Abstract
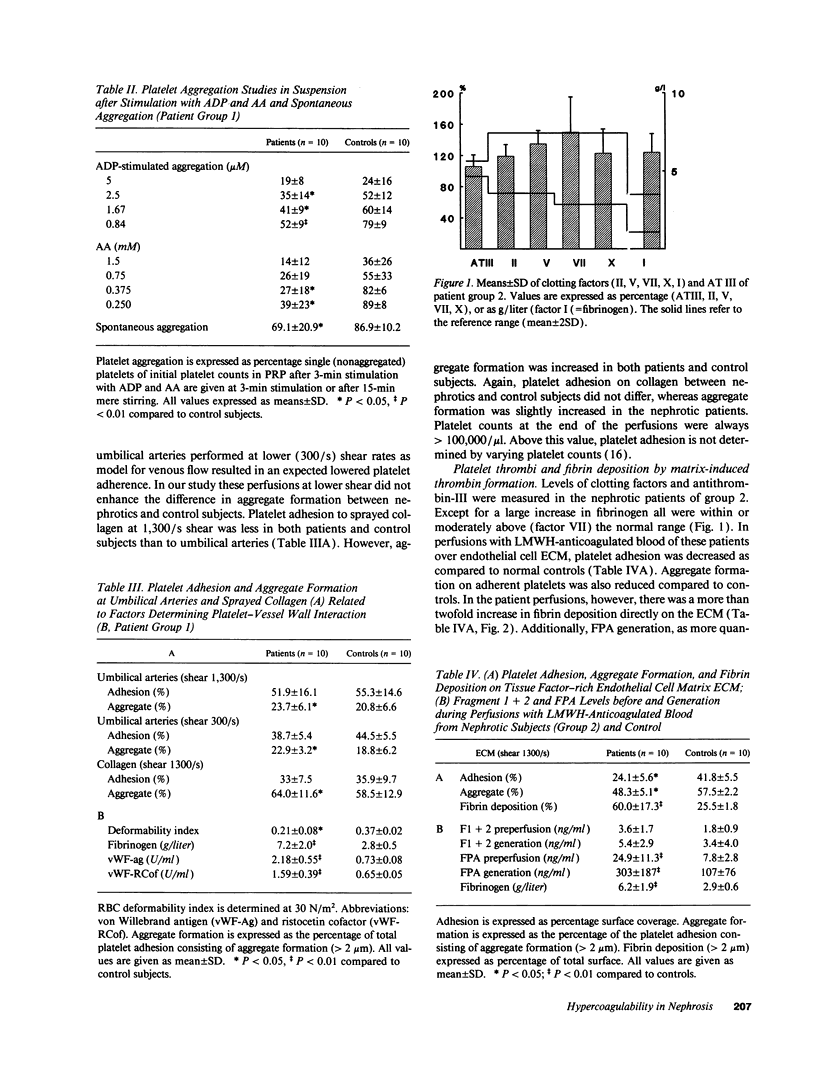
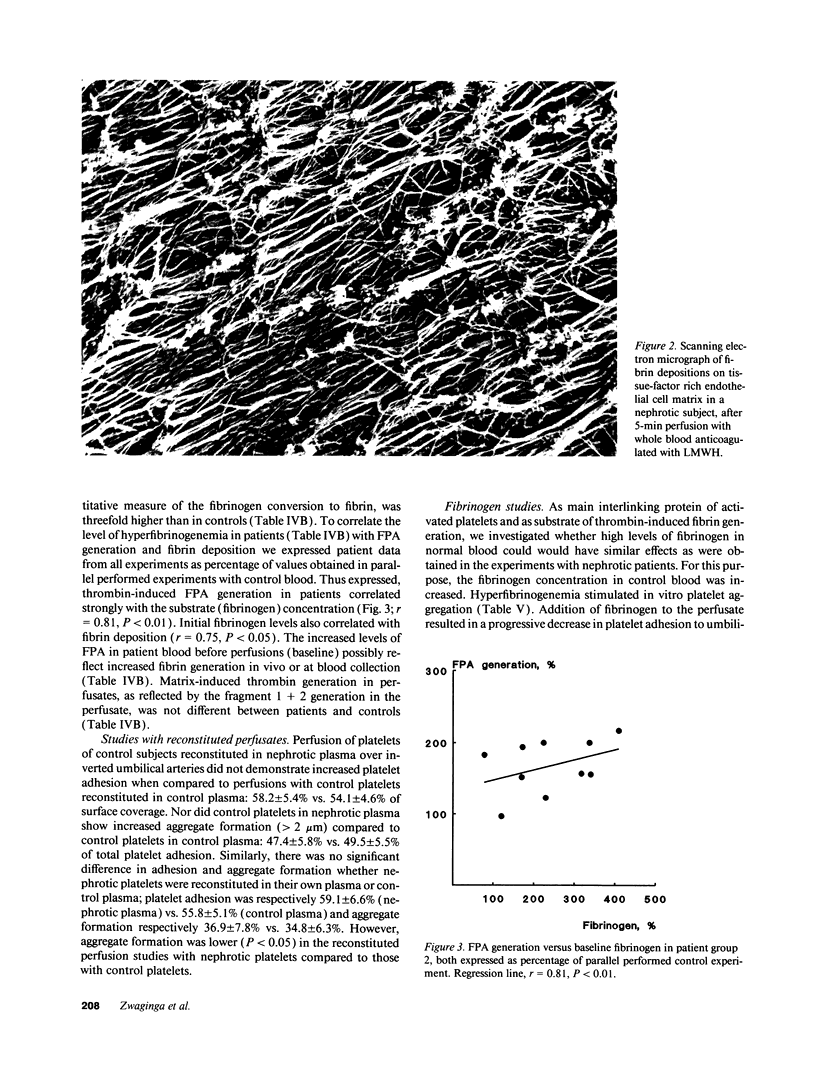
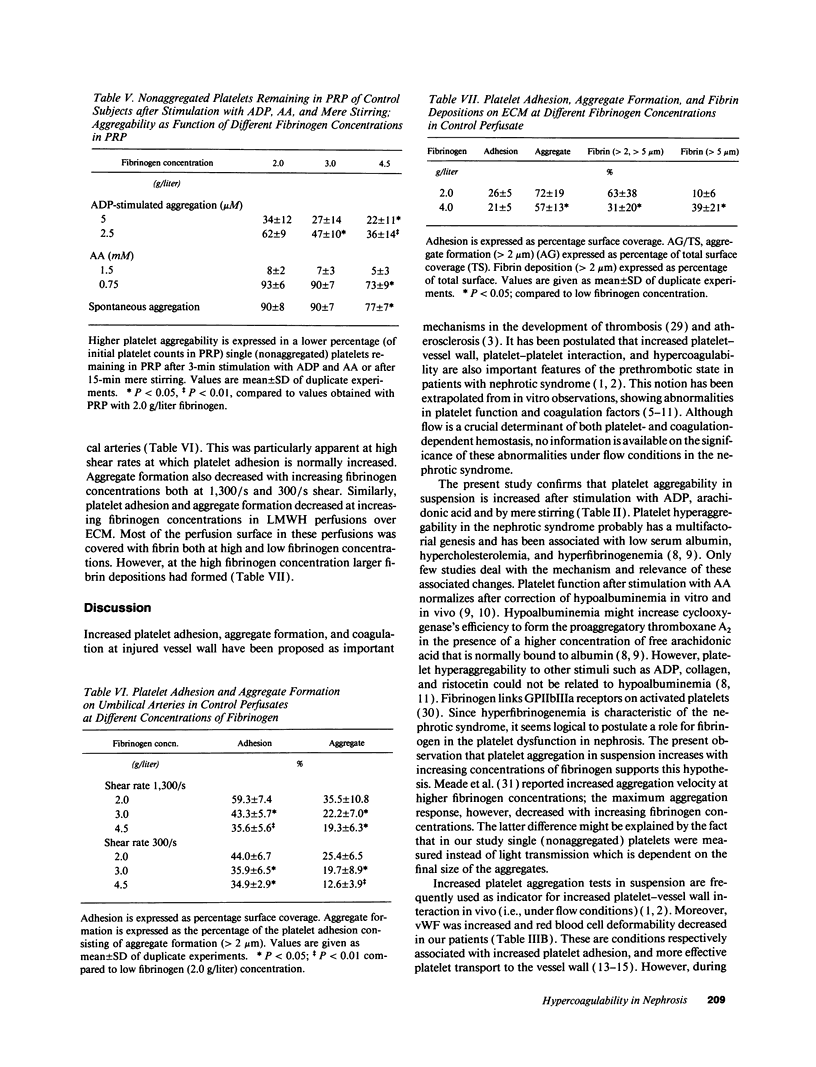
Increased in vitro platelet aggregability and hypercoagulability are generally held to be main determinants in the prethrombotic state in nephrosis. In vivo, however, thrombotic events depend on the dynamic interaction of flowing blood with the vessel wall. The present study confirms that aggregability of platelets of nephrotic patients is significantly increased by mere stirring or by exogenous stimuli as adenosine diphosphate and arachidonic acid. Moreover, the nephrotic patients have high von Willebrand factor and decreased red blood cell deformability, which normally increase platelet-vessel wall interaction. However, perfusion studies under well-defined flow conditions, in which anticoagulated nephrotic blood was exposed to deendothelialized human umbilical artery segments and sprayed collagen, showed normal platelet adhesion and only a modest increase in the deposition of platelet aggregates. This suggests that some factor counteracts platelet-vessel wall interaction under flow conditions in the nephrotic syndrome. When tissue factor associated with endothelial extracellular matrix (ECM) was allowed to generate thrombin, perfusions with nephrotic blood over this ECM resulted in a strong increase in fibrin generation. The capacity of patient blood to form increased amounts of fibrin appeared strongly correlated with the level of hyperfibrinogenemia. Platelet adhesion as well as aggregation in these experiments was even decreased below control values. This suggests that fibrin coverage may block the direct contact between blood platelets and matrix. We therefore also studied the isolated effect of high fibrinogen on platelet-vessel wall interaction by increasing fibrinogen concentrations in normal blood. Modulation of fibrinogen concentrations in normal blood could mimic all the observations in nephrotic blood: platelet aggregation in suspension increased with increasing concentrations of fibrinogen, while platelet adhesion and aggregate formation under