Abstract
Pre-B-cell expansion is driven by signals from the interleukin-7 receptor and the pre-B-cell receptor and is dependent on cyclin D3 and c-Myc. We have shown previously that interferon regulatory factors 4 and 8 induce the expression of Ikaros and Aiolos to suppress pre-B-cell proliferation. However, the molecular mechanisms through which Ikaros and Aiolos exert their growth inhibitory effect remain to be determined. Here, we provide evidence that Aiolos and Ikaros bind to the c-Myc promoter in vivo and directly suppress c-Myc expression in pre-B cells. We further show that downregulation of c-Myc is critical for the growth-inhibitory effect of Ikaros and Aiolos. Ikaros and Aiolos also induce expression of p27 and downregulate cyclin D3 in pre-B cells, and the growth-inhibitory effect of Ikaros and Aiolos is compromised in the absence of p27. A time course analysis further reveals that downregulation of c-Myc by Ikaros and Aiolos precedes p27 induction and cyclin D3 downregulation. Moreover, downregulation of c-Myc by Ikaros and Aiolos is necessary for the induction of p27 and downregulation of cyclin D3. Collectively, our studies identify a pre-B-cell receptor signaling induced inhibitory network, orchestrated by Ikaros and Aiolos, which functions to terminate pre-B-cell expansion.
Successful recombination of the immunoglobulin (Ig) heavy-chain locus leads to the expression of Igμ and the assembly of the pre-B-cell receptor at the cell surface. In conjunction with signals delivered through the interleukin-7 receptor (IL-7R), pre-B-cell receptor (pre-BCR) expression induces a limited proliferative burst that is dependent upon the cell cycle regulators cyclin D3 and c-Myc (12). In the absence of cyclin D3, but not cyclin D2, the large pre-B-cell pool is greatly diminished and proliferation in both pro- and pre-B cells is impaired (3). Similarly, the deletion of c- and n-Myc induces a block at the pro-B-cell stage that is associated with impaired B-cell progenitor proliferation (10). It is likely that c-Myc also has a differentiative function since constitutive c-Myc expression restores pre-B-cell development in Rag-2−/− mice (10). Collectively, these studies identify both cyclin D3 and c-Myc as central mediators of both pro- and pre-B-cell proliferation.
Cyclin D3 expression is dependent upon both the IL-7R, which induces cyclin D3 mRNA, and the pre-BCR that regulates the stability of the cyclin D3 protein (3). This complementary regulation may underlie the observed synergy in proliferation when both receptors are stimulated (6). In contrast, in pro- and pre-B cells, Erk activation enhances c-Myc expression (38). Furthermore, c-Myc expression rescues defective proliferation in Erk1−/− Erk2−/− B-cell progenitors (38).
For progression beyond the large pre-B-cell stage, cells must exit the cell cycle and initiate light-chain recombination. This transition is associated with the pre-BCR mediated repression of cyclin D3 transcription (21). However, it is not known whether similar molecular processes silence c-Myc expression. Furthermore, the relationships between the repression of cyclin D3 and c-Myc and the induction of cell cycle inhibitor p27 are not known.
Interferon regulatory factor 4 (IRF4) and IRF8 are immune system-specific transcription factors that are critical for the development and function of B, T, and myeloid cells (15, 32). In the absence of IRF4 and IRF8, B-cell development is blocked at the large pre-B-cell stage (16). We and others have shown that IRF4 promotes light-chain rearrangement and transcription in pre-B cells (13, 18, 29). In addition, IRF4 and IRF8 have also been found to negatively regulate pre-B-cell proliferation through inducing the expression of Ikaros and Aiolos (17). Ikaros and Aiolos are members of Ikaros family of transcription factors that are critical for immune system development and function (2, 8). Ikaros and Aiolos bind to the same consensus sequence as either homodimers or heterodimers and can recruit transcriptional repressor complexes to silence gene expression (14, 31). The expression of Ikaros and Aiolos is elevated in pre-B cells, indicating that they may play an important role in pre-B-cell development (24). Indeed, Ikaros and Aiolos directly silence the expression of the surrogate light-chain gene λ5, leading to the downregulation of the pre-BCR (33). Furthermore, there is an expansion of pre-B cells in Aiolos-deficient mice, indicating that Aiolos may negatively regulate pre-B-cell expansion in vivo (35).
Previously, we have shown that Ikaros and Aiolos are expressed at low levels in IRF4 and IRF8 double-deficient pre-B cells (hereafter referred to as DKO pre-B), and reconstituting the expression of Ikaros and Aiolos inhibits their proliferation (17). However, the molecular mechanism through which Ikaros and Aiolos exert their growth-inhibitory effect remains to be determined. Here, we demonstrate that Ikaros and Aiolos directly bind the c-Myc promoter and repress c-Myc expression in pre-B cells. In addition, we demonstrate that the repression of c-Myc by Ikaros and Aiolos is necessary for the induction of p27 and the downregulation of cyclin D3. Collectively, our studies identify a molecular network that orchestrates cell cycle exit in pre-B cells.
MATERIALS AND METHODS
Mice.
IRF4 and IRF8 compound mutant mice have been described (18). p27 mutant mice and Eμ-Myc transgenic mice in the C57B6 background were obtained from the Jackson laboratory (5, 11). The mice were maintained under specific-pathogen-free conditions. Experiments were performed according to guidelines from the National Institutes of Health and with an approved IACUC protocol from the University of Nebraska Medical Center. Mice 5 to 8 weeks of age were used for the present study.
Cell culture and retroviral infection.
Pre-B cells were cultivated as described previously (18). Briefly, B220+ cells were isolated from mouse bone marrow by using a MACS separation column (Miltenyi Biotech). Purified cells were overlaid on top of an irradiated S17 stromal cell layer in Opti-MEM (Gibco) medium containing 5% fetal bovine serum, 50 μM β-mercaptoethanol, 2 mM l-glutamine, 100 U of penicillin-streptomycin, and 5 ng of IL-7 (R&D)/ml. Retroviral vectors expressing Ikaros, Aiolos, and p27 have been described previously (17). Retroviral infection of pre-B cells was conducted as described previously (17). The infected cells were analyzed by fluorescence-activated cell sorting (FACS) at the indicated time points.
FACS analysis and cell sorting.
