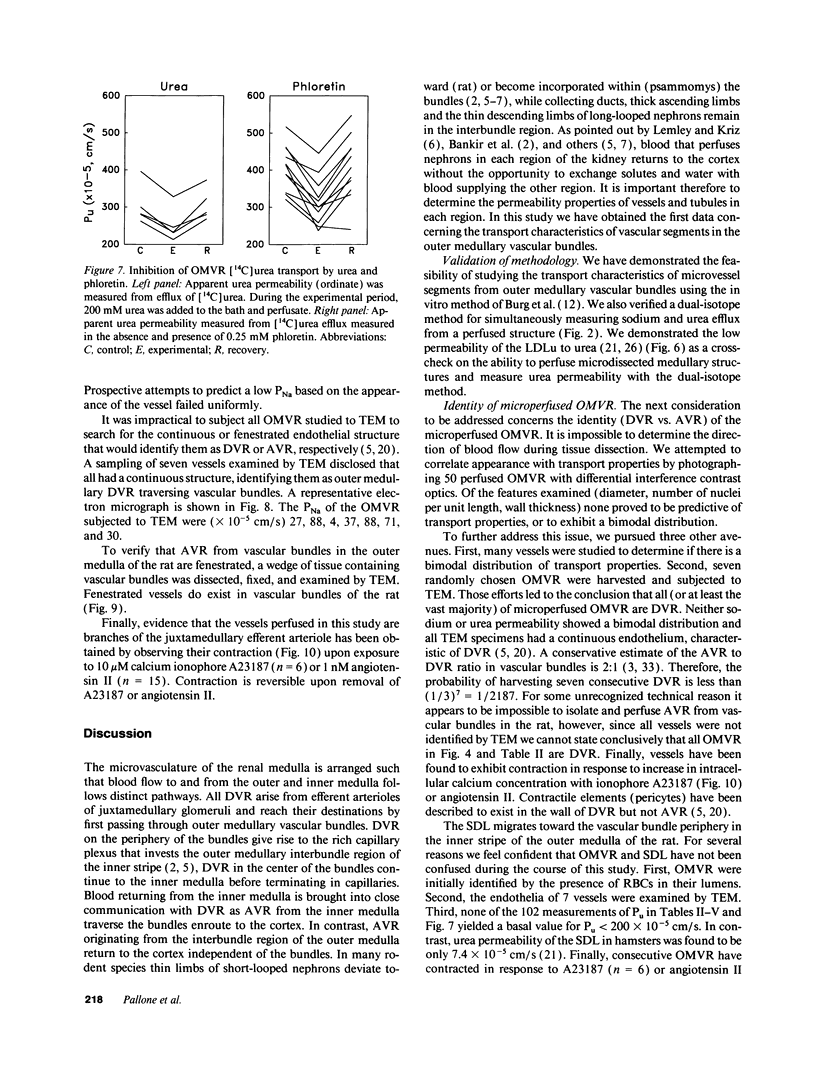
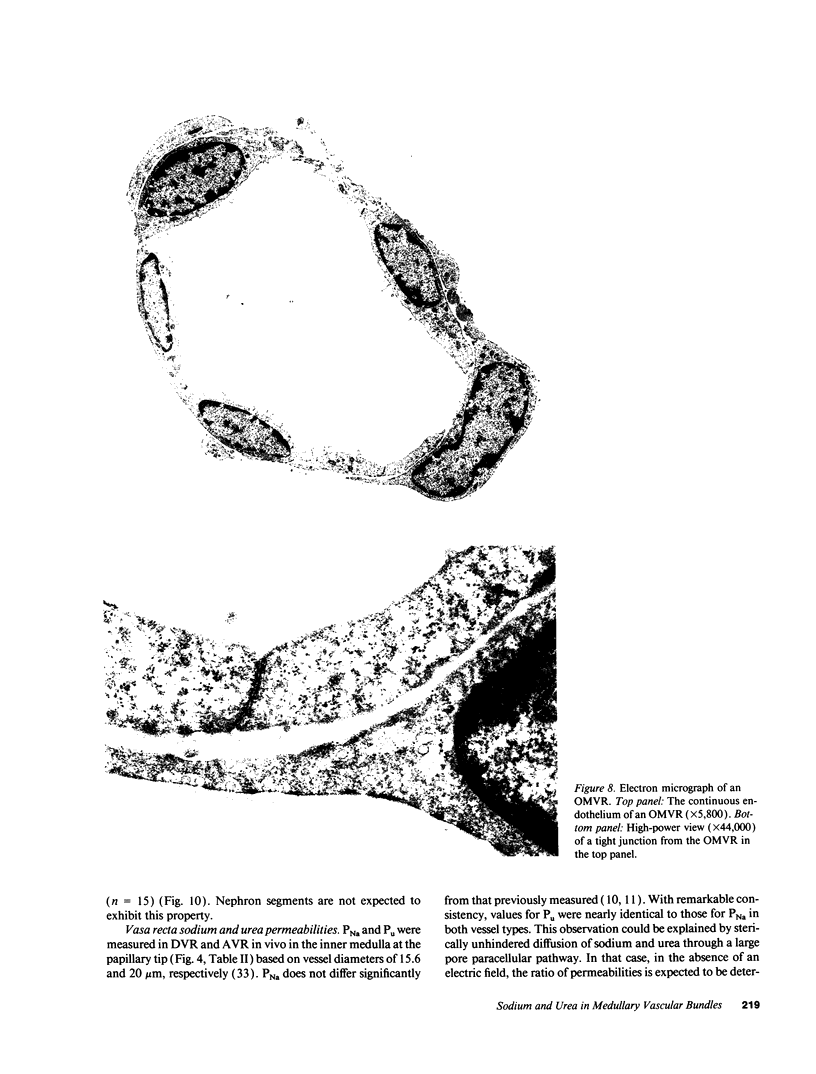
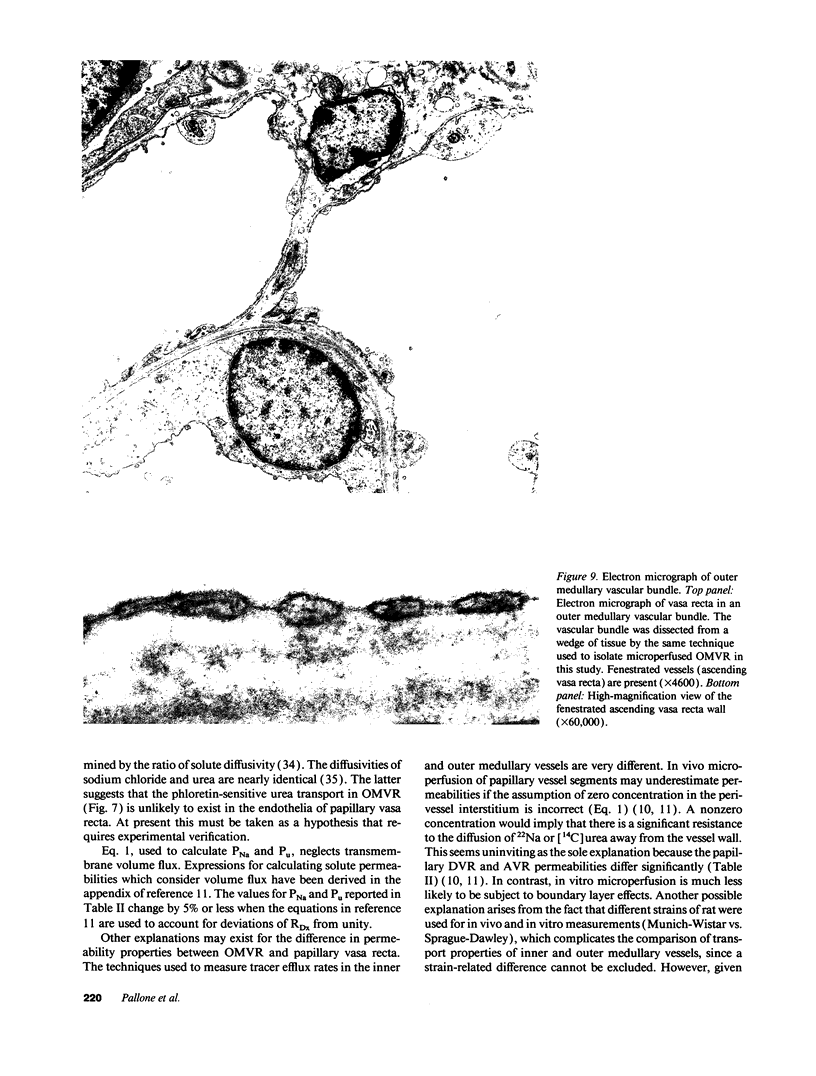
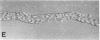Abstract
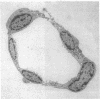
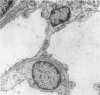
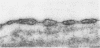
We dissected and perfused outer medullary vasa recta (OMVR) from vascular bundles in the rat. Permeabilities of sodium (PNa) and urea (Pu) were simultaneously determined from the lumen-to-bath efflux of 22Na and [14C]urea. PNa and Pu were also measured by in vivo microperfusion of descending (DVR) and ascending vasa recta (AVR) at the papillary tip of Munich-Wistar rats. In some OMVR PNa was indistinguishable from zero. The mean +/- SE of PNa (x 10(-5), cm/s) in OMVR was 76 +/- 9. Pu in OMVR was always very high (x 10(-5), cm/s), 360 +/- 14. There was no correlation between OMVR PNa and Pu. Inner medullary AVR and DVR had PNa of 115 +/- 10 and 75 +/- 10, respectively, and Pu of 121 +/- 10 and 76 +/- 11, respectively. PNa and Pu in papillary vasa recta were always nearly identical and highly correlated. Transport of [14C] urea in OMVR was reversibly inhibited by addition of unlabeled urea or phloretin to the bath and lumen, providing evidence for carrier-mediated transport. These data suggest that sodium and urea might traverse the wall of inner medullary vasa recta by a paracellular pathway while urea also crosses by a transcellular route in OMVR. Electron microscopic examination of seven in vitro perfused OMVR revealed no fenestrations and exposure of these vessels to 10 microM calcium ionophore A23187 or 1 nM angiotensin II resulted in reversible contraction, suggesting that in vitro perfused OMVR are DVR only.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLINER R. W., LEVINSKY N. G., DAVIDSON D. G., EDEN M. Dilution and concentration of the urine and the action of antidiuretic hormone. Am J Med. 1958 May;24(5):730–744. doi: 10.1016/0002-9343(58)90377-2. [DOI] [PubMed] [Google Scholar]
