35). Such toxic fluids might be dependent on the feeding guild and contribute to the differences detected in the present study. Thus, the factors that might contribute to potential feeding guild-dependent differences in gut biota are complex.
It should be noted that the narG and nosZ primers used in the current study have limitations relative to resolving nitrate-reducing and denitrifying taxa. For example, napA encodes a subunit of a nitrate reductase that is widely spread among bacteria but is not targeted by narG primers (45, 53). In addition, nitrous oxide reductases of Gram-positive denitrifiers are not covered by the nosZ primers. The constraints of these considerations notwithstanding, the collective findings of the present study indicate that the dissimilatory nitrate reducers and denitrifiers in the earthworm gut are largely soil derived and that these soil-derived functional groups are subject to a postingestion stimulation that is influenced by the earthworm feeding guild. These general conclusions must nonetheless be weighed against the fact that only a single species was evaluated per feeding guild, thus pointing toward the need to extend these observations to other earthworm species. The detection of narG and nosZ transcripts that were closely related to sequences of uncultured soil bacteria (Fig. 1 and 5) suggest that novel uncultured narG- and nosZ-containing taxa contribute to the capacity of ingested soil biota to reduce nitrate and produce nitrogenous gases during gut passage, likewise emphasizing the need to bring such organisms into culture so that their response to the in situ conditions of the gut can be assessed at the cellular level.
Supplementary Material
Acknowledgments
Support for this study was provided by the Deutsche Forschungsgemeinschaft (DR310/4-1) and the University of Bayreuth.
Footnotes
Published ahead of print on 23 July 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aira, M., F. Monroy, and J. Dominguez. 2009. Changes in bacterial numbers and microbial activity of pig slurry during transit of epigeic and anecic earthworms. J. Hazard. Mater. 162:1404-1407. [DOI] [PubMed] [Google Scholar]
- 2.Bedmar, E. J., E. F. Robles, and M. J. Delgado. 2005. The complete denitrification pathway of the symbiotic, nitrogen-fixing bacterium Bradyrhizobium japonicum. Biochem. Soc. Trans. 33:141-144. [DOI] [PubMed] [Google Scholar]
- 3.Bertora, C., P. C. J. van Vliet, E. W. J. Hummelink, and J. W. van Groenigen. 2007. Do earthworms increase N2O emissions in ploughed grassland? Soil Biol. Biochem. 39:632-640. [Google Scholar]
- 4.Bonnefoy, V., and J. A. DeMoss. 1994. Nitrate reductases in Escherichia coli. Antonie Van Leeuwenhoek 66:47-56. [DOI] [PubMed] [Google Scholar]
- 5.Borken, W., S. Grundel, and F. Beese. 2000. Potential contribution of Lumbricus terrestris L. to carbon dioxide, methane and nitrous oxide fluxes from a forest soil. Biol. Fertil. Soils 32:142-148. [Google Scholar]
- 6.Bouché, M. B. 1977. Lumbricid strategies, p. 122-132. In U. Lohm and T. Persson (ed.), Soil organisms as components of ecosystems. Ecological Bulletins, vol. 25. Oikos Editorial Office, Lund University, Lund, Sweden. [Google Scholar]
- 7.Brohmer, P. 1984. Fauna von Deutschland, 16th ed. Quelle and Meyer, Heidelberg, Germany.
