Abstract
Background and Purpose
Neurogenesis can arise from neural stem/progenitor cells of the subventricular zone (SVZ) after strokes involving both the cortex and striatum. However, it is controversial whether all types of stroke and strokes of different sizes activate neurogenesis from the SVZ niche. In contrast with cortical/striatal strokes, repair and remodeling after mild cortical strokes may involve to a greater extent local cortical stem/progenitor cells and cells from non-neurogenic niches.
Methods
We compared stem/progenitor cell responses after focal cortical strokes produced by distal Middle Cerebral Artery Occlusion (dMCAO) and cortical/striatal strokes produced by the intraluminal suture model. To label migrating neuroblasts from the SVZ, we injected DiI to the lateral ventricle after dMCAO. By immunohistochemistry, we characterized cells expressing stem/progenitor cell markers in the peri-infarct area. We isolated cortical stem/progenitor cells from the peri-infarct area following dMCAO and assayed their self-renewal and differentiation capacity.
Results
In contrast with cortical/striatal strokes, focal cortical strokes did not induce neuroblast migration from the SVZ to the infarct zone following dMCAO. By immunohistochemistry, we observed sub-populations of reactive astrocytes in the peri-infarct area that co-expressed radial glial cell markers such as Sox2, Nestin, and RC2. Neural spheres clonally-isolated from the peri-infarct area after dMCAO differentiated into neurons, astrocytes, oligodendrocytes, and smooth muscle cells. Notably, neural spheres isolated from the peri-infarct area also expressed RC2 prior to differentiation.
Conclusions
Mild cortical strokes that do not penetrate the striatum activate local cortical stem/progenitor cells but do not induce neuroblast migration from the SVZ niche.
Keywords: Radial Glia, MCAO, Neural Stem Cells, RC2, Reactive Astrocytes
Introduction
Several types of stem/progenitor cells have been reported in the adult brain including: neural stem/progenitor cells (NSCs) in neurogenic niches such as the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the hippocampus, oligodendrocyte progenitor cells (OPCs), progenitor cells from the subcallosal zone, cortical stem/progenitor cells and white matter progenitor cells (WMPCs) 1-5. CNS injury also activates reactive astrocytes in the cortex and white matter, and some reactive astrocytes may act as progenitor cells after injury 6. Activation of the various cell types and their relative abilities to promote repair after CNS injury is a vigorous area of investigation 7-9.
Neurogenesis from neurogenic niches has been documented following stroke and newly born neurons were observed in both the striatum 10-13 and in the cortex 14, 15. However, the origin of new neurons in the cortex after injury remains controversial. Some reports demonstrated neuroblast migration from the SVZ to the injured cortex 14, 15. In contrast, some groups suggested that neuroblast migration from the SVZ to cortex does not occur following stroke 11.
To examine stem/progenitor cell responses to different size strokes, we induced focal cortical infarcts by distal middle cerebral artery occlusion (dMCAO) and compared them with cortical/striatal strokes produced by the intraluminal suture model. In contrast to cortical/striatal strokes induced by the intraluminal model, SVZ-derived neuroblasts did not migrate to the infarct area from the lateral ventricle at 3, 14 or 28 days after dMCAO. By immunohistochemistry, we identified several proliferating cell types in the peri-infarct area such as Sox2-positive cells, Nestin-positive cells, GFAP-positive reactive astrocytes, and RC2-positive reactive astrocytes in the cortex and bordering white matter 3 days following stroke. We isolated local stem/progenitor cells from the peri-infarct region. Clonally-derived neural spheres from these isolates expressed the radial glial cell marker RC2 and differentiated into neurons, astrocytes and oligodendrocytes ex vivo. Despite their multipotency in culture, BrDU tracing studies demonstrated that the local stem/progenitor cells engaged in gliogenesis (astrocytes and oligodendrocytes) but not neurogenesis in vivo. Strategies to enhance neurogenesis from locally-derived cell populations may augment repair after mild cortical stroke.