Cells were preincubated with either 2% rat serum or Fc-Block (2.4G2) and then stained with optimal amounts of specific antibodies, either biotinylated or directly fluorophore conjugated. Antibodies to B220 (RA3-6B2), CD43 (S7), CD25, IgM, and λ5 were purchased from Pharmingen; anti-kappa (H139-52.1) antibody was obtained from Southern Biotech. FACS analysis was performed with a FACSCalibur flow cytometer. The infected pre-B cells were sorted based on green fluorescent protein (GFP) expression by using a BD FACSAria sorter. For the present study, only the top 10% GFP highest-expressing cells were analyzed by FACS and were isolated for real-time PCR analysis. Cell cycle analysis with live cells was conducted by using Hoechst 33342 as previously described (17).
ChIP assay.
The chromatin immunoprecipitation (ChIP) analysis with DKO pre-B cells was performed essentially as described previously (18). Briefly, DKO pre-B cells were infected with control or Ikaros- or Aiolos-expressing retrovirus. The infected cells were fixed in 1% paraformaldehyde, lysed, and sonicated to generate chromatin fragments. Chromatin fractions (equivalent to 4 million cells each) were immunoprecipitated with anti-Ikaros (H-100; Santa Cruz Biotech), anti-Aiolos (Aviva system), or isotype-specific control antibodies (rabbit IgG). Isolated DNA fragments were subjected to real-time PCR analysis with primer sets that target different regions of the c-Myc gene. The intensity of the amplified signals was expressed as a percentage of the input.
Real-time PCR analysis.
Total RNA was extracted by using TRIzol and reverse transcribed with a single-strand cDNA synthesis kit (Amersham). Quantitative real-time PCR analysis was carried out in a 7500 real-time PCR system (ABI) using SYBR green PCR core reagents (ABI). All samples were tested in triplicate, and average threshold cycle (CT) values were calculated and normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Each primer set was independently repeated three times, and average values and standard deviations were calculated. The sequences of the primer sets used in the present study are presented in detail in Table 1.
TABLE 1.
Primer set sequences
| Name | Sequence (5′-3′) |
|
|---|---|---|
| Primer 1 | Primer 2 | |
| C-myc | AGCTGCAGCCGCCCGCGCCCAGT | GGAGAAGTTGCCACCGCCGCCGTC |
| N-myc | AGAGCACAGCCCGGAGCCTTCGAAT | CCACATGCAGTCCTGAAGGATGACC |
| Cyclin D3 | CTGCTTGGGGACCAGCGTGT | GCAGGACAGGTAGCGATCCAGGTAG |
| λ5 | CTTCCCGGCAGGCTCCTGTT | CTCACCAAACACACTACGTGTGGCC |
| p27 | ATCCCTTGTCCCGACTCACTCG | AAGTGTACTGGAGGGCGGGGAA |
| Aiolos | AGCGAAGCCATACTGGTGAACGCCC | TGCCCCGTGAGCGCATCT |
| GAPDH | TGTGTCCGTCGTGGATCTGA | CCTGCTTCACCACCTTCTTGAT |
| p21 | GTG AGC AGT TGC GCC GTG AT | GGG AAT CTT CAG GCC GCT CA |
| Cyclin E | CCC CAG GAC TGC ATT TCA GC | TGA CGC TGC AGA AAG TGC TCA |
| Cyclin D2 | CCAAGCTGAAAGAGACCATCCCG | GCTCAATGAAGTCGTGAGGGGTGAC |
| Upstream ChIP | ACCACCCAATTCGTAAGCAGTCGGG | ATCCCACCACACCTGGCCTTATGC |
| Downstream ChIP | TGGAGAAAGAGGAGGGCGGAGA | CCACCTGAAGGAGAAAGGCGAGAG |
| Ikaros1 ChIP | GCTGCGCCCGAACAACCGTACAGAA | TTTTTCCTCCTCTCGCTTCCCCGCC |
| Ikaros2 ChIP | ATTCCAGCGAGAGACAGAGGGAGTG | CCGCTGCAATGGGCAAAGTTTCC |
Western blot analysis.
Pre-B cells were lysed and used for Western blot analysis. The signals were visualized by using a SuperSignal West Dura HRP detection kit (Pierce). Antibodies against c-Myc was obtained from Cell Signaling; antibodies to cyclin D3, p27, and β-actin (AC-15) were obtained from Santa Cruz Biotech.
RESULTS
Ikaros and Aiolos suppress c-Myc expression in pre-B cells.
Since c-Myc and cyclin D3 are important regulators of pre-B-cell expansion, the effects of Ikaros and Aiolos on c-Myc and cyclin D3 expression were examined in DKO pre-B cells. Control, Ikaros-infected, and Aiolos-infected DKO pre-B cells were isolated by sorting after 24 h. A previously described loss of function Ikaros mutant (IkarosDN) was used to infect DKO pre-B cells and served as an additional control. The infected cells coexpress GFP, and only the cells expressing a high level of GFP were sorted (top 10%). As shown in Fig. 1 A, the expression of c-Myc was reduced by ∼5-fold in the presence of either Ikaros or Aiolos. In contrast, the expression of cyclin D3 was not affected by Ikaros and Aiolos after 24 h (Fig. 1D). Ikaros and Aiolos were also able to suppress the expression of surrogate light-chain λ5, a known target of Ikaros and Aiolos (Fig. 1B). Pre-B cells also express n-Myc (25). However, the expression of n-Myc was not affected by Ikaros and Aiolos (Fig. 1C). In addition, the expression of cyclin D2 was moderately reduced, whereas the expression of cyclin E and p21 was not significantly affected by Aiolos (Fig. 1E, F, and G). In contrast, the overexpression of IkarosDN in DKO pre-B cells had no significant effect on the expression of cell cycle regulators. The infected cells (GFPhi) express Ikaros at a level that is ∼3-fold higher than the that of the control wild-type pre-B cells (Fig. 1H). This result indicates that Ikaros was not grossly overexpressed in the sorted cells. It should be pointed out that the cultured wild-type and DKO pre-B cells used here are exclusively cycling pre-B cells (data not shown). If Ikaros and Aiolos are critical for c-Myc downregulation, c-Myc should be expressed at a higher level in DKO pre-B cells, which have been shown to express low levels of endogenous Ikaros and Aiolos. Since DKO pre-B cells are primarily cycling pre-B cells, we decided to compare c-Myc expression between DKO pre-B cells and sorted wild-type large pre-B cells. Indeed, in DKO pre-B cells, c-Myc was expressed at a level that is ∼2.5-fold higher than that of wild-type large pre-B cells (Fig. 1I). In summary, our results show that Ikaros and Aiolos suppress c-Myc expression in DKO pre-B cells.
FIG. 1.
