Abstract
Objective
To investigate the risk of uterine fibroids and other reproductive risk factors in women with hereditary leiomyomatosis and renal cell cancer (HLRCC).
Design
Case-control study.
Setting
National Institutes of Health, Rockville, Maryland.
Patients
A family-based case-control study was conducted between July 1, 2004, and June 30, 2006, including 105 women from families with HLRCC ascertained throughout North America. A telephone interview was conducted with all participants using a standardized questionnaire that elicited information about their menstrual, pregnancy, uterine fibroid, and hormonal contraceptive use history. Diagnosis of uterine fibroids was confirmed by pathologic diagnosis and by medical record review. DNA was extracted from blood samples and was screened for germline mutations in the fumarate hydratase (FH) gene.
Main Outcome Measures
FH germline mutation status, presence of uterine fibroids, age at diagnosis, and symptoms and treatment of uterine fibroids.
Results
Of 105 women, 77 reported a history of uterine fibroids. Regardless of uterine fibroid status, 75 of 105 women had a germline mutation in FH (FHmut positive). The risk of uterine fibroids in FHmut-positive women was statistically significantly increased compared with that in FHmut-negative women (odds ratio [OR], 7.6; 95% confidence interval [CI], 2.9–20.0), as it was among women clinically affected with HLRCC compared with those clinically unaffected with HLRCC (8.6; 3.1–24.0). The median age at uterine fibroid diagnosis for FHmut-positive women (28 years) was significantly younger than that for FHmut-negative women (38 years) (P=.03). Women with a germline mutation in FH or clinically affected with HLRCC reported younger age at menarche (P < .004) compared with FHmut-negative women (P =.02) or women who were clinically unaffected with HLRCC. Women with HLRCC were more likely to have had treatment for uterine fibroids (OR, 4.6; 95% CI, 1.4–15.8), including hysterectomy (P=.02) at an earlier age compared with women who were clinically unaffected with HLRCC.
Conclusions
This study provides the first evidence (to our knowledge) that women with germline mutations in FH and with clinical HLRCC have an increased risk of developing uterine fibroids. These women also have a younger age at uterine fibroid diagnosis and are more likely to have treatment for uterine fibroids at a younger age than women without HLRCC in their families.
Uterine leiomyomas (Fibroids), benign smooth muscle tumors of the uterus, are the most common gynecologic tumors in reproductive-aged women, reported in up to 77%.1–3 Approximately 25% of women with uterine fibroids report severe pain, menorrhagia, infertility, and pregnancy complications.1,4,5 As a result, uterine fibroids are the most frequent indication for hysterectomy in the United States and are a major public health problem for women of reproductive age.3,6,7 Uterine fibroids increase in prevalence with age, such that women aged 40 to 44 years are at highest risk.8 The etiology of uterine fibroids is largely unknown. However, uterine fibroids are more common among women who are obese, nulliparous, or African American or who experience menarche before age 11 years.9–12 Smoking and oral contraceptive exposure have been inconsistently reported as risk factors.8,13–18
In 1854, Virchow19 first described cutaneous leiomyomas as rare smooth muscle neoplasms of the skin. Cutaneous leiomyomas are benign mesenchymal tumors with smooth muscle differentiation. Multiple piloleiomyomas are the most common type. The other 2 main types of cutaneous leiomyomas are angioleiomyomas, which occur most frequently in the subcutis and less often in the deep dermis, and genital leiomyomas, which occur in the scrotum, vulva, and nipple areas. Cutaneous leiomyomas may manifest as a solitary lesion; as a clustered, linear, segmental arrangement sometimes involving a whole extremity; or as bilateral, symmetrical, or disseminated lesions.20,21 Cutaneous leiomyomas are found most frequently on the extremities, followed by the trunk.22 Most solitary lesions are sporadic, but in patients with a family history of cutaneous leiomyomas, this tumor represents a manifestation of an inherited predisposition. Most leiomyomas do not spontaneously regress, they may gradually increase in size, and new lesions usually develop over time.
About a century after the first description by Virchow,19 Kloepfer et al23 proposed that cutaneous leiomyomas occur in individuals from families exhibiting an autosomal dominant inheritance pattern with incomplete penetrance. In 1973, Reed et al24 described 2 kindreds in which cutaneous and uterine leiomyomas cosegregated in multiple members during 3 generations in an autosomal dominant pattern. Since then, the association between cutaneous and uterine leiomyomas has been known as Reed syndrome. Other names commonly used to describe this association include multiple cutaneous and uterine leiomyomatosis, leiomyomatosis cutis et uteri, and multiple leiomyomatosis. 22 Numerous unrelated families having 2 or more individuals with cutaneous leiomyomas have been described worldwide. In 2001, Launonen et al25 described 2 families in whom cutaneous and uterine leiomyomas and a form of papillary renal cell cancer (RCC) segregated together, which they named hereditary leiomyomatosis and renal cell cancer (HLRCC[OMIM605839]). Hereditary leiomyomatosis and RCC is recognized as the autosomal dominant predisposition to the development of cutaneous and/or uterine leiomyomas and RCC.25–27 The susceptibility gene for HLRCC was mapped to the long arm of chromosome 1 in band q42 and was subsequently identified as the fumarate hydratase (FH) gene (Genbank NM000143), which encodes the FH enzyme.25–28 In the identification of FH as the HLRCC susceptibility gene, cutaneous leiomyomas were clinically used as a marker of disease status, as these cutaneous lesions are rare in the general population but are the most sensitive and specific clinical marker of the disease among the clinical manifestations of HLRCC.25–28 In 2003, investigators at the National Cancer Institute described the first 35 families in North America affected with HLRCC,27 and 55 families have been described by the National Cancer Institute group.29,30 Approximately 76% of families with HLRCC have individuals with cutaneous leiomyoma (1 to >100 lesions).27,30 Therefore, there is variable expression of cutaneous manifestations in HLRCC, ranging from absent to severe involvement. However, cutaneous leiomyomas are pathognomonic for HLRCC. Previous studies27,30 showed that 98% to 100% of women with cutaneous leiomyomas had uterine leiomyomas. Most important, women with HLRCC were diagnosed as having uterine fibroids at a mean age of 30 years, and more than 50% underwent hysterectomy for symptomatic uterine fibroids at or before age 30 years.27
Previously, the association of cutaneous and uterine leiomyomas was assumed based on descriptions of families with cosegregation of both traits, but the presumed association was never evaluated formally.24–28 Therefore, we designed a family-based case-control study to investigate whether women with a germline mutation in FH have an increased risk of uterine fibroids, report a younger age at uterine fibroid diagnosis and treatment, and have more treatments, as well as whether they have more uterine fibroid symptoms compared with women in their families without HLRCC or without a germline mutation in FH. Assessing the risk of uterine leiomyomas in women with a germline mutation in FH is important because the prevalence of uterine fibroids is high among the general population. To our knowledge, this is the first epidemiologic study to evaluate the risk and severity of uterine fibroids in women with HLRCC.