Methods
Distal Permanent Middle Cerebral Artery Occlusion (dMCAO)
All animal work was approved by the University of Vermont Office of Animal Care in accordance with National Institutes of Health guidelines. Adult male C57/BL6 mice (Taconic, 6-8 weeks) were anesthetized with isoflurane (1-5%; Webster Veterinary, Sterling, MA). Body temperature was maintained with a heated pad. Focal cerebral ischemia was produced by permanently ligating the middle cerebral artery (MCA) through a small burr hole in the skull 16, 17.
Intraluminal MCAO surgery
A tip of 7-0 nylon suture was heated and a round ball was formed. The suture was dipped into poly-L-lysine solution, dried for 30 minutes, and cut to 1.5cm in length. Adult male C57/BL6 mice were anesthetized with isoflurane and body temperature was maintained with a heated pad. The external carotid artery and common carotid artery was occluded by 8-0 suture. The internal carotid artery was occluded loosely. The 7-0 round tip nylon (tipped) suture was introduced into the internal carotid artery and advanced 8–10 mm distal to the carotid bifurcation to occlude the MCA. The 7-0 suture was tied by the 6-0 suture at the internal carotid artery. Mice that did not show any neurological deficit 1 h after ischemia were excluded from the experiment.
Stereotaxic surgery
Stereotaxic surgery was performed immediately after dMCAO surgery. Stereotaxic surgery was used to deliver 50 μM DiI (CellTracker™ CM-DiI, Invitrogen) in 5 μl of PBS into the lateral ventricle (Lateral ventricle coordinates: A/P, -0.3mm, M/L ±1.0 mm, and D/V -3.0mm). The solution was delivered at a rate of 1 μl/min. Three days or 14 days later, mice were euthanized.
BrDU (5-Bromo-2’-deoxyuridine) administration
Mice received 50 mg/kg of BrDU (5-Bromo-2’-deoxyuridine, Sigma) by an intraperitoneal injection for 3 or 5 consecutive days from day 1 following dMCAO. Mice were euthanized on day 14 or 28 after dMCAO.
Neural stem/progenitor cell isolation
One or three days following dMCAO, brains were removed, placed into a polyacrylic brain block (Acrylic Matrices, RBMA-200C, World Precision Instruments, Sarasota, FL), and cut coronally in 1 mm sections. The peri-infarct area was dissected under a light microscope (Fig. 2A). Tissues from the anterior lateral ventricle, posterior lateral ventricle, hippocampal arch, dentate gyrus and third ventricle were carefully excluded. The tissue was harvested, diced into 1 mm3 pieces and digested in enzyme digestion solution (200 units papain [Worthington, Lakewood, NJ], 20 μg/ml DNase [Worthington], 1.5 mM EDTA [Fisher Scientific, Fair Lawn, NJ], 1.5 mM CaCl2 [Sigma], 2 mg L-Cysteine [Sigma], DMEM/F12 media [Invitrogen, Carsbad, CA]) at 37 C° for 30 min. Following enzymatic digestion, the tissues were triturated 3 times in DMEM/F12 medium with 15 mg trypsin inhibitor [Invitrogen], 15 mg BSA [Fisher Scientific]). Cells were resuspended in DMEM/F12 medium containing 0.9 M sucrose and centrifuged to remove myelin (750 rpm for 10 minutes). Cells were grown in NSC medium (10 ng/ml EGF [BD Biosciences, Bedford, MA], 10 ng/ml FGF [BD Biosciences], 2 μg/ml Heparin [Sigma], B27 supplement [Invitrogen], 2 mM L-Glutamaine [Mediatech], and 100 units/ml Penicillin/100 μg/ml Streptomycin, in Neurobasal A [Invitrogen]) in a 100 mm2 dish (Nunc, Thermo Fisher Scientific, Rochester, NY). The resulting neural spheres were cultured for 1-2 weeks prior to passage and differentiation.
Figure 2.