- 8.Brown, G. G. 1995. How do earthworms affect microfloral and faunal community diversity? Plant Soil 170:209-231. [Google Scholar]
- 9.Byzov, B. A., T. Y. Nechtitaylo, B. K. Bumazhkin, A. V. Kurakov, P. N. Golyshin, and D. G. Zvyagintsev. 2009. Culturable microorganisms from the earthworm digestive tract. Microbiology 78:360-368. [PubMed] [Google Scholar]
- 10.Byzov, B. A., N. V. Khomyakov, S. A. Kharin, and A. V. Kurakov. 2007. Fate of soil bacteria and fungi in the gut of earthworms. Eur. J. Soil Biol. 43:149-156. [Google Scholar]
- 11.Chèneby, D., S. Hallet, M. Mondon, F. Martin-Laurent, J. C. Germon, and L. Philippot. 2003. Genetic characterization of the citrate reducing community based on narG nucleotide sequence analysis. Microb. Ecol. 46:113-121. [DOI] [PubMed] [Google Scholar]
- 12.Conrad, R. 1996. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 60:609-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel, O., and J. M. Anderson. 1992. Microbial biomass and activity in contrasting soil materials after passage through the gut of the earthworm Lumbricus rubellus Hoffmeister. Soil Biol. Biochem. 24:465-470. [Google Scholar]
- 14.Deiglmayr, K., L. Philippot, D. Tscherko, and E. Kandeler. 2006. Microbial succession of nitrate-reducing bacteria in the rhizosphere of Poa alpina across a glacier foreland in the Central Alps. Environ. Microbiol. 8:1600-1612. [DOI] [PubMed] [Google Scholar]
- 15.Drake, H. L., and M. A. Horn. 2006. Earthworms as a transient heaven for terrestrial denitrifying microbes: a review. Eng. Life Sci. 6:261-265. [Google Scholar]
- 16.Drake, H. L., and M. A. Horn. 2007. As the worm turns: the earthworm gut as a transient habitat for soil microbial biomes. Annu. Rev. Microbiol. 61:169-189. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, C. A., and P. J. Bohlen. 1996. Biology and ecology of earthworms, 3rd ed. Chapman & Hall, London, United Kingdom.
- 18.Egert, M., and M. W. Friedrich. 2003. Formation of pseudo-terminal restriction fragments, a PCR-related bias affecting terminal restriction fragment length polymorphism analysis of microbial community structure. Appl. Environ. Microbiol. 69:2555-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egert, M., S. Marhan, B. Wagner, S. Scheu, and M. W. Friedrich. 2004. Molecular profiling of 16S rRNA genes reveals diet-related differences of microbial communities in soil, gut, and casts of Lumbricus terrestris L. (Oligochaeta: Lumbricidae). FEMS Microbiol. Ecol. 48:187-197. [DOI] [PubMed] [Google Scholar]
- 20.Enwall, K., L. Philippot, and S. Hallin. 2005. Activity and composition of the denitrifying bacterial community respond differently to long-term fertilization. Appl. Environ. Microbiol. 71:8335-8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eschbach, M., H. Möbitz, A. Rompf, and D. Jahn. 2003. Members of the genus Arthrobacter grow anaerobically using nitrate ammonification and fermentation processes: anaerobic adaption of aerobic bacteria abundant in soil. FEMS Microbiol. Lett. 223:227-230. [DOI] [PubMed] [Google Scholar]
- 22.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 23.Fischer, O. A., L. Matlova, J. Bart. L. Dvorska, P. Svastova, P. du Maine, I. Melicharek, M. Bartos, and I. Pavlik. 2003. Earthworms (Oligochaeta, Lumbricidae) and mycobacteria. Vet. Microbiol. 91:325-338. [DOI] [PubMed] [Google Scholar]
- 24.Furlong, M. A., D. R. Singleton, D. C. Coleman, and W. B. Whitman. 2002. Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl. Environ. Microbiol. 68:1265-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodfellow, M. 1992. The family Nocardiaceae, p. 1188-1213. In A. Balows, H. Trüper, M. Dworkin, W. Harer, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, New York, NY.
