Abstract
Protein tyrosine kinase 6 (PTK6) is a nonmyristoylated Src-related intracellular tyrosine kinase. Although not expressed in the normal mammary gland, PTK6 is expressed in a majority of human breast tumors examined, and it has been linked to ErbB receptor signaling and AKT activation. Here we demonstrate that AKT is a direct substrate of PTK6 and that AKT tyrosine residues 315 and 326 are phosphorylated by PTK6. Association of PTK6 with AKT occurs through the SH3 domain of PTK6 and is enhanced through SH2 domain-mediated interactions following tyrosine phosphorylation of AKT. Using Src, Yes, and Fyn null mouse embryonic fibroblasts (SYF cells), we show that PTK6 phosphorylates AKT in a Src family kinase-independent manner. Introduction of PTK6 into SYF cells sensitized these cells to physiological levels of epidermal growth factor (EGF) and increased AKT activation. Stable introduction of active PTK6 into SYF cells also resulted in increased proliferation. Knockdown of PTK6 in the BPH-1 human prostate epithelial cell line led to decreased AKT activation in response to EGF. Our data indicate that in addition to promoting growth factor receptor-mediated activation of AKT, PTK6 can directly activate AKT to promote oncogenic signaling.
Protein tyrosine kinase 6 (PTK6; also known as the breast tumor kinase BRK) is an intracellular Src-related tyrosine kinase (9, 48). Human PTK6 was identified in cultured human melanocytes (32) and breast tumor cells (39), while its mouse orthologue was cloned from normal small intestinal epithelial cell RNA (50). Although PTK6 shares overall structural similarity with Src family tyrosine kinases, it lacks an N-terminal myristoylation consensus sequence for membrane targeting (39, 51). As a consequence, PTK6 is localized to different cellular compartments, including the nucleus (14, 15). PTK6 is expressed in normal differentiated epithelial cells of the gastrointestinal tract (34, 42, 51), prostate (14), and skin (51-53). Expression of PTK6 is upregulated in different types of cancers, including breast carcinomas (6, 39, 54), colon cancer (34), ovarian cancer (47), head and neck cancers (33), and metastatic melanoma cells (16). The significance of apparent opposing signaling roles for PTK6 in normal differentiation and cancer is still poorly understood.
In human breast tumor cells, PTK6 enhances signaling from members of the ErbB receptor family (10, 29, 30, 36, 40, 49, 54). In the HB4a immortalized human mammary gland luminal epithelial cell line, PTK6 promoted epidermal growth factor (EGF)-induced ErbB3 tyrosine phosphorylation and AKT activation (29). In response to EGF stimulation, PTK6 promoted phosphorylation of the focal adhesion protein paxillin and Rac1-mediated cell migration (10). PTK6 can be activated by the ErbB3 ligand heregulin and promotes activation of extracellular signal-regulated kinase 5 (ERK5) and p38 mitogen-activated protein kinase (MAPK) in breast cancer cells (40). PTK6 can also phosphorylate p190RhoGAP-A and stimulate its activity, leading to RhoA inactivation and Ras activation and thereby promoting EGF-dependent breast cancer cell migration and proliferation (49). Expression of PTK6 has been correlated with ErbB2 expression in human breast cancers (4, 5, 54).
AKT (also called protein kinase B) is a serine-threonine kinase that is activated downstream of growth factor receptors (38). It is a key player in signaling pathways that regulate energy metabolism, proliferation, and cell survival (7, 45). Aberrant activation of AKT through diverse mechanisms has been discovered in different cancers (2). AKT activation requires phosphorylation of AKT on threonine residue 308 and serine residue 473. The significance of phosphorylation of AKT on tyrosine residues is less well understood. Src has been shown to phosphorylate AKT on conserved tyrosine residues 315 and 326 near the activation loop (11). Substitution of these two tyrosine residues with phenylalanine abolished AKT kinase activity stimulated by EGF (11). Use of the Src family inhibitor PP2 impaired AKT activation following IGF-1 stimulation of oligodendrocytes (13). The RET/PTC receptor tyrosine kinase that responds to glial cell-line-derived neurotrophic factor also phosphorylated AKT tyrosine residue 315 promoting activation of AKT (28). AKT tyrosine residue 474 was phosphorylated when cells were treated with the tyrosine phosphatase inhibitor pervanadate, and phosphorylation of tyrosine 474 contributed to full activation of AKT (12). Recently, the nonreceptor tyrosine kinase Ack1 was shown to regulate AKT tyrosine phosphorylation and activation (37).
Here we show that AKT is a cytoplasmic substrate of the intracellular tyrosine kinase PTK6. We identify the tyrosine residues on AKT that are targeted by PTK6, and we demonstrate that tyrosine phosphorylation plays a role in regulating association between PTK6 and AKT. In addition, we show that PTK6 promotes AKT activation and cell proliferation in a Src-independent manner.
MATERIALS AND METHODS
Reagents.
Anti-human PTK6 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) or Millipore (Bedford, MA). Anti-mouse PTK6 and antiphosphotyrosine (PY20) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine clone 4G10 antibody was purchased from Millipore (Bedford, MA). Antibodies directed against AKT, phospho-AKT (Ser-473), phospho-AKT (Thr-308), Myc-tag (9B11), and phospho-p44/p42 MAPK (20G11) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies directed against α-tubulin and β-actin (AC-15) were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies directed against the hemagglutinin (HA) tag and glutathione S-transferase (GST) tag were purchased from Covance (Cumberland, VA). Donkey anti-rabbit or sheep anti-mouse antibodies conjugated to horseradish peroxidase were used as secondary antibodies (Amersham Biosciences, Piscataway, NJ) and detected by chemiluminescence with SuperSignal 165 West Dura extended-duration substrate from Pierce (Rockford, IL).
Construction of expression vectors.
Full-length human PTK6 and PTK6-YF in the pcDNA3 vector containing a Myc epitope tag were described in a previous study (41). Full-length wild-type mouse PTK6, PTK6-YF and PTK6-KM constructs in the pLXSN vector were described previously (52). PTK6-YF has a mutation of the regulatory tyrosine (Y) at position 447 of wild-type PTK6 to phenylalanine (F), resulting in a constitutively active mutant of the kinase. PTK6 KM has a mutation of a critical lysine (K219) in the ATP binding site of wild-type PTK6 to methionine (M), resulting in a kinase-dead mutant. Coding sequences from the pLXSN constructs were subcloned into the pBABE-puro vector (Cell Biolabs, Inc., San Diego, CA). The GST-PTK6 (mouse) constructs were previously described (51). Coding sequences from pcDNA3-human PTK6 were subcloned into the green fluorescent protein (GFP) vector pEGFP-C1 (Clontech, Palo Alto, CA).
Full-length wild-type HA-tagged mouse AKT and mAKT, in the mammalian expression vector pcDNA3, were kindly provided by Nissim Hay (University of Illinois at Chicago, Chicago, IL) (18, 19). The AKT mutants with mutations at tyrosine residues 215, 315, 326, and 474 and at proline residues 424, 427, 467, and 470 were created using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) as described by the manufacturer with pcDNA3 HA-AKT plasmid as a template. Mutagenic primers used for mouse AKT included Y215F (forward, 5′-CCTTACGGCCCTCAAGTTCTCATTCCAGACCCACG-3′; reverse, 5′-CGTGGGTCTGGAATGAGAACTTGAGGGCCGTAAGG-3′), Y315F (forward, 5′-CTGCGGAACGCCGGAGTTCCTGGCCCCTGAGGTG-3′; reverse, 5′-CACCTCAGGGGCCAGGAACTCCGGCGTTCCGCAG-3′), Y326F (forward, 5′-GCTGGAGGACAACGACTTCGGCCGTGCAGTGGAC-3′; reverse, 5′-GTCCACTGCACGGCCGAAGTCGTTGTCCTCCAGC-3′), Y474F (forward, 5′-CTTCCCCCAGTTCTCCTTCTCAGCCAGTGGCACAG-3′; reverse, 5′-CTGTGCCACTGGCTGAGAAGGAGAACTGGGGGAAG-3′), Y315F/Y326F (forward, 5′-GAACGCCGGAGTTCCTGGCCCCTGAGGTGCTGGAGGACAACGACTTCGGCCGTGCAG-3′; reverse, 5′-CTGCACGGCCGAAGTCGTTGTCCTCCAGCACCTCAGGGGCCAGGAACTCCGGCGTTC-3′), Y315E/Y326E (forward, 5′-GAACGCCGGAGGAGCTGGCCCCTGAGGTGCTGGAGGACAACGACGAGGGCCGTGCAG-3′; reverse, 5′-CTGCACGGCCCTCGTCGTTGTCCTCCAGCACCTCAGGGGCCAGCTCCTC CGGCGTTC-3′), P424A/P427A (forward, 5′-GCTGAGCCCAGCTTTCAAGGCCCAGGTCACCTC-3′; reverse, 5′-GAGGTGACCTGGGCCTTGAAAGCTGGGCTCAGC-3′), and P467A/P470A (forward, 5′-GTGAGCGGAGGGCGCACTTCGCCCAGTTCTCCTAC-3′; reverse, 5′-GTAGGAGAACTGGGCGAAGTGCGCCCTCCGCTCAC-3′). All constructs were sequenced to verify point mutations. Coding sequences from wild-type AKT and the AKT Y315F/Y326F mutant were subcloned into the bacterial expression vector pGEX-2TK (GE Healthcare Bio-Sciences, Piscataway, NJ).
Cell culture and transfections.