Ikaros and Aiolos suppress c-Myc expression in pre-B cells. DKO pre-B cells were cultivated in the presence of IL-7 (5 ng/ml). The cells were infected with retrovirus expressing Ikaros, Aiolos, and IkarosDN. Infected cells were isolated 24 h later by sorting. Empty vector-infected cells were analyzed as a control. The expression of c-Myc (A), λ5 (B), n-Myc (C), cyclin D3 (D), cyclin D2 (E), p21 (F), and cyclin E (G) was analyzed in the sorted cells by real-time PCR. (H) Expression levels of Ikaros in wild-type pre-B and infected DKO pre-B cells. Cultured wild-type pre-B cells and DKO pre-B cells were lysed for protein analysis. The Ikaros-infected DKO pre-B cells which express a high level of GFP were sorted at 24 h after infection for Western blot analysis. (I) DKO pre-B cells express a higher level of c-Myc than wild-type large pre-B cells. Pre-B cells (B220+ CD43− IgM−) were isolated by sorting from DKO mice. Pre-B cells in wild-type control mice were further separated based on the forward scatter, and only the large pre-B cells were isolated by sorting. Real-time PCR analysis was carried out to examine c-Myc expression in isolated wild-type large pre-B cells and DKO pre-B cells. The values shown are averages and standard deviations from three independent experiments. The statistical significance was determined by a Student t test (*, P < 0.05; **, P < 0.01).
Ikaros and Aiolos bind the c-Myc promoter in pre-B cells.
Since Ikaros and Aiolos are well-characterized transcriptional repressors, one simple scenario for our findings would be that Ikaros and Aiolos directly repress c-Myc. To examine this possibility, we analyzed the promoter region of the murine c-Myc gene. The c-Myc gene has two major transcription initiation sites, P1 and P2, and P2 is 187 bp downstream of P1 (Fig. 2 A) (36). It has been shown that Ikaros binds to a core consensus sequence containing TGGGAA (23). A few potential Ikaros binding sites were identified on the 4-kb region upstream of P2. Since important regulatory elements tend to be conserved between species, we compared human and murine c-Myc promoters to narrow down the list of potential Ikaros binding sites. Two conserved Ikaros binding sites were identified. One site is located 75 bp upstream of P1 site (Ikaros1), and the other site is located 219 bp downstream of P2 (Ikaros2) (Fig. 2A). The site Ikaros1 which exists in a reverse orientation is 100% identical between mouse and human (Fig. 2B). Interestingly, it lies immediately adjacent to an Sp1 site that has been shown to play an important role in activation of the c-Myc promoter (1, 7). The site Ikaros2 on the murine and human c-Myc promoter consists of an identical CTGGGAA sequence located 480 bp downstream from Ikaros1. The identification of these highly conserved Ikaros binding sites on the c-Myc promoter strongly suggests that c-Myc is a potential target of Ikaros and Aiolos in pre-B cells.
FIG. 2.
Aiolos and Ikaros bind c-Myc promoter in pre-B cells in vivo. (A) Schematic diagram of the murine c-Myc promoter. Two major transcription starting sites, P1 and P2, are indicated. Two highly conserved Ikaros binding sites Ikaros1 and Ikaros2 are indicated with black bars. The shaded rectangle represents the first exon of c-Myc gene. (B) Sequence alignment of Ikaros1 and surrounding sequences between the human and mouse c-Myc promoters. (C and D) Aiolos and Ikaros bind to the c-Myc promoter in vivo. DKO pre-B cells were infected with control, Aiolos, or Ikaros. A ChIP assay was performed 24 h later to determine whether Aiolos and Ikaros bind to c-Myc promoter in vivo. The empty-vector-infected cells were analyzed as a control (Vector). The binding of control antibody (rabbit IgG), Aiolos antibody (Aiolos Ig), and Ikaros antibody (Ikaros Ig) to the Ikaros1 and Ikaros2 sites on c-Myc promoter was analyzed by real-time PCR. The binding of Aiolos and Ikaros to regions either 2 kb upstream or downstream of c-Myc promoter was also examined. The intensity of the signals is expressed as a percentage of the input. The values are averages and standard deviations from three independent experiments. (E) Ikaros binds to c-Myc promoter in sorted wild-type pre-B cells. Pre-B cells (B220+ CD43− IgM−) were isolated via sorting from wild-type mice. A total of 107 pre-B cells were used for ChIP analysis.
To determine whether Ikaros and Aiolos bind to the c-Myc promoter in vivo, Aiolos, Ikaros, and control vector-infected DKO pre-B cells were isolated for ChIP analysis. The sonicated protein-DNA complexes were immunoprecipitated with anti-Aiolos, anti-Ikaros, or control antibodies, and the immunoprecipitated DNA fragments were amplified by real-time PCR with primers targeting the Ikaros1 and Ikaros2 sites individually. As shown in Fig. 2C and D, Aiolos- and Ikaros-infected cells yielded signals that were 20- to 30-fold higher than the control infected cells, indicating that Aiolos and Ikaros bind to c-Myc promoter region in vivo. No significant changes in Aiolos or Ikaros binding were observed in regions either 2 kb upstream or 2 kb downstream of c-Myc promoter, indicating that Aiolos and Ikaros predominantly bind to the promoter region (Fig. 2C and D). To further determine whether Ikaros binds to c-Myc promoter in wild-type pre-B cells under physiological conditions, we isolated pre-B cells via sorting from the bone marrow of wild-type mice and performed ChIP analysis. Our result shows that Ikaros indeed bound to the Ikaros1 and Ikaros2 sites in wild-type pre-B cells, since anti-Ikaros antibody yielded signals that are ∼8-fold higher compared to the control antibody at the Ikaros1 and Ikaros2 sites (Fig. 2E). It should be pointed out that we did not analyze the binding of Aiolos and Ikaros to other regions of c-Myc locus, so it is possible that there are other binding sites of Aiolos and Ikaros on the c-Myc locus. Nevertheless, our results confirm that Aiolos and Ikaros bind to the c-Myc promoter in vivo in pre-B cells.
Downregulation of c-Myc is critical for Aiolos and Ikaros to exert their growth-inhibitory effect on pre-B cells.