METHODS
PATIENT ASCERTAINMENT AND STUDY DESIGN
Fifty-six families with HLRCC from the United States and Canada were ascertained on the basis of the presence of cutaneous leiomyomas (52 families) or a history of RCC (4 families). All subjects were enrolled in a protocol studying HLRCC approved by the institutional review board of the National Cancer Institute, Rockville, Maryland. Each patient provided written informed consent before participation. From October 26, 2004, to June 1, 2006, women from these families were invited by mail to participate in a reproductive health study that included a telephone interview, medical record review, and blood drawing. If a woman had not responded after 2 weeks, she was contacted by telephone. If a woman declined participation, no further contact occurred. If she agreed to participate, a telephone interview was scheduled. Of 131 women identified through review of the family medical histories, 12 women had died (10 of RCC and 2 of other causes), and 2 women could not be located. Of 117 eligible women, 10 declined participation, and 107 agreed to participate (initial response rate, 91.5%). Ultimately, 105 women were interviewed (final response rate, 89.7%).
DEFINITIONS
A family with HLRCC was defined as a family of 2 or more individuals (first- and second-degree relatives) having at least 1 individual with clinical HLRCC. A case was defined as a woman from a family with at least 1 blood relative with HLRCC who reported having a diagnosis of uterine fibroids, whereas a control was defined as a woman from the same family who did not report a history of uterine fibroids. Exposure groups were based on FH germline mutation status: exposed had a germline mutation in FH (FHmut-positive women), while unexposed did not (FHmut-negative women). In addition, each woman was classified as having or as not having a clinical diagnosis of HLRCC. The clinical diagnosis of HLRCC was defined by the presence of pathologically confirmed cutaneous leiomyomas or RCC with specific histologically confirmed HLRCC.27 All women having a clinical diagnosis of HLRCC had an FH germline mutation.
SEQUENCING OF THE FH GENE
Blood samples were obtained from interviewed women, and DNA was extracted from peripheral blood leukocytes according to standard procedures.27 The genomic sequence containing FH was determined by BLAT (BLAST [basic local alignment search tool]-like alignment tool) alignment of the mitochondrial FH precursor complementary DNA (with the assembled genomic sequence (Building 34; National Center for Biotechnology Information, Bethesda, Maryland). Methods for identification of exon and intron boundaries and for high-throughput DNA sequencing were as previously described.27
TELEPHONE INTERVIEW
A telephone interview was conducted using a standardized questionnaire that elicited information regarding various aspects of reproductive history, including pregnancy, uterine fibroids, uterine surgery, hormonal contraceptive use, and menstruation (age at onset, duration, and symptoms). The menstrual history section asked women about their early reproductive life before pregnancy or hormonal contraceptive use. Questions were also asked about demographics, smoking, hypertension, and cutaneous leiomyoma and RCC history. To evaluate the morbidity associated with uterine fibroids, women were asked about their age at diagnosis, uterine fibroid symptoms, age at symptom manifestation, uterine fibroid treatment and age at treatment, and the presence of single or multiple uterine fibroids at diagnosis. Two interviewers (L.S. and an experienced assistant) blinded to the patient’s germline mutation and uterine fibroid status administered the questionnaire, which took approximately 30 to 45 minutes. Medical records were obtained for review of radiologic, surgical, or pathologic reports, and histologic slides were obtained to confirm uterine fibroid diagnosis.
STATISTICAL ANALYSIS
Questionnaire data were coded and double-keyed by Westat, Inc (Rockville, Maryland). Analyses were conducted using 2-sided tests (SAS 8.0; SAS Institute Inc, Cary, North Carolina). Participant characteristics were compared between exposure groups using χ2 tests. Confounding variables were chosen from previously published associations in the literature or from significant differences noted in the study. For variables that showed statistical differences, effect modification was assessed. Kruskal-Wallis tests were used to determine the statistical significance of variables such as age at diagnosis and age at first surgical treatment. Age group comparisons were based on published data when they were available as a continuous variable, or if no published data were available, the median of the control population was used. Logistic regression analysis was used to identify whether combined factors were jointly associated with the presence or absence of uterine fibroids. We conducted 2 main analyses. The first analysis compared women with and without a germline mutation in FH to evaluate the relationship between uterine fibroids and the presence or absence of an FH germline mutation. The second analysis compared women with and without a clinical diagnosis of HLRCC. All women in the study who had a clinical diagnosis of HLRCC had an FH germline mutation.
RESULTS
MUTATION SCREENING
We identified FH germline mutations in 100.0% (56 of 56) of families with HLRCC.27,29,30 Each missense mutation cosegregated with disease and was absent in more than 160 controls. Seventy-five were FHmut-positive women, while 30 were FHmut-negative women.
GENERAL PATIENT CHARACTERISTICS
Regardless of uterine fibroid status, 75 women were FHmut positive; 60 of them had clinical features of HLRCC, and the other 15 had no clinical evidence of HLRCC. None of 30 women without germline mutations in FH had cutaneous leiomyomas or HLRCC-associated RCC. Of 60 women clinically affected with HLRCC, 59 had cutaneous leiomyomas (9 of whom also had HLRCC-associated RCC), and 1 had HLRCC-associated RCC but no cutaneous leiomyomas. Forty-five women (median age, 42.5 years), including all 30 FHmut-negative women, were clinically unaffected with cutaneous leiomyomas or RCC.