The human embryonic kidney cell line 293 (HEK-293) (ATCC CRL-1573) and the mouse embryonic fibroblast cell line SYF (ATCC CRL-2459) were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The benign prostatic hyperplasia epithelial cell line BPH-1 (kindly provided by Simon Hayward [Vanderbilt University, Nashville, TN]) was cultured in RPMI 1640 containing 5% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (23). Transfections of HEK-293 cells were performed using the Lipofectamine 2000 transfection reagent (Invitrogen Corp, Carlsbad, CA) as per the manufacturer's instructions. Transfections of SYF cells were performed using the Lipofectamine transfection reagent in combination with PLUS reagent (Invitrogen Corp, Carlsbad, CA) as per the manufacturer's instructions.
In vitro kinase assays.
Recombinant human PTK6 (Invitrogen Corp, Carlsbad, CA) (50 ng) alone or in combination with 1 μg recombinant human AKT (Millipore, Bedford, MA) or GST-tagged mouse AKT or the GST-tagged mouse AKT Y315F/Y326F mutant was incubated in 30 μl kinase buffer (10 mM HEPES, pH 7.5, 150 mM NaCl, 2.5 mM dithiothreitol [DTT], 0.01% Triton X-100, 10 mM MnCl2) with or without 200 μM ATP for 10 min at 30°C. The reaction was stopped by adding 30 μl of 2× reducing Laemmli sample buffer and boiling. Samples were subjected to SDS-PAGE on 8% gels and transferred onto Immobilon-P membranes (Millipore, Bedford, MA) for immunoblotting.
Identification of phosphorylation sites by mass spectrometry.
Several AKT phosphotyrosine sites were detected by various tandem mass spectrometry methods. Tyrosine phosphorylation at Y215 was detected after recombinant AKT (Invitrogen Corp, Carlsbad, CA) was subjected to in-gel digestion using a combination of trypsin and chymotrypsin followed by C18 reversed-phase microcapillary liquid chromatography-tandem mass spectrometry (LC/MS/MS) using a LTQ 2D linear ion trap mass spectrometer (Thermo Scientific) in positive-ion data-dependent acquisition mode. MS/MS spectra were searched against the reversed and concatenated Swiss-Prot protein database using the Sequest algorithm (Proteomics browser; Thermo Scientific) with differential modifications for STY phosphorylation (+79.97) and methionine oxidation (+15.99). Phosphorylation sites were identified if they initially passed the following Sequest scoring thresholds: 2+ ions, Xcorr ≥ 1.90, Sf ≥ 0.4, P > 0; 3+ ions, Xcorr ≥ 2.55, Sf ≥ 0.5, P > 0 against the forward database. Peptides with gas phase charges of 1+ and 4+ were generally not accepted due to difficulty of interpretation. Passing MS/MS spectra were then manually validated to ensure that all b- and y-fragment ions aligned with the assigned modified protein sequence. Determination of the exact sites of phosphorylation was aided using GraphMod software (Proteomics browser, Thermo Scientific), resulting in a false-positive identification rate of less than 1.5%. Tyrosine phosphorylation at Y326 was detected from a tryptic digestion from recombinant AKT using a targeted-ion LC/MS/MS (TIMM) experiment with a high-resolution/mass accuracy hybrid LTQ-Orbitrap XL mass spectrometer (Thermo Scientific) by setting the ion trap to filter for the phosphorylated Y326 peptide, a method that enhances sensitivity of detection. Validation of the site was similar to the method described above in addition to the added criteria that the identified phosphopeptide be present within a ±5-ppm mass accurate window.
Protein lysates and immunoprecipitations.
Transfected cells were rinsed twice with cold phosphate-buffered saline (PBS) and lysed in 1% Triton X-100 lysis buffer (1% Triton X-100, 20 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM Na-pyrophosphate, 100 mM NaF, 5 mM iodoacetic acid, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], protease inhibitor cocktail) 18 to 24 h after transfection. Immunoprecipitation was performed with 500 μg of total cell lysates and 0.5 μg specific antibodies under overnight incubation at 4°C. Then, 30 μl protein A-Sepharose CL-4B beads (GE Healthcare Bio-Sciences, Piscataway, NJ) was added and incubated for 1 h, following which the beads were washed four times in wash buffer (1% Triton X-100, 20 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM Na-pyrophosphate). After the supernatant was removed from the final wash, samples were resuspended in 30 μl of 2× reducing Laemmli sample buffer and boiled for 5 min. The proteins retained on the protein A beads were subjected to SDS-PAGE on 8% gels and transferred onto Immobilon-P membranes (Millipore, Bedford, MA) for immunoblotting.
Immunostaining.
HEK-293 cells were plated in 4-well chamber slides and were cotransfected with Myc-PTK6-YF and HA-AKT or HA-mAKT plasmids, as per the manufacturer's instructions. Cells were washed with PBS and fixed in Carnoy's solution (ethanol-chloroform-acetic acid = 6:3:1). Then cells were blocked with goat serum for 1 h and were incubated with mouse anti-Myc-tag and rabbit anti-AKT primary antibodies overnight. After washing, samples were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse and biotinylated anti-rabbit secondary antibodies and then incubated with rhodamine-conjugated avidin. Slides were mounted in Vectashield fluorescent mount media containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). Images were collected with a Zeiss LSM510 confocal microscope.
Expression of GST fusion proteins and GST pulldown assays.
The GST tag, GST-PTK6FL (full-length), GST-PTK6-SH2, GST-PTK6-SH3, and GST-PTK6-SH2/SH3 fusion proteins were expressed in BL21 cells (Stratagene, La Jolla, CA). Protein purification was performed as described previously (41). The GST-AKT and GST-AKT Y315F/Y326F fusion proteins were prepared similarly.
For GST pulldown assays, 6 μg of the purified GST fusion proteins GST-PTK6FL, GST-PTK6-SH2, GST-PTK6-SH3, and GST-PTK6-SH2/SH3 were incubated with 25 μl glutathione-Sepharose 4B beads for 30 min and then incubated with 500 μg of lysates overexpressing AKT alone or in combination with PTK6-YF overnight at 4°C, following which the beads were washed four times in wash buffer (1% Triton X-100, 20 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM Na-pyrophosphate). After removal of the supernatant in the final wash, samples were resuspended in 30 μl of 2× Laemmli sample buffer and boiled for 5 min. The proteins retained on the GST beads were resolved by SDS-PAGE. Binding was compared with that of 10% of the lysates added to the pulldown reactions.
Retrovirus production and transduction of SYF cells.
pBABE-PTK6-WT, pBABE-PTK6-YF, pBABE-PTK6-KM, or pBABE-vector plasmids were transfected in Phoenix Eco cells (murine) by Lipofectamine 2000, and DMEM with 10% fetal bovine serum (FBS) was changed after 24 h. Retrovirus was collected 24 h later. SYF cells were infected by retrovirus carrying pBABE-PTK6-WT, pBABE-PTK6-YF, pBABE-PTK6-KM, or pBABE-vector at a multiplicity of infection (MOI) of 500 for 24 h. Stable cell lines were selected in DMEM containing 10% FBS and 2 μg/ml puromycin for a week.
Cell proliferation assays.
Subconfluent cells (2 × 103) were plated in DMEM containing 10% FBS in each well in 48-well plates. Cell number was measured by the CellTiter-Glo luminescent cell viability assay (Promega, Madison, WI) as per the manufacturer's instructions. The average cell population doubling time (hours) was calculated between 1 and 5 days. Results were formatted as means ± standard deviations from three independent experiments.
Knockdown of PTK6 in BPH-1 cells.
Lentivirus expressing MISSION TRC PTK6 short hairpin RNA (shRNA) TRCN0000021552 (Sigma-Aldrich, St. Louis, MO) and empty PLKO-1 vector were produced in the HEK293FT packaging cell line by cotransfection with compatible packaging plasmids HIV-trans and VSVG (provided by Bin He [University of Illinois at Chicago, Chicago, IL]). BPH-1 cells were infected with retrovirus (50% viral supernatant and 50% growth medium containing 5 μg/ml Polybrene) and placed in selection medium containing 2 μg/ml puromycin for 2 weeks.
RESULTS
Active PTK6 induces tyrosine phosphorylation of AKT.
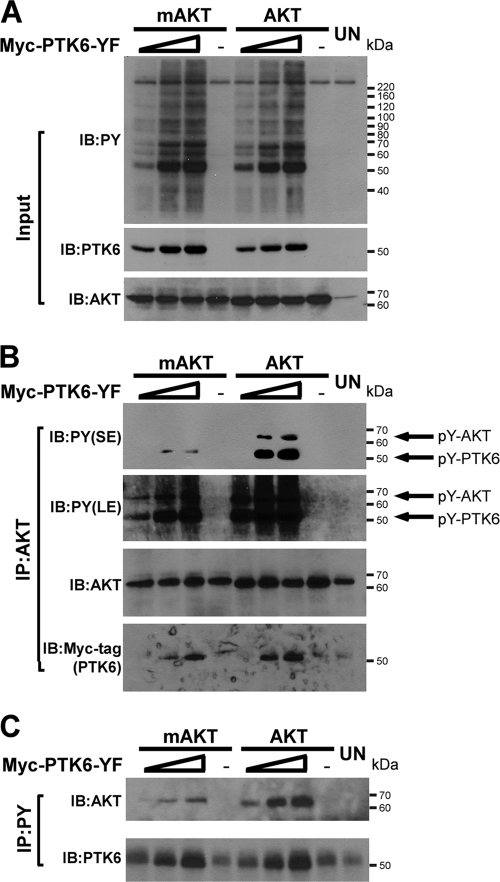
Previous studies have demonstrated that PTK6 is involved in regulating AKT (20, 21, 29, 55). To explore the relationship between PTK6 and AKT, HEK-293 cells were cotransfected with increasing amounts (1, 2, and 3 μg) of Myc-tagged PTK6-YF plasmid and 1 μg HA (hemagglutinin)-tagged AKT or myristoylated AKT (mAKT) plasmids. mAKT represents an active form of AKT that is targeted to the cellular membrane and activated independently of phosphatidylinositol 3-kinase (PI3-kinase) activation (18). PTK6-YF has a substitution of the PTK6 negative regulatory carboxy-terminal tyrosine at position 447 to phenylalanine and is a constitutively active form of the kinase (15, 43). The expression of active forms of PTK6 leads to a dramatic increase in tyrosine-phosphorylated proteins as assessed by immunoblotting total cell lysates with antiphosphotyrosine (PY) antibodies (Fig. 1A, PY). Immunoblot analysis of immunoprecipitated AKT showed that both AKT and mAKT were phosphorylated on tyrosine residues in cells expressing active forms of PTK6 and that the level of phosphorylation is dependent on the level of PTK6 expression [Fig. 1B, PY(LE), upper band]. However, mAKT shows less tyrosine phosphorylation than AKT [Fig. 1B, PY(SE), upper band]. Probing the AKT immunoprecipitates with an anti-Myc-tag antibody revealed that both AKT and mAKT associated with active PTK6, but there is less PTK6 associating with mAKT than with AKT (Fig. 1B, Myc-tag). In addition, immunoprecipitation using antiphosphotyrosine antibodies was performed to monitor AKT tyrosine phosphorylation in a reverse manner. Although there was an increasing amount of AKT and mAKT in the immunoprecipitated phosphotyrosine protein complex, as the expression of active PTK6 increased, the level of mAKT was much less than that of AKT (Fig. 1C, AKT), which is probably due to less tyrosine phosphorylation on mAKT.