flow conditions decreased. In perfusions over tissue factor-rich matrix, fibrin deposition increased. Therefore, our observations indicate that nephrotic hyperaggregability in suspension is not associated with increased platelet vessel wall-interaction under flow conditions. The latter is probably counteracted by high levels of fibrinogen. Our observations further suggest that hyperfibrinogenemia may be a major thrombotic risk factor in nephrosis by inducing more fibrin depositions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarts P. A., Heethaar R. M., Sixma J. J. Red blood cell deformability influences platelets--vessel wall interaction in flowing blood. Blood. 1984 Dec;64(6):1228–1233. [PubMed] [Google Scholar]
- Adler A. J., Lundin A. P., Feinroth M. V., Friedman E. A., Berlyne G. M. Beta-thromboglobulin levels in the nephrotic syndrome. Am J Med. 1980 Oct;69(4):551–554. doi: 10.1016/0002-9343(80)90466-0. [DOI] [PubMed] [Google Scholar]
- Barnes J. L., Venkatachalam M. A. The role of platelets and polycationic mediators in glomerular vascular injury. Semin Nephrol. 1985 Mar;5(1):57–68. [PubMed] [Google Scholar]
- Baumgartner H. R., Muggli R., Tschopp T. B., Turitto V. T. Platelet adhesion, release and aggregation in flowing blood: effects of surface properties and platelet function. Thromb Haemost. 1976 Feb 29;35(1):124–138. [PubMed] [Google Scholar]
- Baumgartner H. R. The role of blood flow in platelet adhesion, fibrin deposition, and formation of mural thrombi. Microvasc Res. 1973 Mar;5(2):167–179. doi: 10.1016/0026-2862(73)90069-1. [DOI] [PubMed] [Google Scholar]
- Bennett A., Cameron J. S. Platelet hyperaggregability in the nephrotic syndrome which is not dependent on arachidonic acid metabolism or on plasma albumin concentration. Clin Nephrol. 1987 Apr;27(4):182–188. [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Cameron J. S. Coagulation and thromboembolic complications in the nephrotic syndrome. Adv Nephrol Necker Hosp. 1984;13:75–114. [PubMed] [Google Scholar]
- Clark M. R., Mohandas N., Shohet S. B. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood. 1983 May;61(5):899–910. [PubMed] [Google Scholar]