Of 105 women, 77 reported a history of uterine fibroids, and 28 did not. The diagnosis of uterine fibroids was confirmed in 97.4% (75 of 77) by medical records and in 89.6% (69 of 77) by pathologic review of slides obtained after hysterectomy or myomectomy (Figure). Medical records were unavailable for the 2 women whose uterine fibroid diagnosis was not confirmed. Mild histologic atypia was reported in 6 uterine fibroid cases, but there were no reports of leiomyosarcoma of the uterus. Renal cell cancer was present in 10 women; all cases occurred before ascertainment of the families and were histologically confirmed. The diagnosis of cutaneous leiomyoma was histologically confirmed in all 59 women who reported having cutaneous leiomyomas. Table 1 gives the demographic characteristics for all participants. More than 90% of women were white, and the age at interview ranged from 19 to 81 years.
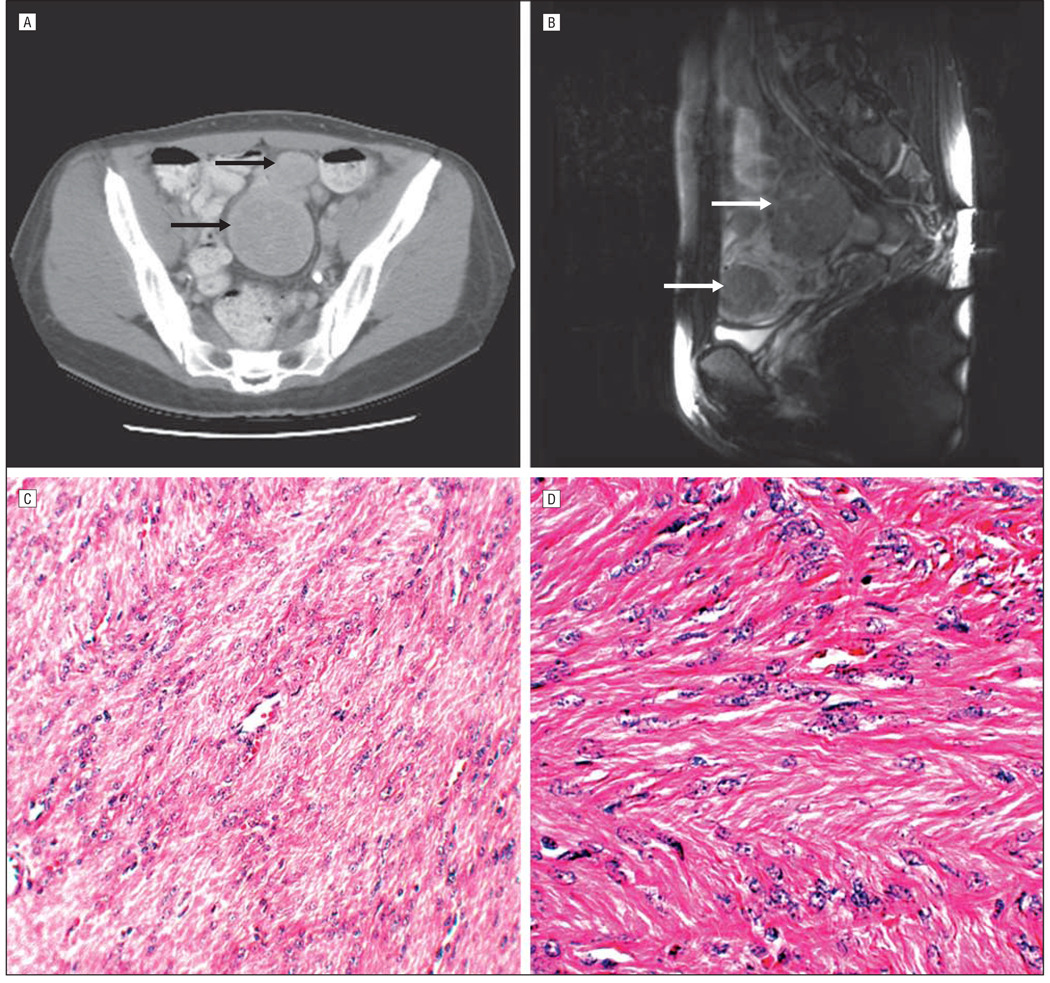
Figure.
Uterine leiomyomas in women with hereditary leiomyomatosis and renal cell cancer in the study. Magnetic resonance images show axial view (A) and sagittal view (B) of multiple uterine leiomyomas. Arrows indicate the masses that represent uterine leiomyomas. C and D, Histopathologic findings of uterine leiomyomas. C, Microscopic examination shows interlacing fascicles of bland smooth muscle cells (hematoxylin-eosin, original magnification ×20). D, Microscopic examination shows interlacing smooth muscle cells with eosinophilic cytoplasm and uniform blunt-ended cigar-shaped nuclei (hematoxylin-eosin, original magnification ×40).
Table 1.