FIG. 1.
PTK6 induces phosphorylation of AKT on tyrosine residues. (A) Immunoblot analysis of total cell lysates of HEK-293 cells coexpressing increasing amounts (1, 2, and 3 μg) of Myc-PTK6-YF plasmid and 1 μg HA-AKT or HA-mAKT plasmid. Untreated (UN) HEK-293 cells served as a control. Total cell lysates were analyzed by immunoblotting with antiphosphotyrosine (PY), anti-PTK6, and anti-AKT antibodies. (B) Immunoblot analysis of AKT immunoprecipitated (IP) from lysates of HEK-293 cells coexpressing AKT or mAKT and PTK6-YF. AKT tyrosine phosphorylation was analyzed using anti-PY antibodies. Both short exposure (SE) and long exposures (LE) are shown. Arrows point at tyrosine-phosphorylated AKT and PTK6. The membrane was reprobed with antibodies directed against AKT and Myc-tagged PTK6. (C) Immunoblot analysis of phosphotyrosine-containing proteins that immunoprecipitated from lysates of HEK-293 cells coexpressing AKT or mAKT and PTK6-YF. The levels of AKT and PTK6 were analyzed using anti-AKT and anti-PTK6 antibodies.
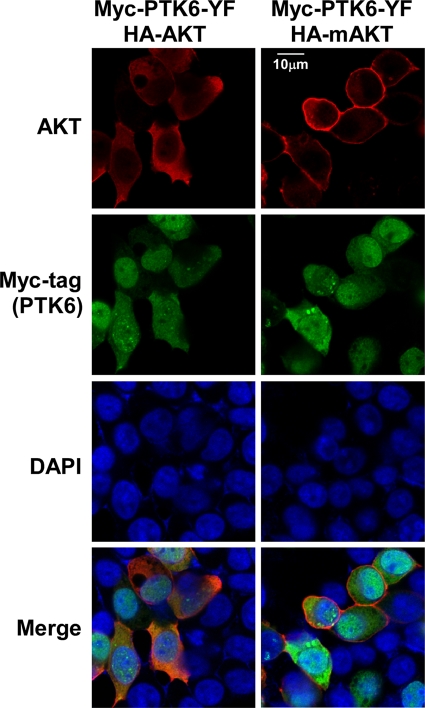
We hypothesized that the reduced level of mAKT phosphorylation on tyrosine compared with AKT might be due to differences in intracellular localization of PTK6-YF, mAKT, and AKT. This led us to examine the localization of these proteins in HEK-293 cells following transfection using immunofluorescence staining. PTK6-YF (green, FITC) and AKT (red, rhodamine) were predominantly colocalized in the cytoplasm (Fig. 2, left panel). In contrast, much of the mAKT (red, rhodamine) was at the cellular membrane (Fig. 2, right panel) and did not colocalize with PTK6-YF. Views shown depict cells expressing both PTK6 and AKT and cells that were not cotransfected with both constructs. Overexpression of mAKT was not sufficient to bring PTK6 to the cell membrane.
FIG. 2.
PTK6 colocalizes with AKT in transfected HEK-293 cells. Intracellular localization of AKT, mAKT and PTK6 was examined by indirect immunofluorescence in HEK-293 cells coexpressing PTK6-YF and AKT or mAKT. Myc-tagged PTK6 immunoreactivity is visualized with FITC (green), while HA-tagged AKT and mAKT are detected with rhodamine (red). Cells were counterstained with DAPI (blue). Transfected PTK6 and AKT colocalize in the cytoplasm (left panels), whereas mAKT is localized at the membrane (right panels). Size bar denotes 10 μm.
PTK6 directly phosphorylates AKT in vitro.
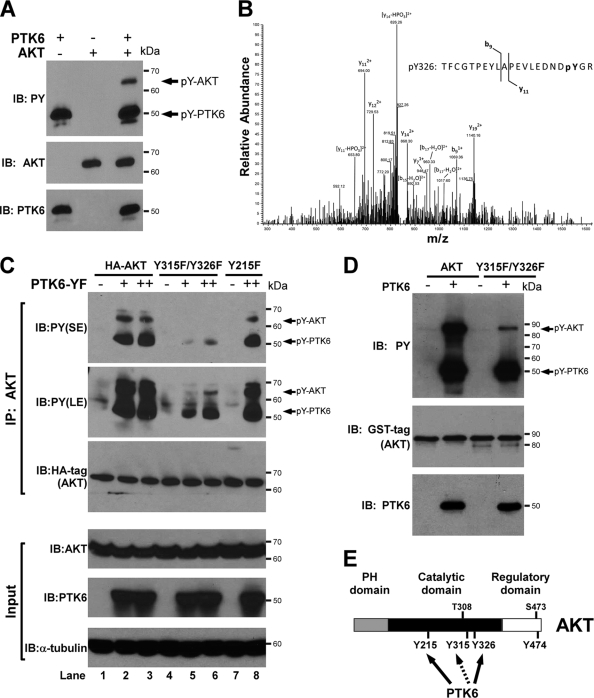
To address whether PTK6 directly phosphorylates AKT, in vitro kinase assays were performed using active recombinant human PTK6 and recombinant human AKT. Reactions were performed in the presence of ATP for 10 min at 30°C. Activated PTK6 was detected using antiphosphotyrosine antibodies (Fig. 3A, PY, lower band). Tyrosine-phosphorylated wild-type AKT was detected only in the presence of PTK6 (Fig. 3A, PY, upper band). Mass spectrometry was utilized to identify the specific tyrosine site(s) targeted by PTK6, and tyrosine phosphorylation events at AKT tyrosine residues 215 (Y215) and 326 (Y326) were identified. The MS/MS spectrum for the AKT peptide containing phosphotyrosine at residue 326 is shown (Fig. 3B). Tyrosine 215 is a novel site, while tyrosine 326 was previously reported to be phosphorylated by Src (11). Phosphorylation on tyrosine 315 (Y315), another tyrosine residue targeted by Src (11), and tyrosine 474 (Y474), which is phosphorylated after treatment of cells with the phosphatase inhibitor pervanadate (12), was not detected by mass spectrometry. However, we determined that the tyrosine residue 315 is also targeted by PTK6 (see below). A schematic diagram of AKT shows tyrosine residues targeted by PTK6 (Fig. 3E).
FIG. 3.
PTK6 directly phosphorylates AKT on tyrosine residues 315 and 326. (A) PTK6 directly phosphorylates AKT in an in vitro kinase reaction. Recombinant human PTK6 and human AKT were incubated in kinase buffer in the presence of ATP for 10 min at 30°C and then subjected to SDS-PAGE and immunoblot analysis with anti-PY, -AKT, and -PTK6 antibodies. (B) The MS/MS spectrum of the AKT peptide TFCGTPEYLAPEVLEDNDpYGR showing phosphorylation at Tyr326. The fragment ions at b9 and y11 minus phosphate prove that phosphorylation is present on the tyrosine residue on the C-terminal end of the peptide. (C) Immunoblot analysis of AKT immunoprecipitated (IP) from lysates of HEK-293 cells coexpressing PTK6-YF and wild-type AKT or AKT mutants (Y215F and Y315F/Y326F). AKT tyrosine phosphorylation was analyzed using anti-PY antibodies. Both short exposure (SE) and long exposures (LE) are shown. Arrows point at tyrosine-phosphorylated AKT and PTK6. The membrane was reprobed with an antibody directed against the HA tag. Total cell lysates were analyzed by immunoblotting with anti-AKT, -PTK6, and -α-tubulin antibodies as input. (D) Reduced phosphorylation of GST-AKT Y315F/Y326F in an in vitro kinase reaction. Recombinant human PTK6 and purified GST-tagged wild-type AKT or AKT Y315F/Y326F mutant were incubated in kinase buffer in the presence of ATP for 10 min at 30°C and subjected to SDS-PAGE and immunoblot analysis with anti-PY, -GST tag, and -PTK6 antibodies. (E) Schematic representation of tyrosine-phosphorylated AKT. Tyrosine residues 215 and 326 were identified as sites phosphorylated by PTK6 using tandem mass spectrometry (solid arrow). Tyrosine residue 315 was found to be targeted by PTK6 through point mutation studies (dashed arrow). Phosphorylation on tyrosine residue 474 was reported in reference 12.