- 26.Gregory, L. G., P. L. Bond, D. J. Richardson, and S. Spiro. 2003. Characterization of a nitrate-respiring bacterial community using the nitrate reductase gene (narG) as a functional marker. Microbiology 149:229-237. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths, R. I., A. S. Whiteley, A. G. O'Donnell, and M. J. Bailey. 2000. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA and rRNA-based microbial community composition. Appl. Environ. Microbiol. 66:5488-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn, M. A., J. Ihssen, C. Matthies, A. Schramm, G. Acker, and H. L. Drake. 2005. Dechloromonas denitrificans sp. nov., Flavobacterium denitrificans sp. nov., Paenibacillus anaericanus sp. nov., and Paenibacillus terrae strain MH72, N2O-producing bacteria isolated from the gut of the earthworm Aporrectodea caliginosa. Int. J. Syst. Evol. Microbiol. 55:1255-1265. [DOI] [PubMed] [Google Scholar]
- 29.Horn, M. A., A. Schramm, and H. L. Drake. 2003. The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms. Appl. Environ. Microbiol. 69:1662-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn, M. A., H. L. Drake, and A. Schramm. 2006. Nitrous oxide reductase genes (nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar. Appl. Environ. Microbiol. 72:1019-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn, M. A., R. Mertel, M. Kästner, M. Gehre, and H. L. Drake. 2006. In vivo emission of dinitrogen by earthworms via denitrifying bacteria in the gut. Appl. Environ. Microbiol. 72:1013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ihssen, J., M. A. Horn, C. Matthies, A. Gößner, A. Schramm, and H. L. Drake. 2003. N2O-producing microorganisms in the gut of the earthworm Aporrectodea caliginosa are indicative of ingested soil bacteria. Appl. Environ. Microbiol. 69:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karsten, G., and H. L. Drake. 1995. Comparative assessment of the aerobic and anaerobic microfloras of earthworm guts and forest soils. Appl. Environ. Microbiol. 61:1039-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karsten, G. R., and H. L. Drake. 1997. Denitrifying bacteria in the earthworm gastrointestinal tract and in vivo emission of nitrous oxide (N2O) by earthworms. Appl. Environ. Microbiol. 63:1878-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khomyakov, N. V., S. A. Kharin, T. Y. Nechitailo, P. N. Golyshin, A. V. Kurakov, B. A. Byzov, and D. G. Zvyagintsev. 2007. Reaction of microorganisms to the digestive fluid of earthworms. Microbiology 76:45-54. [PubMed] [Google Scholar]
- 36.Knapp, B. A., J. Seeber, S. M. Podmirseg, E. Meyer, and H. Insam. 2008. Application of denaturing gradient gel electrophoresis for analysing the gut microflora of Lumbricus rubellus Hoffmeister under different feeding conditions. Bull. Entomol. Res. 98:271-279. [DOI] [PubMed] [Google Scholar]
- 37.Knapp, B. A., S. M. Podmirseg, J. Seeber, E. Meyer, and H. Insam. 2009. Diet-related composition of gut microbiota of Lumbricus rubellus as revealed by a molecular fingerprinting technique and cloning. Soil Biol. Biochem. 41:2299-2307. [Google Scholar]
- 38.Kocur, M., W. E. Kloos, and K.-H. Schleifer. 1992. The genus Micrococcus, p. 1301-1311. In A. Balows, H. Trüper, M. Dworkin, W. Harer, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, New York, NY.
- 39.Krištůfek, V., K. Ravasz, and V. Pizl. 1992. Changes in densities of bacteria and microfungi during gut transit in Lumbricus rubellus and Aporrectodea caliginosa (Oligochaeta, Lumbricidae). Soil Biol. Biochem. 24:1499-1500. [Google Scholar]
- 40.Lubbers, I. M., L. Brussaard, W. Otten, and J. W. van Groenigen. Earthworm-induced N mineralization in fertilized grassland increases both N2O emission and crop-N uptake. Eur. J. Soil Sci., in press.