Distribution and Significance Testing of Demographic Characteristics and Smoking History, FH Germline Mutation Status, and Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) Clinical Status Among 105 Women
| Germline Mutation Status, No. (%) | HLRCC Clinical Status, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable |
FH Positive (n=75) |
FH Negative (n=30) |
P Value |
Affected (n=60) |
Unaffected (n=45) |
P Value |
||
| Age at questionnaire, y | ||||||||
| <30 | 12 (16.0) | 5 (16.7) | .30 | 3 (5.0) | 14 (31.1) | .001 | ||
| 30–39 | 12 (16.0) | 8 (26.7) | 10 (16.7) | 10 (22.2) | ||||
| 40–49 | 22 (29.3) | 4 (13.3) | 19 (31.7) | 7 (15.6) | ||||
| ≥50 | 29 (38.7) | 13 (43.3) | 28 (46.7) | 14 (31.1) | ||||
| Median | 45 | 42.5 | 48 | 39 | ||||
| Mean (SD) | 46.5 (14.3) | 45.9 (17.2) | 49.6 (13.3) | 42.4 (16.4) | ||||
| Minimum/maximum | 19/79 | 19/81 | 19/79 | 19/81 | ||||
| Race/ethnicitya | ||||||||
| American Indian | 4 (5.3) | 0 | .18 | 4 (6.7) | 0 | .07 | ||
| Asian | 0 | 1 (3.3) | 0 | 1 (2.2) | ||||
| White | 68 (90.7) | 29 (96.7) | 55 (91.7) | 42 (93.3) | ||||
| Hispanic | 2 (2.7) | 0 | 0 | 2 (4.4) | ||||
| Smoking history | ||||||||
| Yes | 34 (45.3) | 12 (40.0) | .62 | 25 (41.7) | 21 (46.7) | .61 | ||
| No | 41 (54.7) | 18 (60.0) | 35 (58.3) | 24 (53.3) | ||||
| Duration of smoking, y | ||||||||
| ≤11 | 11 (36.7) | 3 (27.3) | .58 | 4 (19.0) | 10 (50.0) | .04 | ||
| >11 | 19 (63.3) | 8 (72.7) | 17 (81.0) | 10 (50.0) | ||||
Some totals do not sum to column totals because of missing data.
COMPARISON BY FH GERMLINE MUTATION STATUS
Smoking, Menstrual, Pregnancy, and Hormonal Contraceptive Use History
Smoking history among FHmut-positive and FHmut-negative women did not differ for ever or never smoking, number of cigarettes per day, smoking regularly for at least 1 year, total number of years smoking (Table 1), or pack-years (data not shown).
Menstrual, pregnancy, and hormonal contraceptive use history are given in Table 2. The age of onset of menarche was significantly younger in FHmut-positive women compared with FHmut-negative women (P=.02). Similarly, the age of onset of menarche was significantly younger in women clinically affected with HLRCC compared with those clinically unaffected with HLRCC (P =.004).However, no differences were observed in menstrual cycle pattern or symptoms or in contraceptive use or duration between comparison groups. Approximately 75% of women in each comparison group used hormonal contraceptives for at least 1 year. Most of these women used a combination of estrogen and progesterone.
Table 2.
Distribution and Significance Testing of Menstrual, Contraceptive Use, and Pregnancy History Characteristics by FH Germline Mutation Status and by Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) Clinical Status Among 105 Womena
| Germline Mutation Status | HLRCC Clinical Status | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable |
FH Positive (n=75) |
FH Negative (n=30) |
P Value |
Affected (n=60) |
Unaffected (n=45) |
P Value |
||
| Age at menarche, No. (%), y | ||||||||
| ≤12 | 23 (46.9) | 6 (27.3) | .02 | 20 (52.6) | 9 (27.3) | .004 | ||
| 13–15 | 19 (38.8) | 16 (72.7) | 12 (31.6) | 23 (69.7) | ||||
| >15 | 7 (14.3) | 0 | 6 (15.8) | 1 (3.0) | ||||
| Self-reported period flow, No. (%) | ||||||||
| Light | 7 (9.5) | 4 (13.3) | .74 | 5 (8.5) | 6 (13.3) | .57 | ||
| Medium | 45 (60.8) | 16 (53.3) | 37 (62.7) | 24 (53.3) | ||||
| Heavy | 22 (29.7) | 10 (33.3) | 17 (28.8) | 15 (33.3) | ||||
| Self-reported pain during menstruation, No. (%) | ||||||||
| None | 19 (25.3) | 5 (16.7) | .15 | 16 (26.7) | 8 (17.8) | .47 | ||
| Mild | 15 (20.0) | 11 (36.7) | 13 (21.7) | 13 (28.9) | ||||
| Moderate | 20 (26.7) | 10 (33.3) | 15 (25.0) | 15 (33.3) | ||||
| Severe | 21 (28.0) | 4 (13.3) | 16 (26.7) | 9 (20.0) | ||||
| Irregular cycles, No. (%) | ||||||||
| Always regular | 58 (77.3) | 20 (66.7) | .44 | 49 (81.7) | 29 (64.4) | .14 | ||
| Sometimes irregular | 7 (9.3) | 3 (10.0) | 4 (6.7) | 6 (13.3) | ||||
| Always irregular | 10 (13.3) | 7 (23.3) | 7 (11.7) | 10 (22.2) | ||||
| Used contraceptives ≥1 y, No. (%) | ||||||||
| Yes | 62 (82.7) | 26 (86.7) | .62 | 50 (83.3) | 38 (84.4) | .88 | ||
| No | 13 (17.3) | 4 (13.3) | 10 (16.7) | 7 (15.6) | ||||
| Contraceptive type, No. (%) | ||||||||
| Hormonal | 57 (91.9) | 22 (84.6) | .30 | 45 (90.0) | 34 (89.5) | .94 | ||
| Nonhormonal | 5 (8.1) | 4 (15.4) | 5 (10.0) | 4 (10.5) | ||||
| Used contraceptives, No. (%) | ||||||||
| Hormonal | 57 (76.0) | 22 (73.3) | .78 | 45 (75.0) | 34 (75.6) | .95 | ||
| Never used hormonal or nonhormonal contraceptives | 18 (24.0) | 8 (26.7) | 15 (25.0) | 11 (24.4) | ||||
| Duration of contraceptive use, y | ||||||||
| Median | 6.5 | 6.0 | 6.0 | 6.5 | ||||
| Mean (SD) | 7.2 (5.6) | 7.4 (5.5) | 7.3 (5.8) | 7.2 (5.1) | ||||
| Minimum/maximum | 1/23 | 1/20 | 1/23 | 1/20 | ||||
| Duration of hormone use, No. (%), y | ||||||||
| ≤6.5 | 25 (50.0) | 10 (52.6) | .85 | 20 (51.3) | 15 (50.0) | .92 | ||
| >6.5 | 25 (50.0) | 9 (47.4) | 19 (48.7) | 15 (50.0) | ||||
| History of pregnancy, No. (%) | ||||||||
| Yes | 58 (77.3) | 20 (66.7) | .26 | 44 (73.3) | 34 (75.6) | .80 | ||
| No | 17 (22.7) | 10 (33.3) | 16 (26.7) | 11 (24.4) | ||||
| Trouble getting pregnant among those trying to get pregnant, No. (%) | ||||||||
| Yes | 15 (23.8) | 6 (21.4) | .80 | 12 (25.0) | 9 (20.9) | .65 | ||
| No | 48 (76.2) | 22 (78.6) | 36 (75.0) | 34 (79.1) | ||||
| Parity, No. (%) | ||||||||
| Nulliparous | 27 (36.0) | 10 (33.3) | .79 | 22 (36.7) | 15 (33.3) | .72 | ||
| Parous | 48 (64.0) | 20 (66.7) | 38 (63.3) | 30 (66.7) | ||||
| No. of full-term or near-full-term live births | ||||||||
| Median | 2.0 | 2.5 | 2.0 | 2.0 | ||||
| Mean (SD) | 2.0 (1.3) | 2.7 (1.5) | 2.1 (1.3) | 2.2 (1.5) | ||||
| Minimum/maximum | 0/5 | 1/7 | 0/5 | 0/7 | ||||
| No. of live births delivered by cesarean section, No. (%) | ||||||||
| 0 | 33 (68.8) | 15 (75.0) | .61 | 26 (68.4) | 22 (73.3) | .66 | ||
| ≥1 | 15 (31.3) | 5 (25.0) | 12 (31.6) | 8 (26.7) | ||||
| Pregnancy complications, No. (%) | ||||||||
| Yes | 22 (38.6) | 10 (52.6) | .29 | 15 (34.9) | 17 (51.5) | .15 | ||
| No | 35 (61.4) | 9 (47.4) | 28 (65.1) | 16 (48.5) | ||||
Some variable totals do not sum to column totals because of missing data. Percentages are based on the variable totals.