Identification of AKT tyrosine residues 315 and 326 as key sites phosphorylated by PTK6.
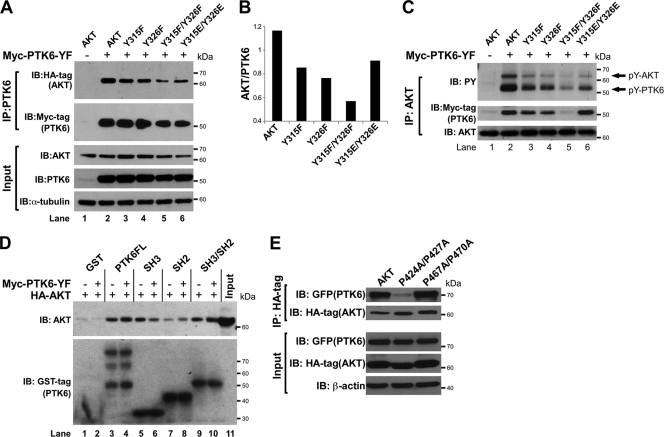
While mass spectrometry is a powerful analytical tool with high precision and sensitivity, it still has its limitations in identifying target sites in low-abundance peptides. Therefore, to determine which tyrosine residues in AKT are physiologically relevant targets of PTK6, we examined the significance of all four candidate tyrosine residues on AKT and converted individual and combinations of tyrosine residues (Y215, Y315, Y326, Y474, Y315/Y326, and Y215/Y474) to phenylalanine using site-directed mutagenesis. HEK-293 cells were cotransfected with increasing amounts (0 μg, 1 μg, 3 μg) of Myc-PTK6-YF plasmid and 1 μg of wild-type HA-AKT plasmid or mutant plasmids with a single tyrosine mutation (Y215F) or double tyrosine mutations (Y315F/Y326F). Immunoblot analysis of immunoprecipitated AKT showed that the AKT Y215F mutant was still phosphorylated by active PTK6, like wild-type AKT (Fig. 3C, PY, upper band, lanes 2, 3, and 8). Similar experiments were performed with the AKT Y474F and AKT Y215F/Y474F mutants, and mutation of these residues had no impact on PTK6-mediated AKT phosphorylation (data not shown). However, when either tyrosine residue 315 or 326 of AKT was mutated to phenylalanine, there was a moderate reduction in the level of tyrosine phosphorylation of AKT (Fig. 4C, PY, upper band, lanes 3 and 4). When both tyrosine residues 315 and 326 were mutated to phenylalanine, the level of tyrosine phosphorylation of the AKT double mutant Y315F/Y326F was greatly reduced (Fig. 3C, PY, lanes 5 and 6; Fig. 4C, PY, lane 5), indicating that tyrosine 315 and tyrosine 326 are the major sites phosphorylated by PTK6. We also performed in vitro kinase assay experiments using wild-type GST-AKT or mutant AKT (Y315F/Y326F) fusion proteins as substrates of active PTK6, and much less tyrosine phosphorylation was detected on the GST-AKT Y315F/Y326F mutant protein (Fig. 3D, PY, upper band). These data confirm that AKT tyrosine residues 315 and 326 are the primary sites phosphorylated by PTK6.
FIG. 4.
Interactions between PTK6 and AKT are mediated by the PTK6 SH2 and SH3 domains. (A) Immunoblot analysis of PTK6 immunoprecipitated (IP) from lysates of HEK-293 cells coexpressing PTK6-YF and wild-type AKT or AKT mutants (Y315F, Y326F, Y315F/Y326F and Y315E/Y326E). AKT association was analyzed using an anti-HA tag antibody. The membrane was stripped and reprobed with an antibody directed against Myc tag. Total cell lysates were analyzed by immunoblotting with anti-AKT, -PTK6, and -α-tubulin antibodies as input. (B) A column graph showing the ratio of AKT (HA-tag) to PTK6 (Myc-tag) band density quantified by NIH ImageJ software. (C) Immunoblot analysis of AKT immunoprecipitated (IP) from lysates of HEK-293 cells coexpressing PTK6-YF and wild-type AKT or AKT mutants (Y315F, Y326F, Y315F/Y326F, and Y315E/Y326E). The levels of tyrosine-phosphorylated AKT and PTK6 (pY-AKT and pY-PTK6) were analyzed using anti-PY antibodies (arrow). The level of PTK6 that interacts with AKT was analyzed using an anti-Myc tag antibody. The membrane was reprobed with an antibody directed against AKT. (D) GST pulldown assay using lysates of HEK-293 cells expressing AKT alone or in combination with PTK6-YF. Glutathione-Sepharose 4B beads, which bind purified GST tag, GST-PTK6, GST-SH2, GST-SH3, or GST-SH2/SH3 protein, were used to pull down AKT from HEK-293 lysates. Levels of bound AKT were analyzed using an anti-AKT antibody. Ten percent of the lysates added to the pulldown reaction served as input. (E) Immunoblot analysis of HA tag IP from lysates of HEK-293 cells coexpressing GFP-PTK6 and wild-type AKT or AKT mutants (P424A/P427A and P467A/P470A). PTK6 association was analyzed using an anti-GFP antibody. The membrane was stripped and reprobed with an antibody directed against HA tag. Total cell lysates were analyzed by immunoblotting with anti-GFP, -HA tag, and -β-actin antibodies as input.
The SH2 and SH3 domains of PTK6 are involved in mediating association between PTK6 and AKT.
PTK6 shares structural homology with Src kinases and has characterized SH2 and SH3 domains that mediate protein associations through recognition of specific phosphotyrosines or proline-rich sequences, respectively (24, 43, 44). In previous experiments, we found that less association between PTK6 and AKT is coupled with less tyrosine phosphorylation on AKT. mAKT that is less phosphorylated on tyrosine showed less interaction with active PTK6 (Fig. 1B, Myc-tag). Also, there was less tyrosine-phosphorylated PTK6 detected in AKT immunoprecipitates when AKT tyrosine residues 315 and 326 were mutated to phenylalanine [Fig. 3C, PY(SE), lower band, lanes 2, 3, 5, and 6]. These data led us to hypothesize that tyrosine phosphorylation is critical for interaction between PTK6 and AKT. To test this, HEK-293 cells were cotransfected with Myc-PTK6-YF and HA-tagged wild-type AKT or AKT mutants (Y315F, Y326F, Y315F/Y326F, Y315E/Y326E), and PTK6 immunoprecipitations were performed. An anti-HA tag immunoblot showed that the interaction between PTK6 and AKT was slightly decreased when single tyrosine residue 315 or 326 was mutated to phenylalanine but largely decreased when both sites were mutated to phenylalanine (Fig. 4A, HA-tag). AKT Y315E/Y326E served as a tyrosine phosphomimetic form, and the interaction between PTK6 and this AKT form is relatively higher than between PTK6 and AKT Y315F/Y326F (Fig. 4A, HA-tag, lanes 5 and 6), suggesting that tyrosine phosphorylation promotes the association of PTK6 and AKT. The column graph shows the level of AKT binding to PTK6 after normalization to the total amount of immunoprecipitated PTK6 (Fig. 4B). The reverse immunoprecipitation was also performed using an anti-AKT antibody. Consistent with previous results, there was a slight reduction in the amount of PTK6 that coimmunoprecipitated with AKT when single tyrosine residue 315 or 326 was mutated to phenylalanine and a striking reduction when both tyrosine residues were mutated to phenylalanine (Fig. 4C, Myc-tag). The interaction between PTK6 and the AKT Y315E/Y326E phosphomimetic form was comparable to that with wild-type (WT) AKT (Fig. 4C, Myc-tag). In addition, probing blots with antiphosphotyrosine antibodies showed that tyrosine phosphorylation of AKT (Fig. 4C, PY, upper band) correlated well with the association of AKT with PTK6 (Fig. 4A, HA-tag; Fig. 4C, Myc-tag), further indicating the importance of AKT tyrosine phosphorylation in regulating the association of these two proteins.
We hypothesized that the PTK6 SH2 domain might recognize phosphorylated tyrosine residues on AKT and thus regulate the association between PTK6 and AKT. To test this, GST tag, GST-PTK6FL (full-length), GST-PTK6-SH2, GST-PTK6-SH3, and GST-PTK6-SH2/SH3 fusion proteins were prepared and GST pulldown assays were performed using HEK-293 cell lysates expressing AKT alone or in combination with active PTK6. GST pulldown assays using cell lysate lacking active PTK6 showed that the SH3 domain is important for PTK6 to interact with AKT, while the SH2 domain interacted weakly with AKT (Fig. 4D, AKT, lanes 1, 3, 5, 7, and 9). Reduced association of the PTK6 SH3 domain with tyrosine-phosphorylated AKT in lysates with overexpression of active PTK6 might be caused by conformational change of AKT (Fig. 4D, AKT, lanes 5 and 6). In contrast, SH2 domain-containing GST fusion proteins (GST-PTK6-SH2, GST-PTK6-SH2/SH3, GST-PTK6FL) are all able to pull down more tyrosine-phosphorylated AKT (Fig. 4D, AKT, compare lanes 3 and 4, 7 and 8, and 9 and 10). These data indicate that the PTK6 SH2 domain is able to recognize tyrosine phosphorylation on AKT induced by PTK6-YF, promoting a more stable association between AKT and PTK6.
To investigate the role of the PTK6 SH3 domain in mediating association between PTK6 and AKT, two proline-rich PXXP motifs at the C terminus of AKT were mutated. Wild-type HA-tagged AKT or mutant HA-AKT P424A/427A or HA-AKT P467A/P470A were coexpressed with GFP-PTK6 in HEK-293 cells, and HA-tag immunoprecipitations were performed, followed by immunoblotting with anti-GFP to detect PTK6 association. A dramatic decrease in PTK6 binding was observed when proline residues 424 and 427 were mutated to alanine, while mutation of proline residues 467 and 470 to alanine did not affect AKT interaction with PTK6. Consistent with the pulldown data shown in Fig. 4D, these data support a role for the PTK6 SH3 domain in regulating association between PTK6 and AKT, through its interaction with the proline-rich sequence P424XXP427 at the AKT C terminus.