To determine what role the downregulation of c-Myc plays in Aiolos-mediated growth inhibition, DKO pre-B cells were infected simultaneously with retroviruses encoding Aiolos (YFP) and c-Myc fused to the estrogen receptor ligand-binding domain (MycER, GFP). The MSCV-MycER-GFP plasmid has been described elsewhere (27). Two days after infection, FACS analysis revealed four populations of cells: (i) uninfected cells, (ii) cells infected by Aiolos alone (Aiolos), (iii) cells infected by MycER alone (MycER), and (iv) cells successfully infected by both Aiolos and MycER (Aiolos+MycER) (Fig. 3 A). This approach has been described in detail in our previous studies (17). As expected, 62% of pre-B cells expressing MycER were cycling compared to 43% of uninfected cells, indicating that a high level of c-Myc promotes pre-B-cell proliferation (Fig. 3A). Only 18% of cells infected with Aiolos were cycling, a finding consistent with our previous report that Aiolos inhibits pre-B-cell proliferation (Fig. 3A). However, 48% of pre-B cells infected with both Aiolos and MycER were still cycling, indicating that restoring c-Myc expression antagonizes the growth-inhibitory effect of Aiolos (Fig. 3A).
FIG. 3.
Downregulation of c-Myc is critical for Aiolos and Ikaros to exert their growth-inhibitory effect on pre-B cells. (A) Reconstituting c-Myc expression antagonizes Aiolos-mediated growth inhibition. DKO pre-B cells were infected simultaneously with virus expressing MycER and Aiolos. At 12 h after infection, Tamoxifen at 1 μM was added to activate the MycER protein. After 48 h, the infected cells were incubated with Hoechst dye, and the cell cycle status of the infected cells was determined by FACS. The numbers indicate the percentages of cells in the cycle. The result is representative of at least three independent experiments. (B) Wild-type (Wt) and Eμ-Myc pre-B cells were cultivated in the presence of IL-7 until they were homogenous B220+ pre-BCR+ IgM−. The cells were infected with Aiolos and Ikaros, and the impact of Aiolos and Ikaros on cell cycle progression was analyzed 48 h later by FACS. The infected cells expressing high levels of GFP-positive (GFP+) and GFP-negative (GFP−) cells were analyzed separately. The result is a representative of at least three independent experiments. (C) The GFP+ and GFP− cells were also isolated by sorting, and real-time PCR analysis was performed to measure λ5 expression. The values are averages and standard deviations from three independent experiments.
To further confirm that downregulation of c-Myc is necessary for Aiolos- and Ikaros-mediated growth inhibition, we overexpressed Aiolos and Ikaros in pre-B cells derived from Eμ-Myc transgenic mice. In the Eμ-Myc transgenic mice, the expression of the c-Myc transgene is under the control of immunoglobulin (Ig) heavy-chain intronic enhancer and thus cannot be repressed by Aiolos (11). Eμ-Myc mice develop leukemia at 5 to 6 months of age; however, the mice used in the present study were 5 to 6 weeks old and were tumor-free. In cultures with stroma cells and IL-7, both Eμ-Myc and wild-type bone marrow yield homogenous populations of cycling IgM− pre-BCR+ pre-B cells (data not shown). We speculated that the reason that kept these cells in cell cycle is that they do not express sufficient amounts of Ikaros and Aiolos. Indeed, overexpression of Aiolos and Ikaros led to growth arrest in wild-type pre-B cells (Fig. 3B). In contrast, the overexpression of Aiolos and Ikaros has no obvious effect on the proliferation of Eμ-Myc pre-B cells (Fig. 3B). Importantly, expression of the surrogate light-chain λ5 is downregulated to the same extent by Aiolos and Ikaros in both wild-type and Eμ-Myc pre-B cells (Fig. 3C), indicating that Aiolos and Ikaros are being expressed at sufficient amounts in both cells. Taken together, our results indicate that downregulation of c-Myc by Aiolos and Ikaros is critical for its growth-inhibitory effect in pre-B cells.
Suppression of c-Myc by Aiolos and Ikaros in pre-B cells precedes induction of p27 and downregulation of cyclin D3.
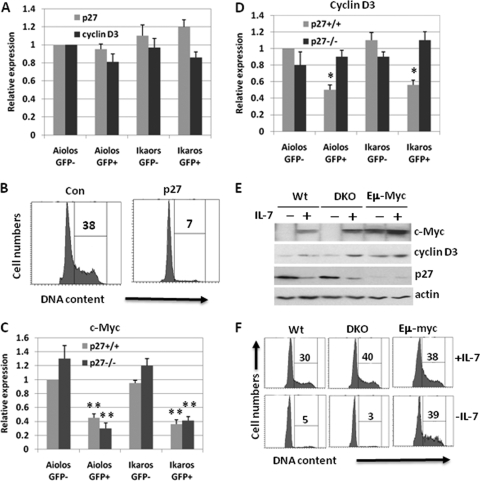
Both cyclin D3 and p27 have been linked to the control of pre-B-cell proliferation (3, 12). Here, we wanted to determine the kinetics of Aiolos- and Ikaros-mediated modulation of c-Myc, p27, and cyclin D3 expression. To this end, Aiolos- and Ikaros-infected DKO pre-B cells were isolated at various times after infection, and the expression of c-Myc, cyclin D3, and p27 proteins was analyzed in the sorted cells (Fig. 4 A). The intensities of c-Myc and cyclin D3 signals were quantified with a densitometer (data not shown). c-Myc protein expression was dramatically reduced in Aiolos- and Ikaros-infected cells. A 5-fold decrease and a 2.5-fold decrease, respectively, were observed as early as 24 h after the infection in Aiolos- and Ikaros-infected cells (Fig. 4A). These results are consistent with our conclusion that Aiolos and Ikaros directly suppress c-Myc expression (Fig. 4A). In addition, Aiolos and Ikaros expression led to cyclin D3 downregulation and p27 induction (Fig. 4A). However, the downregulation of cyclin D3 and induction of p27 only became evident 48 h after infection, indicating that these events occur after c-Myc downregulation. These data suggest that the induction of p27 and downregulation of cyclin D3 are secondary events triggered by Aiolos and Ikaros expression in pre-B cells. However, a greater degree of cell cycle inhibition by Aiolos and Ikaros was observed at 48 h than at 24 h (data not shown), which coincides with cyclin D3 downregulation and p27 induction, indicating that those later events are critical components of Aiolos-mediated growth inhibition.
FIG. 4.

Downregulation of c-Myc by Aiolos and Ikaros in pre-B cells precedes p27 induction and cyclin D3 downregulation. DKO pre-B cells were infected with control and Aiolos- and Ikaros-expressing viruses. The infected cells expressing a high level of GFP were isolated at 24- and 48-h time points. Empty-vector-infected cells were isolated 48 h later as control. (A) Western blot analysis was carried out to determine the expression levels of c-Myc, cyclin D3, and p27 in the infected cells at different time points. (B) The expression of p27 mRNA was examined in the infected cells at different time points by real-time PCR. The result is a representative of at least three independent experiments.