No differences were observed in parity, pregnancy history, trouble getting pregnant, or pregnancy complications between comparison groups. These results are summarized in Table 2.
Uterine Fibroid Risk Assessment
FHmut-positive women had a significantly increased risk of uterine fibroids compared with FHmut-negative women (odds ratio [OR], 7.6; 95% confidence interval [CI], 2.9–20.0) (Table 3). When stepwise logistic regression analysis was conducted for uterine fibroid risk among FHmut-negative vs FHmut-positive women incorporating age at interview, smoking duration, hormonal contraceptive use, and age or school grade at menarche in the model, only age at interview remained, altering the previous OR slightly (OR, 7.9; 95% CI, 2.9–22.0).
Table 3.
Odds Ratios for Uterine Leiomyoma According to FH Germline Mutation Status and Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) Clinical Status Among 105 Women
| No. (%) | |||
|---|---|---|---|
| Variable | Uterine Fibroid Positive (n=77) |
Uterine Fibroid Negative (n=28) |
Odds Ratio (95% Confidence Interval) |
| Germline mutation status | |||
| FH positive | 64 (83.1) | 11 (39.3) | 7.6 (2.9–20.0) |
| FH negative | 13 (16.9) | 17 (60.7) | |
| HLRCC clinical status | |||
| Affected | 54 (70.1) | 6 (21.4) | 8.6 (3.1–24.0) |
| Unaffected | 23 (29.9) | 22 (78.6) | |
| Combined FH germline mutation status and HLRCC clinical status |
|||
| FH positive and HLRCC unaffected |
10 (13.0) | 5 (17.9) | 4.5 (1.0–21.6) |
| FH positive and HLRCC affected |
54 (70.1) | 6 (21.4) | 1 [Reference] |
| FH negative and HLRCC unaffected |
13 (16.9) | 17 (60.7) | 11.8 (3.5–42.1) |
In addition, FHmut-negative women who used hormonal contraceptives showed a reduced risk of uterine fibroids. However, this finding was not statistically significant (OR, 0.3; 95% CI, 0.1–1.8) (data not shown, P =.02). These results should be viewed with caution, and this area needs to be studied further because sufficiently detailed information was unavailable to determine whether hormonal contraceptives were used for treatment of uterine fibroids.
Uterine Fibroid Outcomes, Including Age at Diagnosis, Symptoms, and Treatment
The median age at uterine fibroid diagnosis for FHmut-positive women was significantly younger compared with that for FHmut-negative women (28 vs 38 years, P=.03). Moderate or severe pain was more commonly reported by FHmut-positive women than by FHmut-negative women who underwent myomectomy and hysterectomy (61% vs 39% and 60% vs 25%, respectively) (data not shown). These symptoms may have contributed to the age at uterine fibroid diagnosis. Knowledge of the number of uterine fibroids was unavailable for many women and could not be considered for its effect on risk of treatment.
COMPARISON BY HLRCC CLINICAL STATUS
Women clinically affected with HLRCC were significantly older (P=.001) and reported smoking longer (≤11 vs > 11 years, P=.04) compared with women clinically unaffected with HLRCC (Table 1). Women clinically affected with HLRCC reported younger age at menarche than women clinically unaffected with HLRCC (P=.004) (Table 2). Women with clinical HLRCC who used hormonal contraceptives had a significantly increased risk of uterine fibroids (OR, 7.8; 95% CI, 1.3–48.3). Women without clinical HLRCC who used hormonal contraceptives showed a reduced risk of uterine fibroids, which was not statistically significant (OR, 0.3; 95% CI, 0.05–2.4) (data not shown, P =.20). These results need to be studied further because sufficiently detailed information was unavailable to determine whether hormonal contraceptives were used for treatment of uterine fibroids.
The risk of uterine fibroids in women with a clinical diagnosis of HLRCC was significantly elevated compared with that in women without a clinical diagnosis of HLRCC (OR, 8.6; 95% CI, 3.1–24.0) (Table 3). Compared with women who were HLRCC unaffected and FHmut positive and with women who were HLRCC unaffected and FHmut negative, women who were HLRCC affected and FHmut positive showed a significantly increased risk of uterine fibroids (OR, 4.5; 95% CI, 1.0–21.6; and 11.8; 3.5–42.1, respectively). When stepwise logistic regression analysis was conducted for uterine fibroid risk among women affected with HLRCC vs women unaffected with HLRCC, none of the potential confounding variables contributed to clinical status.