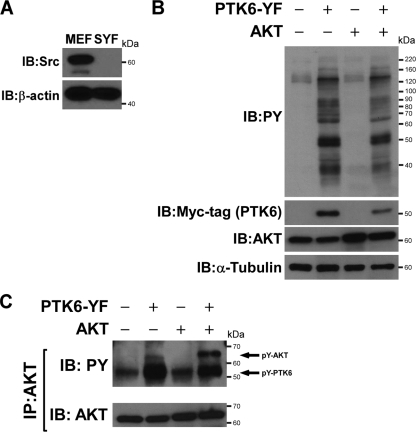
Active PTK6 induces tyrosine phosphorylation of AKT independent of Src in SYF cells.
Tyrosine residues 315 and 326 are also phosphorylated by Src, suggesting possible redundancy between PTK6 and Src (11). Although we showed that PTK6 directly phosphorylates AKT, it is also possible that PTK6 promotes Src-mediated phosphorylation of AKT on tyrosine. To exclude the effect for Src in AKT tyrosine phosphorylation in cells, mouse embryo fibroblasts (MEFs) from mouse embryos harboring functionally null mutations in both alleles encoding the Src family protein tyrosine kinases, Src, Yes, and Fyn, were utilized (SYF cells) (31). In these cells, AKT tyrosine phosphorylation can be examined in a background without Src, Yes, and Fyn activity. Src expression is absent in SYF cells, as expected, but present in wild-type MEF cells (Fig. 5A). To detect whether PTK6-YF expression in SYF cells is able to induce tyrosine phosphorylation on AKT, SYF cells were transfected with different combinations of HA-AKT and Myc-PTK6-YF plasmids. Protein expression was examined by immunoblotting using anti-Myc-tag and AKT antibodies (Fig. 5B). Probing blots with antiphosphotyrosine antibodies showed that phosphotyrosine signaling is induced in the presence of active PTK6 (Fig. 5B). AKT was then immunoprecipitated from those cell lysates, and immunoblotting was performed with antiphosphotyrosine antibodies. Both endogenous and exogenous AKT protein was phosphorylated on tyrosine residues when active PTK6 was present, indicating that PTK6 is able to induce tyrosine phosphorylation of AKT in the absence of Src family kinases (Fig. 5C, PY, upper band).
FIG. 5.
PTK6 induces Src-family independent phosphorylation of AKT on tyrosine residues. (A) Immunoblotting demonstrates the absence of Src expression in SYF cells. Wild-type MEFs express high levels of Src kinase. (B) Immunoblot analysis of the lysates from SYF cells cotransfected with PTK6-YF and AKT plasmids. Total cell lysates were analyzed using antiphosphotyrosine (PY), -AKT, -Myc tag, and -α-tubulin antibodies. (C) Immunoblot analysis of AKT immunoprecipitated (IP) from lysates of SYF cells cotransfected with PTK6-YF and AKT plasmids. Tyrosine phosphorylation of AKT was analyzed using anti-PY antibodies (arrow). The membrane was reprobed with an antibody directed against AKT.
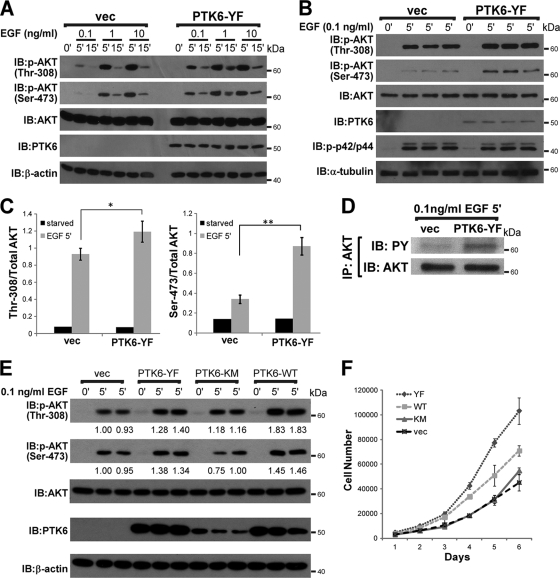
Ectopic PTK6 sensitizes SYF cells to physiological levels of EGF and stimulates SYF cell proliferation.
Others previously showed that Src-mediated phosphorylation of AKT tyrosine residues 315 and 326 played a role in AKT activation by EGF (11). Since our data show that PTK6 also targets AKT on tyrosine residues 315 and 326, we hypothesized that PTK6 might also regulate AKT signaling in response to growth factors. AKT is activated through phosphorylation of Thr-308 in the activation loop of the kinase domain by PDK1 (1) and Ser-473 within the hydrophobic motif of the regulatory domain by mammalian target of rapamycin (mTOR) in a rapamycin-insensitive complex with Rictor and Sin1 (26, 46). Therefore, we examined phosphorylation on Thr-308 and Ser-473 as markers for AKT activation. To exclude potential interference caused by Src, we utilized SYF cells to construct stable cell lines expressing PTK6-YF or empty vector. SYF stable cell lines were serum starved for 48 h and then stimulated with different concentrations of EGF (0.1 ng/ml, 1 ng/ml, 10 ng/ml) for 5 or 15 min. Immunoblot analysis using anti-phospho-Thr-308 and -Ser-473 antibodies showed that the AKT phosphorylation on Thr-308 and -Ser-473 peaks after 5 min of EGF stimulation under all conditions (Fig. 6 A). Interestingly, AKT is able to achieve higher activation in the presence of PTK6-YF when stimulated by physiological levels of EGF (0.1 ng/ml, 1 ng/ml) but not higher concentrations of EGF (10 ng/ml) (Fig. 6A). To confirm this result, we treated both PTK6-YF-expressing SYF cells and control cells with 0.1 ng/ml EGF for 5 min in triplicate. Anti-Thr-308 and -Ser-473 immunoblotting showed that AKT achieves higher activation when PTK6-YF is present, while the phosphorylation of p44/p42 MAPK upon EGF stimulation is not affected, indicating that PTK6 specifically regulates AKT signaling (Fig. 6B). AKT phosphorylation on Thr-308 and Ser-473 was further normalized by total AKT level, and two column graphs were created to show the differences in AKT activation, with P values of 0.031 and 0.0007, respectively (Fig. 6C). Also, increased AKT activation correlated with increased tyrosine phosphorylation of AKT, as is shown in Fig. 6D. There is higher AKT tyrosine phosphorylation in PTK6-YF-expressing SYF cells after 5 min of 0.1 ng/ml EGF treatment than in SYF vector cells (Fig. 6D).
FIG. 6.
PTK6 positively regulates AKT activation stimulated by physiological levels of EGF in SYF cells. (A) Increased activating phosphorylation of AKT on Thr-308 and Ser-473 was detected in SYF cells expressing PTK6-YF following addition of 0.1 ng/ml EGF. SYF cells stably expressing PTK6-YF or empty vector (vec) were serum starved for 48 h and then stimulated with different concentrations of EGF (0.1 ng/ml, 1 ng/ml, 10 ng/ml) for 5 or 15 min. Cell lysates were analyzed by immunoblotting with anti-AKT phospho-Thr-308, -AKT phospho-Ser-473, -AKT, -mouse PTK6, and -β-actin antibodies. (B) SYF cells stably expressing PTK6-YF or empty vector were serum starved for 48 h and then stimulated by 0.1 ng/ml EGF for 5 min. Samples were in triplicate. Cell lysates were analyzed by immunoblotting with anti-AKT phospho-Thr-308, -AKT -phospho-Ser-473, -AKT, -mouse PTK6, -phospho-p42/p44, and -α-tubulin antibodies. (C) The ratio of AKT Thr-308 phosphorylation and Ser-473 phosphorylation band density to total AKT band density was quantified with NIH ImageJ software. Results were formatted as means ± standard deviations of results from three independent experiments. *, P = 0.031; **, P = 0.0007. (D) Immunoblot analysis of AKT immunoprecipitated (IP) from SYF cell lysates described in the legend for panel B. Tyrosine phosphorylation of AKT was analyzed using anti-PY antibodies. (E) SYF cells stably expressing PTK6-YF, PTK6-KM, PTK6-WT, or empty vector were serum starved for 48 h and then stimulated with 0.1 ng/ml EGF for 5 min. Cell lysates were analyzed by immunoblotting with anti-AKT phospho-Thr-308, -AKT phospho-Ser-473, -AKT, -mouse PTK6, and -β-actin antibodies. Relative band densities were quantified with NIH ImageJ Software and are indicated under corresponding bands. (F) Growth curves of SYF cells stably expressing empty vector, PTK6-YF, PTK6-KM, or PTK6-WT.
To investigate whether the regulation of AKT activation by PTK6 is dependent on PTK6 kinase activity, SYF cell lines stably expressing PTK6-WT (wild type), PTK6-YF (constitutively active), PTK6-KM (kinase dead) or empty vector were serum starved for 48 h and then stimulated by 0.1 ng/ml EGF for 5 min. Immunoblot analysis using anti-phospho Thr-308 and Ser-473 antibodies showed that AKT achieves higher activation in the presence of PTK6-WT or PTK6-YF but not PTK6-KM or empty vector (Fig. 6E), indicating the regulation of AKT activation is PTK6 kinase activity dependent. Consistent with previous publications that have focused on the roles of PTK6 in breast cancer cells (11, 29, 30), our data revealed an oncogenic role of PTK6 in regulating AKT signaling.