- Bankir L., Kaissling B., de Rouffignac C., Kriz W. The vascular organization of the kidney of Psammomys obesus. Anat Embryol (Berl) 1979 Jan 30;155(2):149–160. doi: 10.1007/BF00305748. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Lechene C. Renal medullary concentrating process: an integrative hypothesis. Am J Physiol. 1980 Dec;239(6):F578–F588. doi: 10.1152/ajprenal.1980.239.6.F578. [DOI] [PubMed] [Google Scholar]
- Brahm J. Urea permeability of human red cells. J Gen Physiol. 1983 Jul;82(1):1–23. doi: 10.1085/jgp.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973 Mar;224(3):659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- Burg M. B. Perfusion of isolated renal tubules. Yale J Biol Med. 1972 Jun-Aug;45(3-4):321–326. [PMC free article] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Chou C. L., Knepper M. A. In vitro perfusion of chinchilla thin limb segments: segmentation and osmotic water permeability. Am J Physiol. 1992 Sep;263(3 Pt 2):F417–F426. doi: 10.1152/ajprenal.1992.263.3.F417. [DOI] [PubMed] [Google Scholar]
- Chou C. L., Knepper M. A. In vitro perfusion of chinchilla thin limb segments: urea and NaCl permeabilities. Am J Physiol. 1993 Feb;264(2 Pt 2):F337–F343. doi: 10.1152/ajprenal.1993.264.2.F337. [DOI] [PubMed] [Google Scholar]
- Chou C. L., Knepper M. A. Inhibition of urea transport in inner medullary collecting duct by phloretin and urea analogues. Am J Physiol. 1989 Sep;257(3 Pt 2):F359–F365. doi: 10.1152/ajprenal.1989.257.3.F359. [DOI] [PubMed] [Google Scholar]
- Flessner M. F., Wall S. M., Knepper M. A. Permeabilities of rat collecting duct segments to NH3 and NH4+. Am J Physiol. 1991 Feb;260(2 Pt 2):F264–F272. doi: 10.1152/ajprenal.1991.260.2.F264. [DOI] [PubMed] [Google Scholar]