Women affected clinically with HLRCC were significantly more likely to have had surgical treatment for uterine fibroids (OR, 4.6; 95% CI, 1.2–17.8; P=.02) compared with women who were unaffected clinically with HLRCC (Table 4). Women clinically affected with HLRCC were first treated for uterine fibroids at a younger median age than women clinically unaffected with HLRCC (31.5 vs 37.0 years, P=.03), reporting a younger age at hysterectomy (≤42.5 vs >42.5 years, P=.02).
Table 4.
Odds Ratios for Uterine Leiomyoma According to Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) Clinical Status Among 77 Women With a History of Uterine Fibroids
| HLRCC Clinical Status, No. |
Odds Ratio (95% Confidence Interval) |
|||
|---|---|---|---|---|
| Variable | Affected | Unaffected | ||
| Age at diagnosis, ya | ||||
| ≤38 | 44 | 14 | 3.1 (0.9–10.9) | |
| >38 | 9 | 9 | ||
| Treatment | ||||
| Yes | 45 | 12 | 4.6 (1.4–15.8) | |
| No | 9 | 11 | ||
| 1 Surgery | 30 | 8 | 4.6 (1.2–17.8) | |
| No surgery | 9 | 11 | ||
| >1 Surgery | 14 | 3 | 5.7 (1.0–35.6) | |
| No surgery | 9 | 11 | ||
| Myomectomy | ||||
| Yes | 20 | 6 | 0.8 (0.2–3.4) | |
| No | 25 | 6 | ||
| Age at myomectomy, y | ||||
| ≤35.5 | 19 | 3 | 19.0 (1.1–709.5) | |
| >35.5 | 1 | 3 | ||
| Hysterectomy | ||||
| Yes | 38 | 8 | 2.7 (0.5–14.3) | |
| No | 7 | 4 | ||
| Age at hysterectomy, y | ||||
| ≤42.5 | 35 | 4 | 11.7 (1.4–111.7) | |
| >42.5 | 3 | 4 | ||
Only 76 responses were received.
COMMENT
To our knowledge, this is the first epidemiologic study to investigate reproductive health risk factors in women with HLRCC. We showed that women with an FH germline mutation and women with clinical HLRCC (cutaneous leiomyomas or HLRCC kidney cancer) have an 8- to 9-fold increased risk of developing uterine fibroids. Since the original description in 1973 by Reed et al,24 the association of cutaneous and uterine leiomyomas was assumed based on descriptions of families with cosegregation of both traits, but the presumed association was never formally evaluated.25–28
In families with HLRCC, the risk assessment, baseline screening, surveillance, and clinical management depend on accurate identification of individuals at risk. Because cutaneous leiomyomas are the earliest and most common manifestation, dermatologists are frequently the first physicians seen by patients with HLRCC. The presence of multiple cutaneous leiomyomas or family history of RCC, cutaneous leiomyomas, or early-onset symptomatic uterine fibroids should raise a high level of suspicion of HLRCC. Establishing the correct diagnosis of cutaneous leiomyoma is an initial step in the clinical diagnosis of HLRCC. This can be challenging for the dermatologist, especially when patients have a single lesion or a few cutaneous leiomyomas. Quantifying the risk of uterine fibroids is of critical importance to women with HLRCC. We assessed the risk of uterine fibroids by FH germline mutation carrier status and by HLRCC clinical status in women in this study, among whom 80% (60 of 75) of women with an FH germline mutation had cutaneous leiomyomas. Therefore, it is important for dermatologists to be familiar with the quantified risk of uterine fibroids in women with HLRCC and to refer them for appropriate gynecologic evaluation.
The prevalence of uterine fibroids increases with age, becoming most prevalent in the general population among women aged 40 to 44 years.12 However, in this study, uterine fibroids were diagnosed at a significantly younger age in women with a germline mutation in FH compared with those without a germline mutation in FH (median, 28 vs 38 years). This is also significantly younger than the age in the general population.12 Women with HLRCC had a 5-fold increased risk of having treatment for uterine fibroids and were younger than women without HLRCC at myomectomy or hysterectomy. Almost two-thirds of women who had surgical treatment reported moderate to severe pain before surgery. In our study overall, 73.3% of HLRCC-affected women and 74.4% of FHmut-positive women reported dysmenorrhea and pain, symptoms previously described with uterine fibroids31,32 and at much higher rates than the 20% to 50% reported in the general population.1,3,32
In this study, we also found that women with an FH germline mutation, and specifically women with clinical HLRCC, experienced menarche at a younger age than women without an FH germline mutation or women without clinical HLRCC. Early menarche has been previously reported to increase the risk of developing uterine fibroids.8,17,33 Menorrhagia, the most frequently reported symptom in women with uterine fibroids,34 was common among our study population but did not differ between the comparison groups. This is not surprising because uterine bleeding is difficult to quantify accurately by verbal reports. Other menstrual cycle characteristics did not differ between the comparison groups.
Women with HLRCC who used hormonal contraceptives seemed to be at a significantly increased risk of developing uterine fibroids. Given the early age at uterine fibroid diagnosis, the high proportion with symptoms and treatment, and the small sample size of affected with HLRCC, it is difficult to know whether the use of hormonal contraceptives led to the development of uterine fibroids or was merely a treatment for symptoms. Women without an FH germline mutation and women who were clinically unaffected by HLRCC had a lower risk of having uterine fibroids associated with hormonal contraceptive use, an observation similar to that in the general population.8 These findings could be better assessed and confirmed with a longitudinal study rather than a cross-sectional study.
Some evidence suggests that uterine fibroids may cause infertility.15 Neither parity nor infertility was associated with uterine fibroids in this study. Pregnancy has been postulated to be protective against developing uterine fibroids, as increasing parity is inversely associated with uterine fibroids.10 The high proportion of women with clinical HLRCC reporting that they were not trying to get pregnant and the small sample size may have hampered our ability to detect an increased risk of infertility. Consistent with previous findings but in contrast to others,25 women with HLRCC in this study did not develop uterine leiomyosarcoma. This issue requires further study because the risk of leiomyosarcoma has important surveillance and management implications for women considering hysterectomy for uterine fibroids. The sample size and cross-sectional nature of our study precluded further evaluation of this question.