We further delineated the role of PTK6 in regulating SYF cell proliferation. As shown in Fig. 6F, SYF cells expressing PTK6-KM or empty vector proliferated similarly, with a doubling time around 29.0 ± 1.0 h, while PTK6-WT or PTK6-YF expressing SYF cells had a higher proliferation rate, which reduced the doubling time to 25.8 ± 1.1 h or 24.0 ± 0.4 h, respectively. SYF cell growth curves clearly show that PTK6 is able to boost the SYF cell proliferation rate, and this is dependent on PTK6 kinase activity.
Active PTK6 promotes AKT activation through phosphorylation on tyrosine residues 315 and 326.
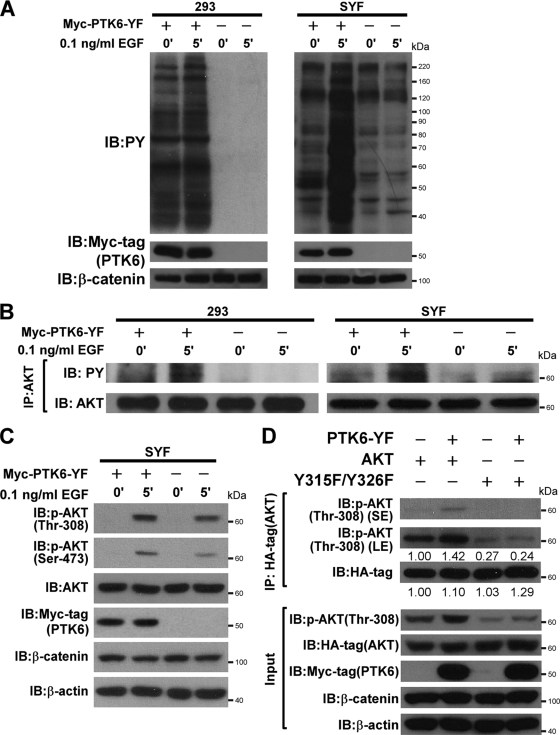
To confirm that AKT activation induced by a low dose of EGF in the presence of PTK6 is correlated with tyrosine phosphorylation of AKT induced by PTK6, we transiently transfected PTK6-YF plasmids into both HEK-293 cells and SYF cells, which significantly increases intracellular phosphotyrosine signaling compared with that of the stable PTK6-YF-expressing cell line. Twenty-four hours posttransfection, cells were serum starved for 24 h and then stimulated by 0.1 ng/ml EGF for 5 min. Twenty-four-hour serum starvation is able to reduce the phosphotyrosine background induced by active PTK6 in SYF cells, and EGF stimulation dramatically induced phosphotyrosine signaling only in the presence of active PTK6 (Fig. 7A, PY, right panel). Phosphotyrosine signaling in HEK-293 cells is more resistant to 24-h serum starvation, but differences under unstimulated (0′) and stimulated (5′) conditions could still be observed in the presence of active PTK6 (Fig. 7A, PY, left panel). To examine AKT tyrosine phosphorylation, AKT protein was immunoprecipitated from cell lysates in panel A, and immunoblotting was performed with antiphosphotyrosine antibodies. In both HEK-293 cells and SYF cells, tyrosine phosphorylation on AKT is dramatically increased in response to EGF stimulation only in the presence of active PTK6 (Fig. 7B, PY). In empty vector-transfected control cells, there is no change in AKT tyrosine phosphorylation before and after EGF stimulation (Fig. 7B, PY). AKT activation was also examined in SYF cells. After EGF treatment, AKT phosphorylation on Thr-308 and Ser-473 is increased in the presence of PTK6-YF (Fig. 7C). These data confirm the positive correlation between AKT tyrosine phosphorylation and AKT activation induced by PTK6-YF overexpression in both stable SYF cell lines (Fig. 6) and transiently transfected SYF cells (Fig. 7).
FIG. 7.
Active PTK6 promotes AKT activation through phosphorylation on tyrosine residues 315 and 326. (A) HEK-293 and SYF cells were transiently transfected with PTK6-YF or empty vector for 24 h, serum starved for another 24 h, and then stimulated with a low dose of EGF (0.1 ng/ml) for 5 min. Cell lysates were analyzed by immunoblotting with anti-PY, -Myc tag, and -β-catenin antibodies. β-Catenin served as a loading control. (B) Immunoblot analysis of AKT immunoprecipitated (IP) from lysates of HEK-293 cells and SYF cells previously described in the legend for panel A. Tyrosine phosphorylation of AKT was analyzed using anti-PY antibodies. The membrane was reprobed with an antibody directed against AKT. (C) SYF cell lysates described in the legend for panel A were analyzed by immunoblotting with anti-phospho-Thr-308, -phospho-Ser-473, -total AKT, -Myc tag, -β-catenin, and -β-actin antibodies. (D) Immunoblot analysis of HA tag immunoprecipitated (IP) from lysates of SYF cells coexpressing PTK6-YF and wild-type AKT or AKT Y315F/Y326F mutant. AKT activation was analyzed using anti-phospho-Thr-308 antibodies. Both short exposure (SE) and long exposures (LE) are shown. The membrane was reprobed with an antibody directed against the HA-tag. Relative band densities were quantified with NIH ImageJ software and are indicated under corresponding bands. Total cell lysates were analyzed by immunoblotting with anti-phospho-Thr-308, -HA tag, -Myc-tag, -β-catenin, and -β-actin antibodies as input. β-Catenin and β-actin served as loading controls.
To further address whether PTK6 directly promotes AKT activation through phosphorylation of tyrosine residues 315 and 326, PTK6-YF was coexpressed with HA-tagged wild-type AKT or AKT Y315F/Y326F mutant in SYF cells. Exogenous AKT was immunoprecipitated using anti-HA-tag antibody, and immunoblotting was performed to detect AKT activation with anti-phospho-Thr-308 antibody. Wild-type AKT is more activated in the presence of active PTK6, while the AKT Y315F/Y326F mutant has much less phosphorylation on Thr-308, and active PTK6 does not promote activation of the AKT Y315F/Y326F mutant (Fig. 7D). These data indicated that PTK6 positively regulates AKT signaling by directly phosphorylating AKT on tyrosine residues 315 and 326.
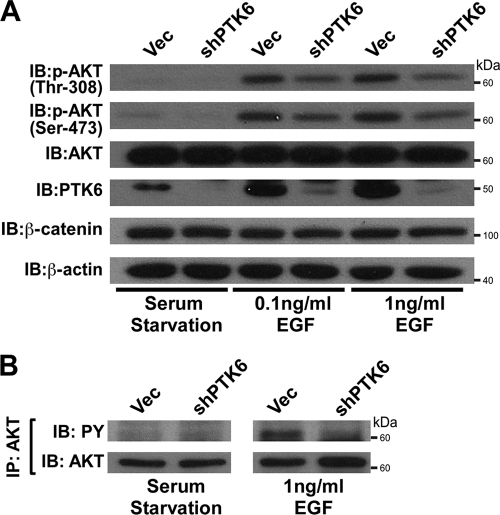
Knockdown of PTK6 in BPH-1 cells impairs AKT activation in response to EGF.
PTK6 is expressed in prostate epithelial cells (14). BPH-1 is a benign prostatic hyperplasia epithelial cell line which expresses PTK6 (23). BPH-1 cells also express high levels of EGF receptor, and EGF is able to induce strong tyrosine phosphorylation in this cell line (17). Therefore, the BPH-1 cell line was utilized to investigate whether loss of PTK6 is able to affect AKT activation in response to EGF. Both BPH-1 PTK6 knockdown and vector cells were serum starved for 48 h and stimulated by 0.1 ng/ml or 1 ng/ml EGF for 5 min. Probing blots with anti-Thr-308 and anti-Ser-473 antibodies showed impaired AKT activation when PTK6 is absent (Fig. 8A). AKT immunoprecipitation followed by immunoblotting was performed to detect AKT tyrosine phosphorylation in BPH-1 cells stably expressing PTK6 shRNA or empty vector. The level of AKT tyrosine phosphorylation in response to 1 ng/ml EGF is decreased in BPH-1 cells with knockdown of PTK6, compared with that in vector cells (Fig. 8B). The correlation between decreased AKT activation and decreased AKT tyrosine phosphorylation in PTK6 knockdown cells further supports a positive role for PTK6 in regulating AKT activation.
FIG. 8.
Knockdown of PTK6 in BPH-1 cells impairs AKT activation in response to EGF. (A) BPH-1 cells stably expressing PLKO-1 vector (vec) or PTK6 shRNA were serum starved for 48 h and then stimulated with 0.1 ng/ml or 1 ng/ml EGF for 5 min. Cell lysates were analyzed by immunoblotting with anti-phospho-Thr-308, -phospho-Ser-473, -total AKT, -PTK6, -β-catenin, and -β-actin antibodies. β-Catenin and β-actin served as loading controls. (B) Immunoblot analysis of AKT immunoprecipitated (IP) from BPH-1 cell lysates previously described in the legend for panel A. Tyrosine phosphorylation of AKT was analyzed using anti-PY antibodies. The membrane was reprobed with an antibody directed against AKT.
DISCUSSION
Although PTK6 shares overall structural similarity with Src, it lacks an N-terminal myristoylation/palmitoylation consensus sequence that is found in all Src family members (51). Lack of such a membrane-targeting signal appears to facilitate its localization to different cellular compartments, including the nucleus (14, 15). In the prostate, PTK6 is localized to nuclei of normal luminal epithelial cells but translocated to the cytoplasm in prostate cancer (14). These data suggested that PTK6 may play an oncogenic role when it is localized to the cytoplasm. This notion was supported by a recent report that showed that cytoplasm/membrane-targeted PTK6 promotes HEK-293 cell proliferation, cell survival, migration, and anchorage-independent growth, while nuclearly targeted PTK6 does not (25). In addition, our group recently demonstrated that PTK6 either positively or negatively regulates β-catenin transcriptional activity depending on its intracellular localization (41). Here we show that PTK6 preferentially phosphorylates AKT in the cytoplasm, compared with that in the membrane compartment (Fig. 1 and 2).