- Good D. W., Vurek G. G. Picomole quantitation of ammonia by flow-through fluorometry. Anal Biochem. 1983 Apr 1;130(1):199–202. doi: 10.1016/0003-2697(83)90670-x. [DOI] [PubMed] [Google Scholar]
- Holliger C., Lemley K. V., Schmitt S. L., Thomas F. C., Robertson C. R., Jamison R. L. Direct determination of vasa recta blood flow in the rat renal papilla. Circ Res. 1983 Sep;53(3):401–413. doi: 10.1161/01.res.53.3.401. [DOI] [PubMed] [Google Scholar]
- Imai M. Function of the thin ascending limb of Henle of rats and hamsters perfused in vitro. Am J Physiol. 1977 Mar;232(3):F201–F209. doi: 10.1152/ajprenal.1977.232.3.F201. [DOI] [PubMed] [Google Scholar]
- Imai M. Functional heterogeneity of the descending limbs of Henle's loop. II. Interspecies differences among rabbits, rats, and hamsters. Pflugers Arch. 1984 Dec;402(4):393–401. doi: 10.1007/BF00583940. [DOI] [PubMed] [Google Scholar]
- Imai M., Hayashi M., Araki M. Functional heterogeneity of the descending limbs of Henle's loop. I. Internephron heterogeneity in the hamster kidney. Pflugers Arch. 1984 Dec;402(4):385–392. doi: 10.1007/BF00583939. [DOI] [PubMed] [Google Scholar]
- Imai M., Kusano E. Effects of arginine vasopressin on the thin ascending limb of Henle's loop of hamsters. Am J Physiol. 1982 Aug;243(2):F167–F172. doi: 10.1152/ajprenal.1982.243.2.F167. [DOI] [PubMed] [Google Scholar]
- Imai M., Taniguchi J., Tabei K. Function of thin loops of Henle. Kidney Int. 1987 Feb;31(2):565–579. doi: 10.1038/ki.1987.37. [DOI] [PubMed] [Google Scholar]
- Imai M., Taniguchi J., Yoshitomi K. Transition of permeability properties along the descending limb of long-loop nephron. Am J Physiol. 1988 Mar;254(3 Pt 2):F323–F328. doi: 10.1152/ajprenal.1988.254.3.F323. [DOI] [PubMed] [Google Scholar]