In this study of women with HLRCC, a germline mutation in FH seems to increase susceptibility for uterine fibroids. Familial clustering of uterine fibroids in the general population suggests that uterine fibroids may have a significant genetic component.35–37 In 2004, genetic linkage analysis of 123 families with uterine fibroids (but not HLRCC) and with at least 1 affected sib pair suggested FH locus linkage to the long arm of chromosome 1 in band q42 among women younger than 40 years at diagnosis.38 Using fluorescent in situ hybridization, a copy of FH was absent in 9 of 11 uterine fibroid samples from women in these families.38 In another study,39 single and biallelic somatic FH inactivation was reported in some sporadic uterine fibroids. Overall, laboratory investigations demonstrate a loss of heterozygosity mapped to the long arm of chromosome 1 in band q42 around the FH locus in HLRCC-associated uterine fibroids, suggesting that FH acts as a tumor suppressor gene.40 Hypoxia pathways may also be involved in the development of uterine fibroids, as fibroids exhibit and upregulation of HIF1 targets such as VEGF (vascular endothelial growth factor) and BNIP1 (BCL2/adenovirus E1B 19-kDa interacting protein 1) and downregulation of TSP1.41 In addition, in vitro investigations indicate that increased intracellular fumarate directly impairs HIF prolyl hydroxylase and leads to accumulation of HIF.42 These data suggest that loss of FH might be an important event in the pathogenesis of a subset of familial uterine fibroids.
Hereditary leiomyomatosis and renal cell cancer is a rare syndrome, which limited the sample size in this study. However, the high response rate (89.7%) allowed us to identify that women with HLRCC have an increased risk of developing uterine fibroids. The small sample size did not allow sufficient power to detect effects by stratification or condition on family in the analyses. Because participants were selected from families with HLRCC evaluated at the National Institutes of Health, our study could be biased toward women who sought medical care or who had symptoms. Furthermore, recall bias is a limitation in studies requiring women to remember specific dates and symptoms of major reproductive events such as menstrual, pregnancy, and contraceptive use history. This was a cross-sectional study of families, and young FH germline mutation carriers may develop uterine fibroids in the future. Prospective follow-up of families with HLRCC will be required to evaluate these issues further and to determine their effects on the results.
CONCLUSIONS
To our knowledge, this study provides the first statistical evidence that women with germline mutations in FH and women with clinical HLRCC have an increased risk of developing uterine fibroids, are diagnosed as having uterine fibroids at an earlier age, and are more likely to have treatment for uterine fibroids at a younger age than their family members without clinical HLRCC or without germline mutations in FH. Therefore, HLRCC-associated uterine fibroids may represent a distinct gynecologic entity. Cutaneous leiomyoma is the most sensitive and specific clinical marker of HLRCC.25–30 Therefore, if genetic testing is unavailable, a dermatologic examination for cutaneous leiomyomas can be used to identify high-risk women with HLRCC. Women with cutaneous leiomyomas may be informed of their uterine fibroid risk. In addition, if genetic testing is available, it may be used to detect high-risk women in these families, especially women without cutaneous manifestations. Awareness of the diagnosis of HLRCC also benefits patients as an alert to the need to screen for renal tumors, as patients with HLRCC are also at risk of aggressive renal cell cancer. Our study contributes to the understanding of the genetic basis of hereditary uterine fibroids. Future investigation of HLRCC will help to elucidate the etiology of HLRCC-associated uterine fibroids and the role of FH germline mutations among women in the general population.
Acknowledgments
Funding/Support: This study was supported by intramural programs (Division of Cancer Epidemiology and Genetics and Center for Cancer Research) of the National Cancer Institute, National Institutes of Health.
Footnotes
Author Contributions: Dr Toro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Stewart, Glenn, Stratton, Tucker, Linehan, and Toro. Acquisition of data: Stewart, Glenn, Linehan, and Toro. Analysis and interpretation of data: Stewart, Glenn, Stratton, Goldstein, Merino, Tucker, and Toro. Drafting of the manuscript: Stewart, Glenn, Stratton, and Toro. Critical revision of the manuscript for important intellectual content: Stewart, Glenn, Stratton, Goldstein, Merino, Tucker, and Toro. Statistical analysis: Stewart and Goldstein. Obtained funding: Tucker and Toro. Administrative, technical, and material support: Stewart, Merino, and Toro. Study supervision: Glenn, Stratton, Tucker, Linehan, and Toro.
Financial Disclosure: None reported.
Publisher's Disclaimer: Disclaimer: All authors work for the US government and do not have conflicts of interest with the content or results in this publication. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Additional Contributions: Members of the American Academy of Dermatology helped in the recruitment of families. We thank the women and their families with HLRCC for their participation in our study.