We demonstrate that AKT is a direct substrate of PTK6, and we have identified tyrosine residues 315 and 326 as the major sites in AKT that are phosphorylated by PTK6. It has been reported that Src also phosphorylates AKT on these two tyrosine residues (11). However, PTK6 is able to induce tyrosine phosphorylation in the absence of Src, Yes, and Fyn kinases in SYF cells. We examined the possible significance of phosphorylation of AKT tyrosine residue 215, which was identified as a target by mass spectrometry in our studies, and tyrosine residue 474, which was phosphorylated after the treatment of tyrosine phosphatase inhibitor pervanadate (12). However, mutation of tyrosine residues 215 and 474 to phenylalanine did not affect tyrosine phosphorylation of AKT induced by active PTK6, indicating that they are not key PTK6 target sites (data not shown). However, since tyrosine phosphorylation of AKT can still be detected after mutation of tyrosine 315 and 326 to phenylalanine (Fig. 3D), it is possible that other tyrosine site(s) are targeted by PTK6.
Similar to Src family kinases, the SH3 and SH2 domains of PTK6 play important roles in substrate recognition (24, 43, 44). Src interacts with AKT through its SH3 domain, which recognizes the proline-rich motif (P424XXP427) located at the AKT C terminus (27). Although the SH3 domain of PTK6 shares only approximately 35% amino acid identity with the Src SH3 domain (3), we found that PTK6 associates with AKT through the same proline-rich motif, P424XXP427, in AKT. Mutation of AKT proline residues 424 and 427 impaired association between PTK6 and AKT (Fig. 4E). We also demonstrated that the SH2 domain of PTK6, which recognizes phosphorylated tyrosines, also plays a role in regulating PTK6-AKT interactions. Mutation of AKT tyrosine residues 315 and 326 to phenylalanine diminishes association between PTK6 and AKT (Fig. 4A and C), and kinase-active PTK6-YF promotes PTK6-AKT association (Fig. 4D). The tyrosine residues targeted by PTK6 are located in the catalytic domain of AKT, and regulated association of PTK6 with AKT through kinase-independent SH3- and/or kinase-dependent SH2-mediated interactions may have an impact on AKT activity.
While phosphorylation of AKT on Ser-473 and Thr-308 is critical for AKT activation, phosphorylation of AKT on tyrosine residues is also becoming recognized as important for modulation of its activities. Both positive and negative roles for tyrosine phosphorylation in regulating AKT activity have been reported (11, 12, 28, 35, 55). We demonstrate a role for PTK6 in sensitizing SYF cells to a physiological concentration of EGF (0.1 ng/ml) leading to increased AKT activation and cell proliferation. This oncogenic role for PTK6 is kinase dependent. These experiments were performed in a background without the Src, Yes, and Fyn kinases, allowing us to delineate the specific roles of PTK6 more clearly. Our data are consistent with a previous publication that showed that phosphorylation of AKT on tyrosine residues 315 and 326 contributes to AKT activation (11) and with the observation that overexpression of PTK6 promotes breast cancer cell growth through stimulating ErbB receptor family signaling and PI3K/AKT signaling (22, 29, 30).
Although PTK6 appears to enhance oncogenic signaling in breast cancer cells, its activities have been correlated with differentiation and stress-induced apoptosis in normal tissues. Disruption of the Ptk6 gene led to enhanced proliferation and delayed enterocyte differentiation in the untreated mouse intestine (20). In addition, radiation-induced apoptosis was impaired in the Ptk6 null mouse (21). Both phenotypes were accompanied by an increase in activation of AKT signaling, leading to the hypothesis that PTK6 acts as an inhibitor of AKT in normal tissues. In addition, PTK6 was found to inhibit AKT in PTK6/AKT complexes in unstimulated cultured cells (55). When these studies were initiated, our goal was to determine how PTK6 inhibited AKT activities in mouse tissues and cells. However, although we determined that AKT is a direct PTK6 substrate, we did not discover a kinase-dependent inhibitory role for PTK6 in AKT regulation. In contrast, we found that PTK6 may stimulate AKT activation and cell proliferation in response to physiological levels of growth factors. The underlying mechanisms for PTK6-mediated inhibition of AKT activation may involve kinase-independent association/sequestration of AKT by PTK6 that is induced during differentiation or stress. Recent studies that are under way suggest that PTK6 may also promote activation of a phosphatase that targets activating phosphorylation of AKT (A. O. Perekatt and A. L. Tyner, unpublished data).
Like several other regulatory proteins, PTK6 functions appear dependent on cellular context, coexpression of other interacting signaling molecules, and its intracellular localization. A correlation between PTK6 and ErbB2 overexpression was revealed in invasive ductal breast carcinomas (8, 40), and PTK6 increases the ErbB2-induced activation of Ras/MAPK signaling to induce cell proliferation in breast cancers (54). Taken together, our studies suggest that PTK6 may also promote ErbB receptor signaling by enhancing AKT activation in response to physiological levels of growth factors. These studies provide insight about the potential benefits of targeting PTK6 as part of a therapeutic regimen to treat different types of cancer that have upregulated PTK6 expression and activity.
Acknowledgments
This work was supported by National Institutes of Health grants DK44525 and DK068503 (to A.L.T.).
We thank Patrick Brauer for reading the manuscript and helpful comments.
Footnotes
Published ahead of print on 6 July 2010.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Altomare, D. A., H. You, G. H. Xiao, M. E. Ramos-Nino, K. L. Skele, A. De Rienzo, S. C. Jhanwar, B. T. Mossman, A. B. Kane, and J. R. Testa. 2005. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene 24:6080-6089. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubele, M., G. Auer, A. K. Walch, A. Munro, M. J. Atkinson, H. Braselmann, T. Fornander, and J. M. Bartlett. 2007. PTK (protein tyrosine kinase)-6 and HER2 and 4, but not HER1 and 3 predict long-term survival in breast carcinomas. Br. J. Cancer 96:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubele, M., A. K. Walch, N. Ludyga, H. Braselmann, M. J. Atkinson, B. Luber, G. Auer, S. Tapio, T. Cooke, and J. M. Bartlett. 2008. Prognostic value of protein tyrosine kinase 6 (PTK6) for long-term survival of breast cancer patients. Br. J. Cancer 99:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker, K. T., L. E. Jackson, and M. R. Crompton. 1997. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene 15:799-805. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa, A., C. C. Kumar, A. Di Cristofano, and J. R. Testa. 2005. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv. Cancer Res. 94:29-86. [DOI] [PubMed] [Google Scholar]
- 8.Born, M., L. Quintanilla-Fend, H. Braselmann, U. Reich, M. Richter, P. Hutzler, and M. Aubele. 2005. Simultaneous over-expression of the Her2/neu and PTK6 tyrosine kinases in archival invasive ductal breast carcinomas. J. Pathol. 205:592-596. [DOI] [PubMed] [Google Scholar]
- 9.Brauer, P. M., and A. L. Tyner. 2010. Building a better understanding of the intracellular tyrosine kinase PTK6—BRK by BRK. Biochim. Biophys. Acta 1806:66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, H. Y., C. H. Shen, Y. T. Tsai, F. C. Lin, Y. P. Huang, and R. H. Chen. 2004. Brk activates Rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol. Cell. Biol. 24:10558-10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, R., O. Kim, J. Yang, K. Sato, K. M. Eisenmann, J. McCarthy, H. Chen, and Y. Qiu. 2001. Regulation of Akt/PKB activation by tyrosine phosphorylation. J. Biol. Chem. 276:31858-31862. [DOI] [PubMed] [Google Scholar]
- 12.Conus, N. M., K. M. Hannan, B. E. Cristiano, B. A. Hemmings, and R. B. Pearson. 2002. Direct identification of tyrosine 474 as a regulatory phosphorylation site for the Akt protein kinase. J. Biol. Chem. 277:38021-38028. [DOI] [PubMed] [Google Scholar]
- 13.Cui, Q. L., W. H. Zheng, R. Quirion, and G. Almazan. 2005. Inhibition of Src-like kinases reveals Akt-dependent and -independent pathways in insulin-like growth factor I-mediated oligodendrocyte progenitor survival. J. Biol. Chem. 280:8918-8928. [DOI] [PubMed] [Google Scholar]
- 14.Derry, J. J., G. S. Prins, V. Ray, and A. L. Tyner. 2003. Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene 22:4212-4220. [DOI] [PubMed] [Google Scholar]
- 15.Derry, J. J., S. Richard, H. Valderrama Carvajal, X. Ye, V. Vasioukhin, A. W. Cochrane, T. Chen, and A. L. Tyner. 2000. Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol. Cell. Biol. 20:6114-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Easty, D. J., P. J. Mitchell, K. Patel, V. A. Florenes, R. A. Spritz, and D. C. Bennett. 1997. Loss of expression of receptor tyrosine kinase family genes PTK7 and SEK in metastatic melanoma. Int. J. Cancer 71:1061-1065. [DOI] [PubMed] [Google Scholar]