The induction of p27 mRNA by Aiolos and Ikaros was further confirmed by real-time PCR analysis in the infected cells (Fig. 4B). Consistent with the protein analysis, induction of p27 mRNA was delayed and only became obvious after 48 h. In summary, our results identify two critical events that happen in a sequential manner in Aiolos- and Ikaros-infected pre-B cells: an initial suppression of c-Myc, which is followed by p27 induction and cyclin D3 downregulation.
The growth-inhibitory effect of Aiolos and Ikaros on pre-B cells is impaired in the absence of p27.
The induction of p27 by Aiolos and Ikaros in pre-B cells suggests that p27 may play a role in Aiolos- and Ikaros-mediated growth inhibition. To examine this question, we infected cultured p27+/+ and p27−/− pre-B cells with Aiolos and Ikaros. The infected cells were analyzed by FACS 48 h later. As expected, Aiolos and Ikaros dramatically inhibited the proliferation of p27+/+ pre-B cells: the percentages of cycling cells decreased from 24 to 5% and from 25 to 6%, respectively (Fig. 5 A). In contrast, the proliferation of p27−/− cells was only moderately affected by Aiolos and Ikaros: the percentages of cycling cells decreased from 26 to 18% and from 28 to 16%, respectively. Infected cells appeared to express sufficient amounts of Aiolos and Ikaros, since the expression of λ5 was suppressed by Aiolos and Ikaros to the same extent in p27+/+ and p27−/− pre-B cells (Fig. 5B). Collectively, these results indicate that the growth-inhibitory effect of Aiolos and Ikaros on pre-B cells is partially dependent on p27 induction.
FIG. 5.
The growth-inhibitory effects of Aiolos and Ikaros on pre-B cells are compromised in the absence of p27. p27+/+ and p27−/− pre-B cells were infected with Aiolos and Ikaros. (A) The effect of Aiolos and Ikaros on cell cycle progression was analyzed 48 h later by FACS. Infected cells expressing a high level of GFP (GFP+) and the GFP-negative (GFP−) cells were analyzed separately. (B) The expression of λ5 was determined in GFP+ and GFP− cells by real-time PCR analysis. (C) Bone marrow cells from p27+/+ and p27−/− mice were stained with antibodies to B220, CD25, and IgM. After surface staining, the cells were incubated with Hoechst dye 33342 and analyzed by FACS. The cell cycle status of the pre-B cells (B220+ CD25+ IgM−) was examined. In addition, pre-B cells were further fractionated into small pre-B and large pre-B categories based on the forward scatter. The numbers indicate the percentages of large pre-B cells in p27+/+ and p27−/− mice. The result is representative of at least three independent experiments. The statistical significance was determined by a Student t test (*, P < 0.05; **, P < 0.01).
To determine whether p27 plays a role in pre-B-cell proliferation in vivo, we analyzed pre-B cells isolated from p27−/− and p27+/+ mice. Bone marrow cells were stained with cell surface markers and Hoechst dye 33342, and the cell cycle status of pre-B cells (B220+, CD25+, and IgM−) was examined. As shown in Fig. 5C, 19% of p27−/− pre-B cells were cycling compared to only 11% in p27+/+ pre-B cells, indicating that p27−/− pre-B cells are hyperproliferative in vivo. The percentages of large pre-B to small pre-B cells were also altered in p27−/− mice (Fig. 5C). The percentages of small pre-B and large pre-B cells in p27+/+ mice were 80% ± 3% and 20% ± 3%, respectively, whereas in p27−/− mice, the percentages of large pre-B and small pre-B cells changed to 69% ± 4% and 31% ± 4%, respectively. Taken together, our results indicate that p27 regulates pre-B-cell proliferation and the transition from large pre-B to small pre-B in vivo.
Suppression of c-Myc by Aiolos is necessary for p27 induction and cyclin D3 downregulation in pre-B cells.
Our results indicate that downregulation of c-Myc by Aiolos and Ikaros occurs rapidly via direct suppression, whereas the induction of p27 and the downregulation of cyclin D3 by Aiolos and Ikaros happen later. Although these cell cycle regulatory events are temporally separated, the causal relationships between them are unclear. Therefore, we sought to determine whether downregulation of c-Myc was critical for p27 induction and cyclin D3 downregulation. To this end, Eμ-Myc pre-B cells (described in Fig. 3C) were infected with retroviruses encoding Aiolos and Ikaros. At 48 h later, GFP+ cells were analyzed for the expression of p27 and cyclin D3. As demonstrated in Fig. 6 A, in the presence of sustained c-Myc, the ability of Aiolos and Ikaros to downregulate cyclin D3 and to induce p27 was attenuated. These results indicate that Aiolos and Ikaros indirectly regulate cyclin D3 and p27 by suppressing c-Myc. Although Aiolos and Ikaros were unable to inhibit the proliferation of Eμ-Myc pre-B cells, forced expression of p27 in Eμ-Myc pre-B cells shut down their proliferation (Fig. 6B). This result supports our view that induction of p27 is an important component of the ability of Aiolos and Ikaros to inhibit cell proliferation following c-Myc downregulation.
FIG. 6.
Downregulation of c-Myc by Aiolos and Ikaros is necessary for p27 induction and cyclin D3 downregulation. (A) The induction of p27 and downregulation of cyclin D3 by Aiolos and Ikaros are dependent on c-Myc downregulation in pre-B cells. The expression of p27 and cyclin D3 was examined in mRNA isolated from Aiolos- and Ikaros-infected Eμ-Myc pre-B cells (described in Fig. 3C). (B) Forced expression of p27 is sufficient to inhibit the proliferation of Eμ-Myc pre-B cells. Eμ-Myc pre-B cells were infected with virus expressing p27, and the effect of p27 on cell cycle progression was examined after 48 h. Infected cells expressing a high level of GFP-positive (p27) and GFP-negative (Con) cells were analyzed separately. (C) The downregulation of c-Myc by Aiolos and Ikaros is independent of p27. The expression of c-Myc was examined by using mRNA isolated from the Aiolos- and Ikaros-infected p27+/+ and p27−/− pre-B cells (described in Fig. 5B). (D) Downregulation of cyclin D3 by Aiolos and Ikaros is compromised in p27−/− pre-B cells. The expression of cyclin D3 was examined by using mRNA isolated from the Aiolos- and Ikaros-infected p27+/+ and p27−/− pre-B cells (described in Fig. 5B). (E and F) Sustained c-Myc expression blocks IL-7 withdrawal induced cell cycle arrest and prevents p27 induction and cyclin D3 downregulation. Wild-type (Wt), DKO, and Eμ-Myc pre-B cells were cultivated in the presence or absence of IL-7 for 24 h and then lysed for Western blot analysis with antibodies against indicated proteins (E). The cell cycle status of the cells was also analyzed by FACS (F). The result is representative of at least three independent experiments. The statistical significance was determined by a Student t test (*, P < 0.05; **, P < 0.01).