REFERENCES
- 1.Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 2.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94(4):435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 3.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 4.Brahma PK, Martel KM, Christman GM. Future directions in myoma research. Obstet Gynecol Clin North Am. 2006;33(1):199–224. doi: 10.1016/j.ogc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Casini ML, Rossi F, Agostini R, Unfer V. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006;22(2):106–109. doi: 10.1080/09513590600604673. [DOI] [PubMed] [Google Scholar]
- 6.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99(2):229–234. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 7.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance, United States, 1994–1999. MMWR Morb Mortal Wkly Rep. 2002;51(SS05):1–8. [Google Scholar]
- 8.Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90(6):967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 9.Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study: African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol. 2001;153(1):1–10. doi: 10.1093/aje/153.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz SM. Epidemiology of uterine fibroids. Clin Obstet Gynecol. 2001;44(2):316–326. doi: 10.1097/00003081-200106000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14(2):247–250. doi: 10.1097/01.EDE.0000054360.61254.27. [DOI] [PubMed] [Google Scholar]
- 12.Wise LA, Palmer J, Stewart E, Rosenberg L. Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women’s Health Study. Obstet Gynecol. 2005;105(3):563–568. doi: 10.1097/01.AOG.0000154161.03418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise LA, Palmer JR, Harlow BL, et al. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women’s Health Study. Hum Reprod. 2004;19(8):1746–1754. doi: 10.1093/humrep/deh309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumbiganon P, Rugpao S, Phandhu-fung S, Laopaiboon M, Vudhikamraksa N, Werawatakul Y. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: a multicentre case-control study. Br J Obstet Gynaecol. 1996;103(9):909–914. doi: 10.1111/j.1471-0528.1996.tb09911.x. [DOI] [PubMed] [Google Scholar]
- 15.Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111(8):1037–1054. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz SM, Marshall LM, Baird DD. Epidemiologic contributions to understanding the etiology of uterine leiomyomata. Environ Health Perspect. 2000;108(suppl 5):821–827. doi: 10.1289/ehp.00108s5821. [DOI] [PubMed] [Google Scholar]
- 17.Samadi AR, Lee NC, Flanders WD, Boring JR, III, Parris EB. Risk factors for self-reported uterine fibroids: a case-control study. Am J Public Health. 1996;86(6):858–862. doi: 10.2105/ajph.86.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed) 1986;293(6543):359–362. doi: 10.1136/bmj.293.6543.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virchow R. Uber Makroglossie und pathologische Neubildung quergestreifter Muskelfasern. Virchows Arch Pathol Anat. 1854;7:126–138. [Google Scholar]
- 20.Smith CG, Glaser DA, Leonardi C. Zosteriform multiple leiomyomas. J Am Acad Dermatol. 1998;38(2, pt 1):272–273. doi: 10.1016/s0190-9622(98)70602-4. [DOI] [PubMed] [Google Scholar]
- 21.Holst VA, Junkins-Hopkins JM, Elenitsas R. Cutaneous smooth muscle neoplasms: clinical features, histologic findings, and treatment options. J Am Acad Dermatol. 2002;46(4):477–490. doi: 10.1067/mjd.2002.121358. [DOI] [PubMed] [Google Scholar]
- 22.Stewart L, Glenn G, Toro JR. Cutaneous leiomyomas: a clinical marker of risk for hereditary leiomyomatosis and renal cell cancer. Dermatol Nurs. 2006;18(4):335–341. [PubMed] [Google Scholar]
- 23.Kloepfer HW, Krafchuk J, Derbes V, Burks J. Hereditary multiple leiomyoma of the skin. Am J Hum Genet. 1958;10(1):48–52. [PMC free article] [PubMed] [Google Scholar]
- 24.Reed WB, Walker R, Horowitz R. Cutaneous leiomyomata with uterine leiomyomata. Acta Derm Venereol. 1973;53(5):409–416. [PubMed] [Google Scholar]
- 25.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98(6):3387–3392. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomlinson IP, Alam NA, Rowan AJ, et al. Multiple Leiomyoma Consortium. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30(4):406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 27.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73(1):95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Mir A, Glaser B, Chuang GS, et al. Germline fumarate hydratase mutations in families with multiple cutaneous and uterine leiomyomata. J Invest Dermatol. 2003;121(4):741–744. doi: 10.1046/j.1523-1747.2003.12499.x. [DOI] [PubMed] [Google Scholar]
- 29.Pithukpakorn M, Wei MH, Toure O, et al. Fumarate hydratase enzyme activity in lymphoblastoid cells and fibroblasts of individuals in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43(9):755–762. doi: 10.1136/jmg.2006.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei MH, Toure O, Glenn GM, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43(1):18–27. doi: 10.1136/jmg.2005.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Pugnaire MA, Delgado-Florencio V. Familial multiple cutaneous leiomyomas. Dermatology. 1995;191(4):295–298. doi: 10.1159/000246578. [DOI] [PubMed] [Google Scholar]
- 32.Bukulmez O, Doody KJ. Clinical features of myomas. Obstet Gynecol Clin North Am. 2006;33(1):69–84. doi: 10.1016/j.ogc.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Parazzini F, La Vecchia C, Negri E, Cecchetti G, Fedele L. Epidemiologic characteristics of women with uterine fibroids: a case-control study. Obstet Gynecol. 1988;72(6):853–857. doi: 10.1097/00006250-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Lumsden MA, Wallace EM. Clinical presentation of uterine fibroids. Baillieres Clin Obstet Gynaecol. 1998;12(2):177–195. doi: 10.1016/s0950-3552(98)80060-6. [DOI] [PubMed] [Google Scholar]
- 35.Vikhlyaeva EM, Khodzhaeva ZS, Fantschenko ND. Familial predisposition to uterine leiomyomas. Int J Gynaecol Obstet. 1995;51(2):127–131. doi: 10.1016/0020-7292(95)02533-i. [DOI] [PubMed] [Google Scholar]
- 36.Okolo SO, Gentry CC, Perrett CW, Maclean AB. Familial prevalence of uterine fibroids is associated with distinct clinical and molecular features. Hum Reprod. 2005;20(8):2321–2324. doi: 10.1093/humrep/dei049. [DOI] [PubMed] [Google Scholar]
- 37.Kurbanova MK, Koroleva AG, Sergeev AS. Genetic-epidemiologic analysis of uterine myoma: assessment of repeated risk [in Russian] Genetika. 1989;25(10):1896–1898. [PubMed] [Google Scholar]
- 38.Gross KL, Panhuysen CI, Kleinman MS, et al. Involvement of fumarate hydratase in nonsyndromic uterine leiomyomas: genetic linkage analysis and FISH studies. Genes Chromosomes Cancer. 2004;41(3):183–190. doi: 10.1002/gcc.20079. [DOI] [PubMed] [Google Scholar]
- 39.Lehtonen R, Kiuru M, Vanharanta S, et al. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am J Pathol. 2004;164(1):17–22. doi: 10.1016/S0002-9440(10)63091-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiuru M, Lehtonen R, Arola J, et al. Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res. 2002;62(16):4554–4557. [PubMed] [Google Scholar]
- 41.Pollard P, Wortham N, Barclay E, et al. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol. 2005;205(1):41–49. doi: 10.1002/path.1686. [DOI] [PubMed] [Google Scholar]
- 42.Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8(2):143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]