- 17.El Sheikh, S. S., J. Domin, P. Abel, G. Stamp, and E.-N. Lalani. 2004. Phosphorylation of both EGFR and ErbB2 is a reliable predictor of prostate cancer cell proliferation in response to EGF. Neoplasia 6:846-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eves, E. M., W. Xiong, A. Bellacosa, S. G. Kennedy, P. N. Tsichlis, M. R. Rosner, and N. Hay. 1998. Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol. Cell. Biol. 18:2143-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gingras, A. C., S. G. Kennedy, M. A. O'Leary, N. Sonenberg, and N. Hay. 1998. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 12:502-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haegebarth, A., W. Bie, R. Yang, S. E. Crawford, V. Vasioukhin, E. Fuchs, and A. L. Tyner. 2006. Protein tyrosine kinase 6 negatively regulates growth and promotes enterocyte differentiation in the small intestine. Mol. Cell. Biol. 26:4949-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haegebarth, A., A. O. Perekatt, W. Bie, J. J. Gierut, and A. L. Tyner. 2009. Induction of protein tyrosine kinase 6 in mouse intestinal crypt epithelial cells promotes DNA damage-induced apoptosis. Gastroenterology 137:945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey, A. J., and M. R. Crompton. 2003. Use of RNA interference to validate Brk as a novel therapeutic target in breast cancer: Brk promotes breast carcinoma cell proliferation. Oncogene 22:5006-5010. [DOI] [PubMed] [Google Scholar]
- 23.Hayward, S. W., R. Dahiya, G. R. Cunha, J. Bartek, N. Deshpande, and P. Narayan. 1995. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell. Dev. Biol. Anim. 31:14-24. [DOI] [PubMed] [Google Scholar]
- 24.Hong, E., J. Shin, H. I. Kim, S. T. Lee, and W. Lee. 2004. Solution structure and backbone dynamics of the non-receptor protein-tyrosine kinase-6 Src homology 2 domain. J. Biol. Chem. 279:29700-29708. [DOI] [PubMed] [Google Scholar]
- 25.Ie Kim, H., and S. T. Lee. 2009. Oncogenic functions of PTK6 are enhanced by its targeting to plasma membrane but abolished by its targeting to nucleus. J. Biochem. 146:133-139. [DOI] [PubMed] [Google Scholar]
- 26.Jacinto, E., V. Facchinetti, D. Liu, N. Soto, S. Wei, S. Y. Jung, Q. Huang, J. Qin, and B. Su. 2006. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127:125-137. [DOI] [PubMed] [Google Scholar]
- 27.Jiang, T., and Y. Qiu. 2003. Interaction between Src and a C-terminal proline-rich motif of Akt is required for Akt activation. J. Biol. Chem. 278:15789-15793. [DOI] [PubMed] [Google Scholar]
- 28.Jung, H. S., D. W. Kim, Y. S. Jo, H. K. Chung, J. H. Song, J. S. Park, K. C. Park, S. H. Park, J. H. Hwang, K. W. Jo, and M. Shong. 2005. Regulation of protein kinase B tyrosine phosphorylation by thyroid-specific oncogenic RET/PTC kinases. Mol. Endocrinol. 19:2748-2759. [DOI] [PubMed] [Google Scholar]
- 29.Kamalati, T., H. E. Jolin, M. J. Fry, and M. R. Crompton. 2000. Expression of the BRK tyrosine kinase in mammary epithelial cells enhances the coupling of EGF signalling to PI 3-kinase and Akt, via erbB3 phosphorylation. Oncogene 19:5471-5476. [DOI] [PubMed] [Google Scholar]
- 30.Kamalati, T., H. E. Jolin, P. J. Mitchell, K. T. Barker, L. E. Jackson, C. J. Dean, M. J. Page, B. A. Gusterson, and M. R. Crompton. 1996. Brk, a breast tumor-derived non-receptor protein-tyrosine kinase, sensitizes mammary epithelial cells to epidermal growth factor. J. Biol. Chem. 271:30956-30963. [DOI] [PubMed] [Google Scholar]
- 31.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, S. T., K. M. Strunk, and R. A. Spritz. 1993. A survey of protein tyrosine kinase mRNAs expressed in normal human melanocytes. Oncogene 8:3403-3410. [PubMed] [Google Scholar]
- 33.Lin, H. S., G. J. Berry, W. E. Fee, Jr., D. J. Terris, and Z. Sun. 2004. Identification of tyrosine kinases overexpressed in head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 130:311-316. [DOI] [PubMed] [Google Scholar]
- 34.Llor, X., M. S. Serfas, W. Bie, V. Vasioukhin, M. Polonskaia, J. Derry, C. M. Abbott, and A. L. Tyner. 1999. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin. Cancer Res. 5:1767-1777. [PubMed] [Google Scholar]
- 35.Lodeiro, M., M. Theodoropoulou, M. Pardo, F. F. Casanueva, and J. P. Camina. 2009. c-Src regulates Akt signaling in response to ghrelin via beta-arrestin signaling-independent and -dependent mechanisms. PLoS One 4:e4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukong, K. E., D. Larocque, A. L. Tyner, and S. Richard. 2005. Tyrosine phosphorylation of sam68 by breast tumor kinase regulates intranuclear localization and cell cycle progression. J. Biol. Chem. 280:38639-38647. [DOI] [PubMed] [Google Scholar]
- 37.Mahajan, K., D. Coppola, S. Challa, B. Fang, Y. A. Chen, W. Zhu, A. S. Lopez, J. Koomen, R. W. Engelman, C. Rivera, R. S. Muraoka-Cook, J. Q. Cheng, E. Schonbrunn, S. M. Sebti, H. S. Earp, and N. P. Mahajan. 2010. Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation. PLoS One 5:e9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manning, B. D., and L. C. Cantley. 2007. AKT/PKB signaling: navigating downstream. Cell 129:1261-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell, P. J., K. T. Barker, J. E. Martindale, T. Kamalati, P. N. Lowe, M. J. Page, B. A. Gusterson, and M. R. Crompton. 1994. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene 9:2383-2390. [PubMed] [Google Scholar]
- 40.Ostrander, J. H., A. R. Daniel, K. Lofgren, C. G. Kleer, and C. A. Lange. 2007. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 67:4199-4209. [DOI] [PubMed] [Google Scholar]
- 41.Palka-Hamblin, H. L., J. J. Gierut, W. Bie, P. M. Brauer, Y. Zheng, J. M. Asara, and A. L. Tyner. 2010. Identification of β-catenin as a target of the intracellular tyrosine kinase PTK6. J. Cell Sci. 23:236-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petro, B. J., R. C. Tan, A. L. Tyner, M. W. Lingen, and K. Watanabe. 2004. Differential expression of the non-receptor tyrosine kinase BRK in oral squamous cell carcinoma and normal oral epithelium. Oral Oncol. 40:1040-1047. [DOI] [PubMed] [Google Scholar]
- 43.Qiu, H., and W. T. Miller. 2002. Regulation of the nonreceptor tyrosine kinase Brk by autophosphorylation and by autoinhibition. J. Biol. Chem. 277:34634-34641. [DOI] [PubMed] [Google Scholar]
- 44.Qiu, H., and W. T. Miller. 2004. Role of the Brk SH3 domain in substrate recognition. Oncogene 23:2216-2223. [DOI] [PubMed] [Google Scholar]
- 45.Robey, R. B., and N. Hay. 2009. Is Akt the “Warburg kinase”?—Akt-energy metabolism interactions and oncogenesis. Semin. Cancer Biol. 19:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098-1101. [DOI] [PubMed] [Google Scholar]
- 47.Schmandt, R. E., M. Bennett, S. Clifford, A. Thornton, F. Jiang, R. R. Broaddus, C. C. Sun, K. H. Lu, A. K. Sood, and D. M. Gershenson. 2006. The BRK tyrosine kinase is expressed in high-grade serous carcinoma of the ovary. Cancer Biol. Ther. 5:1136-1141. [DOI] [PubMed] [Google Scholar]
- 48.Serfas, M. S., and A. L. Tyner. 2003. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol. Res. 13:409-419. [DOI] [PubMed] [Google Scholar]
- 49.Shen, C. H., H. Y. Chen, M. S. Lin, F. Y. Li, C. C. Chang, M. L. Kuo, J. Settleman, and R. H. Chen. 2008. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration, and invasion. Cancer Res. 68:7779-7787. [DOI] [PubMed] [Google Scholar]
- 50.Siyanova, E. Y., M. S. Serfas, I. A. Mazo, and A. L. Tyner. 1994. Tyrosine kinase gene expression in the mouse small intestine. Oncogene 9:2053-2057. [PubMed] [Google Scholar]
- 51.Vasioukhin, V., M. S. Serfas, E. Y. Siyanova, M. Polonskaia, V. J. Costigan, B. Liu, A. Thomason, and A. L. Tyner. 1995. A novel intracellular epithelial cell tyrosine kinase is expressed in the skin and gastrointestinal tract. Oncogene 10:349-357. [PubMed] [Google Scholar]
- 52.Vasioukhin, V., and A. L. Tyner. 1997. A role for the epithelial-cell-specific tyrosine kinase Sik during keratinocyte differentiation. Proc. Natl. Acad. Sci. U. S. A. 94:14477-14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, T. C., S. H. Jee, T. F. Tsai, Y. L. Huang, W. L. Tsai, and R. H. Chen. 2005. Role of breast tumour kinase in the in vitro differentiation of HaCaT cells. Br. J. Dermatol. 153:282-289. [DOI] [PubMed] [Google Scholar]
- 54.Xiang, B., K. Chatti, H. Qiu, B. Lakshmi, A. Krasnitz, J. Hicks, M. Yu, W. T. Miller, and S. K. Muthuswamy. 2008. Brk is coamplified with ErbB2 to promote proliferation in breast cancer. Proc. Natl. Acad. Sci. U. S. A. 105:12463-12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, P., J. H. Ostrander, E. J. Faivre, A. Olsen, D. Fitzsimmons, and C. A. Lange. 2005. Regulated association of protein kinase B/Akt with breast tumor kinase. J. Biol. Chem. 280:1982-1991. [DOI] [PubMed] [Google Scholar]