- Kokko J. P., Rector F. C., Jr Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972 Oct;2(4):214–223. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Imai M. Effects of glutaraldehyde fixation on renal tubular function. I. Preservation of vasopressin-stimulated water and urea pathways in rat papillary collecting duct. Pflugers Arch. 1987 May;408(5):479–483. doi: 10.1007/BF00585072. [DOI] [PubMed] [Google Scholar]
- Lemley K. V., Kriz W. Cycles and separations: the histotopography of the urinary concentrating process. Kidney Int. 1987 Feb;31(2):538–548. doi: 10.1038/ki.1987.33. [DOI] [PubMed] [Google Scholar]
- Levine S., Franki N., Hays R. M. A saturable, vasopressin-sensitive carrier for urea and acetamide in the toad bladder epithelial cell. J Clin Invest. 1973 Aug;52(8):2083–2086. doi: 10.1172/JCI107393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. J., Segel L. A. Analysis of countercurrent diffusion exchange in blood vessels of the renal medulla. Am J Physiol. 1971 Sep;221(3):817–828. doi: 10.1152/ajplegacy.1971.221.3.817. [DOI] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol. 1968 Jul;215(1):108–115. doi: 10.1152/ajplegacy.1968.215.1.108. [DOI] [PubMed] [Google Scholar]
- Pallone T. L. Effect of sodium chloride gradients on water flux in rat descending vasa recta. J Clin Invest. 1991 Jan;87(1):12–19. doi: 10.1172/JCI114960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallone T. L., Robertson C. R., Jamison R. L. Renal medullary microcirculation. Physiol Rev. 1990 Jul;70(3):885–920. doi: 10.1152/physrev.1990.70.3.885. [DOI] [PubMed] [Google Scholar]
- Pallone T. L. Transport of sodium chloride and water in rat ascending vasa recta. Am J Physiol. 1991 Sep;261(3 Pt 2):F519–F525. doi: 10.1152/ajprenal.1991.261.3.F519. [DOI] [PubMed] [Google Scholar]
- Pallone T. L., Work J., Jamison R. L. Resistance of descending vasa recta to the transport of water. Am J Physiol. 1990 Oct;259(4 Pt 2):F688–F697. doi: 10.1152/ajprenal.1990.259.4.F688. [DOI] [PubMed] [Google Scholar]