- Gurewich V., Lipinski B., Hyde E. The effect of the fibrinogen concentration and the leukocyte count on intravascular fibrin deposition from soluble fibrin monomer complexes. Thromb Haemost. 1976 Dec 31;36(3):605–614. [PubMed] [Google Scholar]
- Hamer R. J., Koedam J. A., Beeser-Visser N. H., Sixma J. J. Human factor VIII: purification from commercial factor VIII concentrate, characterization, identification and radiolabeling. Biochim Biophys Acta. 1986 Oct 17;873(3):356–366. doi: 10.1016/0167-4838(86)90084-1. [DOI] [PubMed] [Google Scholar]
- Hardeman M. R., Goedhart P., Breederveld D. Laser diffraction ellipsometry of erythrocytes under controlled shear stress using a rotational viscosimeter. Clin Chim Acta. 1987 Jun 15;165(2-3):227–234. doi: 10.1016/0009-8981(87)90166-5. [DOI] [PubMed] [Google Scholar]
- Houdijk W. P., de Groot P. G., Nievelstein P. F., Sakariassen K. S., Sixma J. J. Subendothelial proteins and platelet adhesion. von Willebrand factor and fibronectin, not thrombospondin, are involved in platelet adhesion to extracellular matrix of human vascular endothelial cells. Arteriosclerosis. 1986 Jan-Feb;6(1):24–33. doi: 10.1161/01.atv.6.1.24. [DOI] [PubMed] [Google Scholar]
- Ishida T., Tanaka K. Effects of fibrin and fibrinogen-degradation products on the growth of rabbit aortic smooth muscle cells in culture. Atherosclerosis. 1982 Aug;44(2):161–174. doi: 10.1016/0021-9150(82)90111-3. [DOI] [PubMed] [Google Scholar]
- Jackson C. A., Greaves M., Patterson A. D., Brown C. B., Preston F. E. Relationship between platelet aggregation, thromboxane synthesis and albumin concentration in nephrotic syndrome. Br J Haematol. 1982 Sep;52(1):69–77. doi: 10.1111/j.1365-2141.1982.tb03862.x. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann R. H., Veltkamp J. J., Van Tilburg N. H., Van Es L. A. Acquired antithrombin III deficiency and thrombosis in the nephrotic syndrome. Am J Med. 1978 Oct;65(4):607–613. doi: 10.1016/0002-9343(78)90848-3. [DOI] [PubMed] [Google Scholar]