To further confirm the hierarchical relationship between c-Myc, p27, and cyclin D3, we sought to determine whether the downregulation of c-Myc by Aiolos and Ikaros is independent of p27. To this end, we analyzed c-Myc expression in Aiolos- and Ikaros-infected p27+/+ and p27−/− pre-B cells (described in Fig. 5B), c-Myc expression was downregulated in both p27+/+ and p27−/− pre-B cells, indicating that Aiolos and Ikaros are able to suppress c-Myc expression in the absence of p27 (Fig. 6C). Intriguingly, downregulation of cyclin D3 by Aiolos and Ikaros, however, was impaired in p27−/− pre-B cells (Fig. 6D). Collectively, these results indicate that Aiolos and Ikaros directly repress c-Myc, which in turn leads to the upregulation of p27 and the downregulation of cyclin D3.
Pre-B cells require IL-7 to proliferate in vivo and in vitro. Cell cycle arrest induced by IL-7 withdrawal in wild-type and DKO pre-B cells was associated with the downregulation of c-Myc and cyclin D3 and the induction of p27 (Fig. 6E). However, Eμ-Myc pre-B cells failed to downregulate cyclin D3 and to induce p27 after IL-7 withdrawal owing to sustained c-Myc expression (Fig. 6E). As a result, Eμ-Myc pre-B cells remained in cycle in the absence of IL-7 (Fig. 6F). These results further demonstrate the necessity of c-Myc downregulation in the transition of cycling pre-B to resting pre-B cells.
DISCUSSION
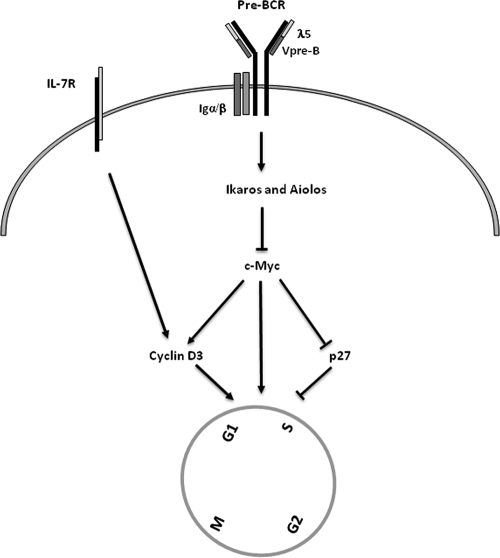
It has been shown that the expression of c-Myc is induced at the cycling pre-B-cell stage and then is downregulated in small resting pre-B cells (38). Herein, we demonstrate that Ikaros and Aiolos bind to the c-Myc promoter and directly suppress c-Myc expression. Repression of c-Myc is critical for Ikaros and Aiolos to exert their growth-inhibitory effect, as Aiolos and Ikaros fail to inhibit pre-B-cell proliferation in the presence of sustained c-Myc expression. Aiolos and Ikaros also inhibit the expression of cyclin D3 and augment p27 expression. However, these latter events depend upon, and follow, c-Myc downregulation (Fig. 7).
FIG. 7.
Schematic diagram depicting components of an inhibitory network that orchestrates the transition from large pre-B cells to small pre-B cells. Pre-B-cell expansion is controlled by IL-7R and pre-BCR signaling. Pre-BCR signaling then induces the expression of Ikaros and Aiolos, which bind the c-Myc promoter and suppress c-Myc expression. The suppression of c-Myc by Ikaros and Aiolos triggers p27 induction and cyclin D3 downregulation. Together, these events lead to cell cycle exit and the transition from cycling pre-B to resting pre-B cells.
The evidence that support c-Myc as a direct target of Ikaros and Aiolos in pre-B cells are the following: (i) we identified highly conserved Ikaros binding sites on the c-Myc promoter, (ii) we detected binding of Ikaros and Aiolos to those sites by ChIP in vivo, and (iii) we observed rapid downregulation of c-Myc transcript by Ikaros and Aiolos in a fashion that is similar to that observed with λ5, a known target of Ikaros and Aiolos. However, we do not have direct functional evidence to demonstrate that the Ikaros binding sites identified here are indeed important for Ikaros- and Aiolos-mediated c-Myc downregulation. Further studies are needed to elucidate precisely how c-Myc is downregulated by Ikaros and Aiolos in pre-B cells. It has been shown that Aiolos downregulates cyclin D3 in pre-B cells (21). However, our results do not support a direct role for Aiolos and Ikaros in suppressing cyclin D3 expression. Whereas the downregulation of c-Myc and λ5 by Aiolos is rapid, the downregulation of cyclin D3 by Aiolos and Ikaros is slow. Furthermore, although the downregulation of cyclin D3 by Ikaros and Aiolos is impaired in Eμ-Myc pre-B cells, the suppression of λ5 expression by Ikaros and Aiolos remains intact. Finally, Ikaros and Aiolos are able to suppress c-Myc and λ5 expression, but not cyclin D3 expression, in p27−/− pre-B cell. Collectively, these results indicate that the downregulation of cyclin D3 by Aiolos occurs through a mechanism that is different from those downregulating of c-Myc and λ5.
Our results indicate that p27 regulates pre-B-cell expansion as p27−/− pre-B cells are hyperproliferative in vivo. However, it is not absolutely required for cell cycle exit and differentiation because the fraction of quiescent small pre-B population, although moderately diminished, is largely intact in p27−/− mice. Nevertheless, induction of p27 by Aiolos and Ikaros still plays an important role, since the growth-inhibitory effects of Aiolos and Ikaros are compromised in p27−/− pre-B cells. Our results further show that induction of p27 by Aiolos and Ikaros is dependent on c-Myc downregulation. It has been demonstrated previously that c-Myc can suppress the transcription of p27 through direct binding to its promoter (37). In addition, c-Myc also negatively regulates the stability of the p27 protein (28). Therefore, it is likely that Aiolos and Ikaros induce p27 indirectly by repressing c-Myc. The finding that p27 expression is induced when the c-Myc gene is deleted in mature B cells is consistent with this view (4).