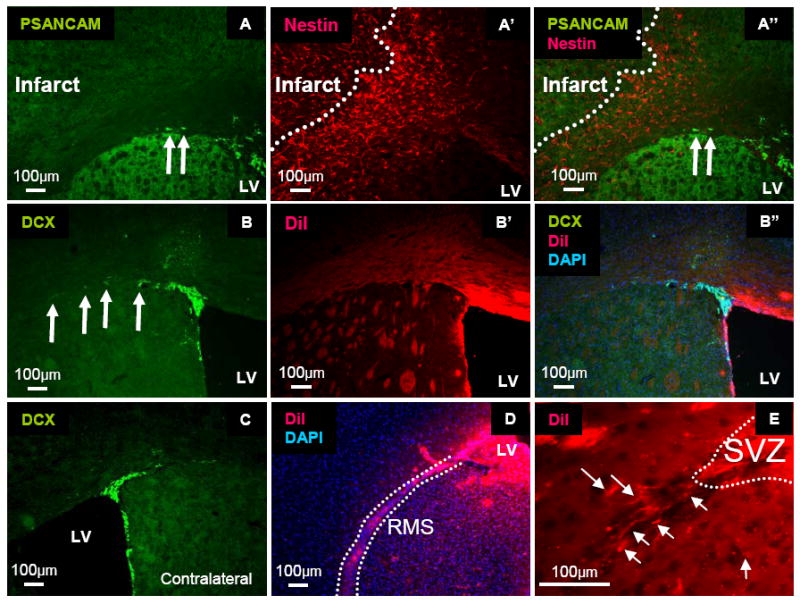
Lack of neuroblast migration from the lateral ventricle to the infarct area. (A, A’, A”) Migrating neuroblasts (PSANCAM, Green) did not reach the infarct area surrounded by reactive astrocytes (Nestin, Red). Arrows: migrating neuroblasts. (B, B’, B”) Migrating neuroblasts (Doublecortin, Red) and DiI-labeled migrating cells (Red) did not reach to the infarct area 3 days after stroke. LV: Lateral Ventricle (C) Doublecortin-positive cells observed in the contralateral side of the lateral ventricle. The relative staining areas for contralateral and ipsilateral (B) doublecortin-positive cells does not differ. (D, E), Successful DiI labeling in the LV was confirmed by DiI-positive cells in the rostral migratory stream (RMS). Dotted line: RMS. Arrows: cells migrating from the SVZ.
For clonal experiments, low density cultures (1,000 cells/ml density) were started directly from a single cell suspension of peri-infarct tissue. Individual small spheres (~ 10 cells per sphere) were pipetted under microscopy into separate 24 well plates. The clonally-isolated neurospheres were expanded and passaged in NSC medium for further studies. For secondary sphere formation assays, primary spheres were dissociated. Cells were plated again in NSC medium (24 well plates, 20 cells/μl density, 500 μl per well). Seven days after plating, sphere number was counted under microscopy. For tertiary sphere formation assays, secondary spheres were dissociated, plated, and counted as above.
Neural stem/progenitor cell differentiation
Twelve mm2 round cover glasses (Electron Microscopy Sciences, Hatfield, PA) were coated with 5 μg/ml laminin/poly-D-lysine (Laminin [BD Biosciences], poly-D-lysine [BD Biosciences]) in 24 well plates (Nunc) for 1 hour. Clonally isolated spheres (passage 0-2) were plated into the 24 well plates and cultured in NSC medium for 1 day. For differentiation, the clonal spheres were cultured for 7 days in Neurobasal A with B27, 2 mM L-Glutamine, 100 units/ml penicillin/100 μg/ml streptomycin and the following: basic medium,1 % FBS; astrocyte/smooth muscle cell medium, 10 % FBS; neuronal medium, 0.1 mM dbcAMP and 10 ng/ml bFGF.
Cresyl Violet staining
After perfusion fixation using 4 % paraformaldehyde (USB, Cleveland, OH), brains were post-fixed in 4 % paraformaldehyde overnight and equilibrated in 30 % sucrose at 4 °C. They were then frozen in OCT compound (Tissue-Tek, Sakura, Tokyo, Japan) and sectioned coronally at 20 μm. Slides were stained with cresyl violet for 15 minutes and cover-slipped. The sections were digitally scanned (EPSON perfection V500 Photo, EPSON, Nagano, Japan).