- 41.Malm, S., Y. Tiffert, J. Micklinghoff, S. Schultze, I. Joost, I. Weber, S. Horst, B. Ackermann, M. Schmidt, W. Wohlleben, S. Ehlers, R. Geffers, J. Reuther, and F.-C. Bange. 2009. The roles of the nitrate reductase NarGHJI, the nitrite reductase NirBD and the response regulator GlnR in nitrate assimilation of Mycobacterium tuberculosis. Microbiology 155:1332-1339. [DOI] [PubMed] [Google Scholar]
- 42.Matthies, C., A. Griesshammer, M. Schmittroth, and H. L. Drake. 1999. Evidence for involvement of gut-associated denitrifying bacteria in emission of nitrous oxide (N2O) by earthworms obtained from garden and forest soils. Appl. Environ. Microbiol. 65:3599-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messing, J. 1983. New M13 vectors for cloning. Methods Enzymol. 101:20-78. [DOI] [PubMed] [Google Scholar]
- 44.Moreno-Vivián, C., P. Cabello, M. Martínez-Luque, R. Blasco, and F. Castillo. 1999. Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 181:6573-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morozkina, E. V., and R. A. Zvyagilskaya. 2007. Nitrate reductases: structure, functions, and effect of stress factors. Biochemistry (Mosc.) 72:1151-1160. [DOI] [PubMed] [Google Scholar]
- 46.Mounier, E., S. Hallet, D. Cheneby, E. Benizri, Y. Gruet, C. Nguyen, S. Piutti, C. Robin, S. Slezack-Deschaumes, F. Martin-Laurent, J. C. Germon, and L. Philippot. 2004. Influence of maize mucilage on the diversity and activity of the denitrifying community. Environ. Microbiol. 6:301-312. [DOI] [PubMed] [Google Scholar]
- 47.Nechitaylo, T. Y., M. M. Yakimov, M. Godinho, K. N. Timmis, E. Belogolova, B. A. Byzov, A. V. Kurakov, D. L. Jones, and P. N. Golyshin. 2010. Effect of the earthworm Lumbricus terrestris and Aporrectodea caliginosa on bacterial diversity in soil. Microb. Ecol. 59:574-587. [DOI] [PubMed] [Google Scholar]
- 48.Palmer, K., H. L. Drake, and M. A. Horn. 2009. Genome-derived criteria for assigning environmental narG and nosZ sequences to operational taxonomic units of nitrate reducers. Appl. Environ. Microbiol. 75:5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philippot, L., S. Piutti, F. Martin-Laurent, S. Hallet, and J. C. Germon. 2002. Molecular analysis of the nitrate-reducing community from unplanted and maize-planted soils. Appl. Environ. Microbiol. 68:6121-6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Philippot, L. 2002. Denitrifying genes in bacterial and archaeal genomes. Biochim. Biophys. Acta 1577:355-376. [DOI] [PubMed] [Google Scholar]
- 51.Pielou, E. C. 1977. Mathematical ecology, p. 385. Wiley Interscience, New York, NY.
- 52.Rich, J. J., R. S. Heichen, J. P. Bottomley, K. Cromack, and D. D. Myrold. 2003. Community composition and functioning of denitrifying bacteria from adjacent meadow and forest soils. Appl. Environ. Microbiol. 69:5974-5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richardson, D. J., B. C. Berks. D. A. Russell, S. Spiro, and C. J. Taylor. 2001. Functional, biochemical and genetic diversity of prokaryotic nitrate reductases. Cell. Mol. Life Sci. 58:165-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rizhiya, E., C. Bertora, P. C. J. van Vliet, P. J. Kuikman, J. H. Faber, and J. W. van Groenigen. 2007. Earthworm activity as a determinant for N2O emission from crop residue. Soil Biol. Biochem. 39:2058-2069. [Google Scholar]
- 55.Römbke, J., A. M. Breure, C. Mulder, and M. Rutgers. 2005. Legislation and ecological quality assessment of soil: implementation of ecological indication systems in Europe. Ecotoxicol. Environ. Saf. 62:201-210. [DOI] [PubMed] [Google Scholar]
- 56.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 57.Sameshima-Saito, R., K. Chiba, J. Hirayama, M. Itakura, H. Mitsui, S. Eda, and K. Minamisawa. 2006. Symbiotic Bradyrhizobium japonicum reduces N2O surrounding the soybean root system via nitrous oxide reductase. Appl. Environ. Microbiol. 72:2526-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scala, D. J., and L. J. Kerkhof. 1998. Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol. Lett. 162:61-68. [DOI] [PubMed] [Google Scholar]