Pre-B cells express both c-Myc and n-Myc; however, only c-Myc has been shown to play a major role in B-cell development. The deletion of n-Myc in B cells has no obvious effect on B-cell development (10, 20). However, n-Myc can compensate for c-Myc, since mice lacking both exhibit a more pronounced defect in pre-B-cell development (10). In addition, previous studies have demonstrated that when inserted into the c-Myc locus, n-Myc can functionally substitute for c-Myc to drive tissue development (19). Therefore, it is likely that c-Myc is more important than n-Myc for B-cell development because more of it is expressed. Nevertheless, since expression of n-Myc is not suppressed by Ikaros and Aiolos, n-Myc may be able to compensate to some extent for the lack of c-Myc in Aiolos- and Ikaros-infected pre-B cells.
The initial pre-B-cell expansion is driven by IL-7- and pre-BCR-mediated signaling; however, pre-BCR signaling then induces the growth inhibitors Aiolos and Ikaros to terminate pre-B-cell expansion. In pre-B cells, the Ras/Erk pathway contributes to Aiolos induction (21). Expression of both Aiolos and Ikaros can also be induced by IRF4, whose expression is dependent on NF-κB signaling (9, 30). Thus, multiple signaling pathways downstream of the pre-BCR are involved in the induction of Aiolos and Ikaros and the termination of pre-B-cell expansion. In addition to the induction of Ikaros and Aiolos, pre-BCR-induced attenuation of IL-7 signaling may also play a role in terminating pre-B-cell expansion. It has been shown that IL-7 signaling in pre-B cells can be attenuated by CXCL12-mediated chemotaxis, which draws CXCR4-expressing pre-B cells away from IL-7-producing stroma cells (13). Interestingly, in this case, expression of CXCR4 is regulated by IRF-4, whose expression is induced by pre-BCR signaling (22). Since expression of cyclin D3 is dependent on IL-7 signaling, the attenuation of IL-7 signaling would inhibit pre-B-cell expansion. Thus, it appears that pre-B cells utilize both intrinsic (Ikaros and Aiolos induction) and extrinsic (attenuation of IL-7 signaling) mechanisms to terminate their own expansion.
The studies presented here identify an inhibitory network, downstream of pre-BCR, which limits pre-B-cell expansion (Fig. 7). Our findings may have important clinical implications. Ikaros has been found to be mutated at a high frequency in pre-B-cell derived acute lymphoblastic leukemia (B-ALL) (26). A recent report has further demonstrated that the pre-BCR induces Ikaros to inhibit the proliferation of Philadelphia chromosome-positive B-ALL cells (34). Thus, the identification of c-Myc as a direct target of Ikaros not only sheds light on the molecular pathogenesis of B-ALL but also offers a potential new target for therapeutic intervention.
Acknowledgments
We are grateful to Harinder Singh for providing reagents. We thank Tim Mckeithan for providing the MSCV-MycER expression plasmid. We also thank Tim Mckeithan, Jason Zhang, and Karen Gould for critical comments on the manuscript.
This study was supported by National Institutes of Health grant AI-67891 (R.L).
Footnotes
Published ahead of print on 21 June 2010.
REFERENCES
- 1.Asselin, C., A. Nepveu, and K. B. Marcu. 1989. Molecular requirements for transcriptional initiation of the murine c-myc gene. Oncogene 4:549-558. [PubMed] [Google Scholar]
- 2.Cobb, B. S., and S. T. Smale. 2005. Ikaros-family proteins: in search of molecular functions during lymphocyte development. Curr. Top. Microbiol. Immunol. 290:29-47. [DOI] [PubMed] [Google Scholar]
- 3.Cooper, A. B., C. M. Sawai, E. Sicinska, S. E. Powers, P. Sicinski, M. R. Clark, and I. Aifantis. 2006. A unique function for cyclin D3 in early B-cell development. Nat. Immunol. 7:489-497. [DOI] [PubMed] [Google Scholar]
- 4.de Alboran, I. M., R. C. O'Hagan, F. Gartner, B. Malynn, L. Davidson, R. Rickert, K. Rajewsky, R. A. DePinho, and F. W. Alt. 2001. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity 14:45-55. [DOI] [PubMed] [Google Scholar]
- 5.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L. H. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 85:733-744. [DOI] [PubMed] [Google Scholar]
- 6.Fleming, H. E., and C. J. Paige. 2001. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity 15:521-531. [DOI] [PubMed] [Google Scholar]
- 7.Geltinger, C., K. Hortnagel, and A. Polack. 1996. TATA box and Sp1 sites mediate the activation of c-myc promoter P1 by immunoglobulin kappa enhancers. Gene Expr. 6:113-127. [PMC free article] [PubMed] [Google Scholar]
- 8.Georgopoulos, K. 2002. Haematopoietic cell-fate decisions, chromatin regulation and Ikaros. Nat. Rev. Immunol. 2:162-174. [DOI] [PubMed] [Google Scholar]
- 9.Grumont, R. J., and S. Gerondakis. 2000. Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor κB. J. Exp. Med. 191:1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habib, T., H. Park, M. Tsang, I. M. de Alboran, A. Nicks, L. Wilson, P. S. Knoepfler, S. Andrews, D. J. Rawlings, R. N. Eisenman, and B. M. Iritani. 2007. Myc stimulates B lymphocyte differentiation and amplifies calcium signaling. J. Cell Biol. 179:717-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris, A. W., C. A. Pinkert, M. Crawford, W. Y. Langdon, R. L. Brinster, and J. M. Adams. 1988. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J. Exp. Med. 167:353-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog, S., M. Reth, and H. Jumaa. 2009. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signaling. Nat. Rev. Immunol. 9:195-205. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, K., T. Hashimshony, C. M. Sawai, J. M. Pongubala, J. A. Skok, I. Aifantis, and H. Singh. 2008. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity 28:335-345. [DOI] [PubMed] [Google Scholar]
- 14.Kim, J., S. Sif, B. Jones, A. Jackson, J. Koipally, E. Heller, S. Winandy, A. Viel, A. Sawyer, T. Ikeda, R. Kingston, and K. Georgopoulos. 1999. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 10:345-355. [DOI] [PubMed] [Google Scholar]