- Kendall A. G., Lohmann R. C., Dossetor J. B. Nephrotic syndrome. A hypercoagulable state. Arch Intern Med. 1971 Jun;127(6):1021–1027. [PubMed] [Google Scholar]
- Kuhlmann U., Steurer J., Rhyner K., von Felten A., Briner J., Siegenthaler W. Platelet aggregation and beta-thromboglobulin levels in nephrotic patients with and without thrombosis. Clin Nephrol. 1981 May;15(5):229–235. [PubMed] [Google Scholar]
- Llach F. Hypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndrome. Kidney Int. 1985 Sep;28(3):429–439. doi: 10.1038/ki.1985.149. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. E., Stibbe J., Kirby E. P., Zucker M. B., Grant R. A., McPherson J. Letter: A method for assaying von Willebrand factor (ristocetin cofactor). Thromb Diath Haemorrh. 1975 Sep 30;34(1):306–308. [PubMed] [Google Scholar]
- Machleidt C., Mettang T., Stärz E., Weber J., Risler T., Kuhlmann U. Multifactorial genesis of enhanced platelet aggregability in patients with nephrotic syndrome. Kidney Int. 1989 Dec;36(6):1119–1124. doi: 10.1038/ki.1989.310. [DOI] [PubMed] [Google Scholar]
- Meade T. W., Vickers M. V., Thompson S. G., Seghatchian M. J. The effect of physiological levels of fibrinogen on platelet aggregation. Thromb Res. 1985 Jun 1;38(5):527–534. doi: 10.1016/0049-3848(85)90185-9. [DOI] [PubMed] [Google Scholar]
- Nievelstein P. F., de Groot P. G., D'Alessio P., Heijnen H. F., Orlando E., Sixma J. J. Platelet adhesion to vascular cells. The role of exogenous von Willebrand factor in platelet adhesion. Arteriosclerosis. 1990 May-Jun;10(3):462–469. doi: 10.1161/01.atv.10.3.462. [DOI] [PubMed] [Google Scholar]
- Niiya K., Hodson E., Bader R., Byers-Ward V., Koziol J. A., Plow E. F., Ruggeri Z. M. Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation. Relationship to the binding of fibrinogen and platelet aggregation. Blood. 1987 Aug;70(2):475–483. [PubMed] [Google Scholar]
- Remuzzi G., Mecca G., Marchesi D., Livio M., de Gaetano G., Donati M. B., Silver M. J. Platelet hyperaggregability and the nephrotic syndrome. Thromb Res. 1979;16(3-4):345–354. doi: 10.1016/0049-3848(79)90082-3. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Sagripanti A., Cupisti A., Ferdeghini M., Pinori E., Barsotti G. Molecular markers of hemostasis activation in nephrotic syndrome. Nephron. 1989;51(1):25–28. doi: 10.1159/000185236. [DOI] [PubMed] [Google Scholar]