- 59.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schönholzer, F., D. Hahn, and J. Zeyer. 1999. Origins and fate of fungi and bacteria in the gut of Lumbricus terrestris L. studied by image analysis. FEMS Microbiol. Ecol. 28:235-248. [Google Scholar]
- 61.Schönholzer, F., D. Hahn, B. Zarda, and J. Zeyer. 2002. Automated image analysis and in situ hybridization as tools to study bacterial populations in food resources, gut and cast of Lumbricus terrestris L. J. Microbiol. Methods 48:53-68. [DOI] [PubMed] [Google Scholar]
- 62.Shoun, H., M. Kano, I. Baba, N. Takaya, and M. Matsuo. 1998. Denitrification by actinomycetes and purification of dissimilatory nitrate reductase and azurin from Streptomyces thioluteus. J. Bacteriol. 180:4413-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sohaskey, C. D., and L. G. Wayne. 2003. Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J. Bacteriol. 185:7247-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stackebrandt, E., and J. Ebers. 2006. Taxonomic parameters revisited: tarnished gold standards. Microbiol. Today 33:152-155. [Google Scholar]
- 65.Stief, P., M. Poulsen, L. P. Nielsen, H. Brix, and A. Schramm. 2009. Nitrous oxide emission by aquatic macrofauna. Proc. Natl. Acad. Sci. U. S. A. 106:4296-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stres, B., I. Mahne, G. Augustin, and J. M. Tiedje. 2004. Nitrous oxidoreductase (nosZ) gene fragments differ between native and cultivated Michigan soils. Appl. Environ. Microbiol. 70:301-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stres, B., T. Danevcic, L. Pal, M. M. Fuka, L. Resman, S. Leskovec, J. Hacin, D. Stopar, I. Mahne, and I. Mandic-Mulec. 2008. Influence of temperature and soil water content on bacterial, archaeal and denitrifying microbial communities in drained fen grassland soil microcosms. FEMS Microbiol. Ecol. 66:110-122. [DOI] [PubMed] [Google Scholar]
- 68.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 69.Thakuria, D., O. Schmidt, D. Finan, D. Egan, and F. M. Doohan. 2010. Gut wall bacteria of earthworms: a natural selection process. ISME J. 4:357-366. [DOI] [PubMed] [Google Scholar]
- 70.Tiedje, J. M. 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium, p. 179-243. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley & Sons, New York, NY.
- 71.Tiedje, J. M., A. J. Sexstone, D. D. Myrold, and J. A. Robinson. 1982. Denitrification: ecological niches, competition and survival. Antonie Van Leeuwenhoek 48:569-583. [DOI] [PubMed] [Google Scholar]
- 72.van Spanning, R. J. M., D. J. Richardson, and S. T. Ferguson. 2007. Introduction to the biochemistry and molecular biology of denitrification, p. 3-20. In H. Bothe, S. J. Ferguson, and W. E. Newton (ed.), Biology of the nitrogen cycle, 1st edition. Elsevier, Amsterdam, Netherlands.
- 73.Weber, I., C. Fritz, S. Ruttkowski, A. Kreft, and F.-C. Bange. 2000. Anaerobic nitrate reductase (narGHJI) activity of Mycobacterium bovis BCG in vitro and its contribution to virulence in immunodeficient mice. Mol. Microbiol. 35:1017-1025. [DOI] [PubMed] [Google Scholar]
- 74.Wolter, C., and S. Scheu. 1999. Changes in bacterial numbers and hyphal length during the gut passage through Lumbricus terrestris (Lumbricidae, Oligochaeta). Pedobiologia 43:891-900. [Google Scholar]
- 75.Wüst, P. K., M. A. Horn, and H. L. Drake. 2009. In situ hydrogen and nitrous oxide as indicators of concomitant fermentation and denitrification in the alimentary canal of the earthworm Lumbricus terrestris. Appl. Environ. Microbiol. 75:1852-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wüst, P. K., M. A. Horn, G. Henderson, P. H. Janssen, B. H. Rehm, and H. L. Drake. 2009. Gut-associated denitrification and in vivo emission of nitrous oxide by the earthworm families Megascolecidae and Lumbricidae in New Zealand. Appl. Environ. Microbiol. 75:3430-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.