- Pallone T. L., Yagil Y., Jamison R. L. Effect of small-solute gradients on transcapillary fluid movement in renal inner medulla. Am J Physiol. 1989 Oct;257(4 Pt 2):F547–F553. doi: 10.1152/ajprenal.1989.257.4.F547. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. M., Knepper M. A. Urea permeability of mammalian inner medullary collecting duct system and papillary surface epithelium. J Clin Invest. 1987 Jan;79(1):138–147. doi: 10.1172/JCI112774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. M., Karnovsky M. J., Vehkatachalam M. A. Ultrastructural differences between rat inner medullary descending and ascending vasa recta;. Lab Invest. 1976 Aug;35(2):161–170. [PubMed] [Google Scholar]
- Stephenson J. L. Concentration of urine in a central core model of the renal counterflow system. Kidney Int. 1972 Aug;2(2):85–94. doi: 10.1038/ki.1972.75. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Tabei K., Imai M. Profiles of water and solute transport along long-loop descending limb: analysis by mathematical model. Am J Physiol. 1987 Mar;252(3 Pt 2):F393–F402. doi: 10.1152/ajprenal.1987.252.3.F393. [DOI] [PubMed] [Google Scholar]
- Wexler A. S., Kalaba R. E., Marsh D. J. Three-dimensional anatomy and renal concentrating mechanism. I. Modeling results. Am J Physiol. 1991 Mar;260(3 Pt 2):F368–F383. doi: 10.1152/ajprenal.1991.260.3.F368. [DOI] [PubMed] [Google Scholar]
- You G., Smith C. P., Kanai Y., Lee W. S., Stelzner M., Hediger M. A. Cloning and characterization of the vasopressin-regulated urea transporter. Nature. 1993 Oct 28;365(6449):844–847. doi: 10.1038/365844a0. [DOI] [PubMed] [Google Scholar]
- de Rouffignac C., Bankir L., Roinel N. Renal function and concentrating ability in a desert rodent: the gundi (Ctenodactylus vali). Pflugers Arch. 1981 May;390(2):138–144. doi: 10.1007/BF00590196. [DOI] [PubMed] [Google Scholar]