- 15.Lu, R. 2008. Interferon regulatory factor 4 and 8 in B-cell development. Trends Immunol. 29:487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu, R., K. L. Medina, D. W. Lancki, and H. Singh. 2003. IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 17:1703-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma, S., S. Pathak, L. Trinh, and R. Lu. 2008. Interferon regulatory factors 4 and 8 induce the expression of Ikaros and Aiolos to downregulate pre-B-cell receptor and promote cell-cycle withdrawal in pre-B-cell development. Blood 111:1396-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma, S., A. Turetsky, L. Trinh, and R. Lu. 2006. IFN regulatory factor 4 and 8 promote Ig light chain kappa locus activation in pre-B cell development. J. Immunol. 177:7898-7904. [DOI] [PubMed] [Google Scholar]
- 19.Malynn, B. A., I. M. de Alboran, R. C. O'Hagan, R. Bronson, L. Davidson, R. A. DePinho, and F. W. Alt. 2000. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 14:1390-1399. [PMC free article] [PubMed] [Google Scholar]
- 20.Malynn, B. A., J. Demengeot, V. Stewart, J. Charron, and F. W. Alt. 1995. Generation of normal lymphocytes derived from N-myc-deficient embryonic stem cells. Int. Immunol. 7:1637-1647. [DOI] [PubMed] [Google Scholar]
- 21.Mandal, M., S. E. Powers, K. Ochiai, K. Georgopoulos, B. L. Kee, H. Singh, and M. R. Clark. 2009. Ras orchestrates exit from the cell cycle and light-chain recombination during early B-cell development. Nat. Immunol. 10:1110-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meixlsperger, S., F. Kohler, T. Wossning, M. Reppel, M. Muschen, and H. Jumaa. 2007. Conventional light chains inhibit the autonomous signaling capacity of the B-cell receptor. Immunity 26:323-333. [DOI] [PubMed] [Google Scholar]
- 23.Molnar, A., and K. Georgopoulos. 1994. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol. Cell. Biol. 14:8292-8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan, B., L. Sun, N. Avitahl, K. Andrikopoulos, T. Ikeda, E. Gonzales, P. Wu, S. Neben, and K. Georgopoulos. 1997. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 16:2004-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow, M. A., G. Lee, S. Gillis, G. D. Yancopoulos, and F. W. Alt. 1992. Interleukin-7 induces N-myc and c-myc expression in normal precursor B lymphocytes. Genes Dev. 6:61-70. [DOI] [PubMed] [Google Scholar]
- 26.Mullighan, C. G., X. Su, J. Zhang, I. Radtke, L. A. Phillips, C. B. Miller, J. Ma, W. Liu, C. Cheng, B. A. Schulman, R. C. Harvey, I. M. Chen, R. J. Clifford, W. L. Carroll, G. Reaman, W. P. Bowman, M. Devidas, D. S. Gerhard, W. Yang, M. V. Relling, S. A. Shurtleff, D. Campana, M. J. Borowitz, C. H. Pui, M. Smith, S. P. Hunger, C. L. Willman, and J. R. Downing. 2009. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N. Engl. J. Med. 360:470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson, J. A., K. H. Maclean, U. B. Keller, H. Pendeville, T. A. Baudino, and J. L. Cleveland. 2004. Mnt loss triggers Myc transcription targets, proliferation, apoptosis, and transformation. Mol. Cell. Biol. 24:1560-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Hagan, R. C., M. Ohh, G. David, I. M. de Alboran, F. W. Alt, W. G. Kaelin, Jr., and R. A. DePinho. 2000. Myc-enhanced expression of Cul1 promotes ubiquitin-dependent proteolysis and cell cycle progression. Genes Dev. 14:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pathak, S., S. Ma, L. Trinh, and R. Lu. 2008. A role for interferon regulatory factor 4 in receptor editing. Mol. Cell. Biol. 28:2815-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito, M., J. Gao, K. Basso, Y. Kitagawa, P. M. Smith, G. Bhagat, A. Pernis, L. Pasqualucci, and R. Dalla-Favera. 2007. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B-cell lymphoma. Cancer Cell 12:280-292. [DOI] [PubMed] [Google Scholar]
- 31.Sun, L., A. Liu, and K. Georgopoulos. 1996. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J. 15:5358-5369. [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura, T., H. Yanai, D. Savitsky, and T. Taniguchi. 2008. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26:535-584. [DOI] [PubMed] [Google Scholar]
- 33.Thompson, E. C., B. S. Cobb, P. Sabbattini, S. Meixlsperger, V. Parelho, D. Liberg, B. Taylor, N. Dillon, K. Georgopoulos, H. Jumaa, S. T. Smale, A. G. Fisher, and M. Merkenschlager. 2007. Ikaros DNA-binding proteins as integral components of B-cell developmental-stage-specific regulatory circuits. Immunity 26:335-344. [DOI] [PubMed] [Google Scholar]
- 34.Trageser, D., I. Iacobucci, R. Nahar, C. Duy, G. von Levetzow, L. Klemm, E. Park, W. Schuh, T. Gruber, S. Herzog, Y. M. Kim, W. K. Hofmann, A. Li, C. T. Storlazzi, H. M. Jack, J. Groffen, G. Martinelli, N. Heisterkamp, H. Jumaa, and M. Muschen. 2009. Pre-B cell receptor-mediated cell cycle arrest in Philadelphia chromosome-positive acute lymphoblastic leukemia requires IKAROS function. J. Exp. Med. 206:1739-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, J. H., N. Avitahl, A. Cariappa, C. Friedrich, T. Ikeda, A. Renold, K. Andrikopoulos, L. Liang, S. Pillai, B. A. Morgan, and K. Georgopoulos. 1998. Aiolos regulates B-cell activation and maturation to effector state. Immunity 9:543-553. [DOI] [PubMed] [Google Scholar]
- 36.Wierstra, I., and J. Alves. 2008. The c-myc promoter: still MysterY and challenge. Adv. Cancer Res. 99:113-333. [DOI] [PubMed] [Google Scholar]
- 37.Yang, W., J. Shen, M. Wu, M. Arsura, M. FitzGerald, Z. Suldan, D. W. Kim, C. S. Hofmann, S. Pianetti, R. Romieu-Mourez, L. P. Freedman, and G. E. Sonenshein. 2001. Repression of transcription of the p27Kip1 cyclin-dependent kinase inhibitor gene by c-Myc. Oncogene 20:1688-1702. [DOI] [PubMed] [Google Scholar]
- 38.Yasuda, T., H. Sanjo, G. Pages, Y. Kawano, H. Karasuyama, J. Pouyssegur, M. Ogata, and T. Kurosaki. 2008. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B-cell expansion. Immunity 28:499-508. [DOI] [PubMed] [Google Scholar]