- Sakariassen K. S., Banga J. D., de Groot P. G., Sixma J. J. Comparison of platelet interaction with subendothelium of human renal and umbilical arteries and the extracellular matrix produced by human venous endothelial cells. Thromb Haemost. 1984 Aug 31;52(1):60–65. [PubMed] [Google Scholar]
- Sixma J. J., Wester J. The hemostatic plug. Semin Hematol. 1977 Jul;14(3):265–299. [PubMed] [Google Scholar]
- Thompson W. D., Smith E. B. Atherosclerosis and the coagulation system. J Pathol. 1989 Oct;159(2):97–106. doi: 10.1002/path.1711590203. [DOI] [PubMed] [Google Scholar]
- Thomson C., Forbes C. D., Prentice C. R., Kennedy A. C. Changes in blood coagulation and fibrinolysis in the nephrotic syndrome. Q J Med. 1974 Jul;43(171):399–407. [PubMed] [Google Scholar]
- Tomura S., Ideura T., Ida T., Osaka Y., Kuriyama R., Abe T., Takeuchi J. Renal vein fibrin degradation products (FDP's) in glomerulonephritis. Clin Nephrol. 1979 Dec;12(6):248–253. [PubMed] [Google Scholar]
- Verhoeven A. J., Mommersteeg M. E., Akkerman J. W. Metabolic energy is required in human platelets at any stage during optical aggregation and secretion. Biochim Biophys Acta. 1984 Aug 21;800(3):242–250. doi: 10.1016/0304-4165(84)90402-1. [DOI] [PubMed] [Google Scholar]
- Willems C., Astaldi G. C., De Groot P. G., Janssen M. C., Gonsalvez M. D., Zeijlemaker W. P., Van Mourik J. A., Van Aken W. G. Media conditioned by cultured human vascular endothelial cells inhibit the growth of vascular smooth muscle cells. Exp Cell Res. 1982 May;139(1):191–197. doi: 10.1016/0014-4827(82)90332-9. [DOI] [PubMed] [Google Scholar]
- Zwaginga J. J., Sixma J. J., de Groot P. G. Activation of endothelial cells induces platelet thrombus formation on their matrix. Studies of new in vitro thrombosis model with low molecular weight heparin as anticoagulant. Arteriosclerosis. 1990 Jan-Feb;10(1):49–61. doi: 10.1161/01.atv.10.1.49. [DOI] [PubMed] [Google Scholar]