Immunohistochemistry
Sections were treated with antisera for immunohistochemical detection (See Supplemental Table 1). Sections were incubated in 100 % cold methanol for 30 minutes and PBS for 15 minutes. For BrDU staining, sections were treated with 2 N HCl for 30 min at 37 °C and washed prior to the incubation with blocking buffer. Sections were then blocked in blocking buffer (5 % normal goat serum, 0.4 % Triton × in PBS) for 1 hour at room temperature. Sections were incubated in the primary antibodies at 4 °C overnight. After 3 × PBS washes, sections were incubated in secondary antisera (Alexa Fluor 488, Alexa Fluor 594 or Alexa Fluor 350, 1:500; Molecular Probes) for 1 hour at room temperature. Cell nuclei were stained with DAPI (Vector Laboratories, Burlingame, CA). Isotype controls, Rabbit IgG (2 μg/ml), Mouse IgG (2 μg/ml), and Mouse IgM (2 μg/ml) were used as negative controls for immunohistochemical stains (Supplemental Fig. 1). Photomicrographs were obtained using an epifluorescence deconvolution microscope with an x,y,z stage (Leica DM6000B, Leica, Wetzlar, Germany) with Leica FW4000 software. For deconvolution, images were obtained every 0.1 μm under 100 × magnification and de-convolved using Leica Deblur software.
Immunocytochemistry
Cells were fixed with 4 % paraformaldehyde for 15 min at room temperature and washed with PBS for 3 times. Cells were blocked with blocking buffer, and incubated with primary antisera. After 3 × PBS washes, cells were incubated in secondary antisera for 1 hour at room temperature. The coverglasses were mounted on a slide with DAPI.
Cell quantification and Statistics
Microbrightfield Bioscience Stereo Investigator Software (Colchester, VT) was used for stereologic sampling for immunohistochemical quantifications. Cell counts were performed by observers blinded to sample identity. Infarct area was defined by tissue autofluorescence. We defined the cortical counting area radially by 200 microns outside the infarct area edge. The white matter counting area was defined by white matter adjacent to the infarct core. All data were expressed as means and standard error means. Comparisons were done by 2 tailed Student’s t-test. Values of p ≤ 0.05 were considered significant.
Results
The SVZ niche is activated after cortical/striatal stroke, but not focal cortical stroke
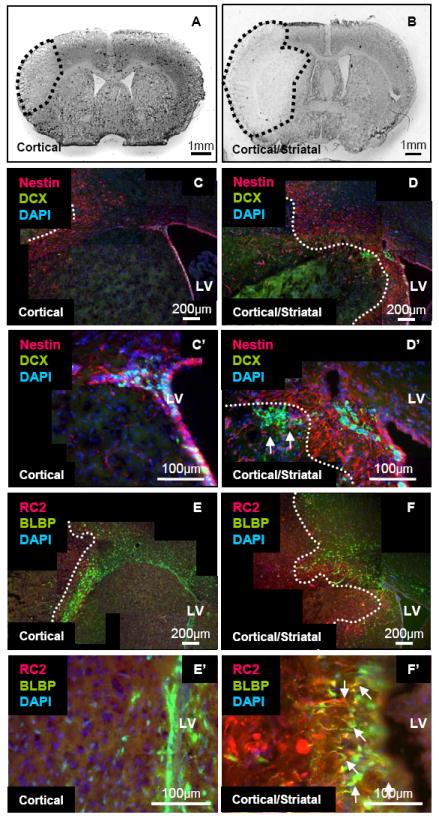
It has been shown that strokes generated with the intraluminal model damage the striatum and induce neurogenesis from the SVZ 18. However, it is unclear whether all types of stroke induce neuroblast migration from the SVZ. Accordingly, we compared neuroblast migration from the SVZ in animals with different infarct sizes produced by the dMCAO model (cortical) and the intraluminal model (cortical/striatal) (Figure 1A and B, respectively). After dMCAO, the infarct region was typically localized to the sensory cortex and a portion of the motor cortex and did not include the striatum (Figure 1A). In brains with intraluminal occlusion of the MCA, the infarct area was observed in both the cortex and the striatum, approaching the lateral ventricle wall (Figure 1B). In agreement with the literature, after cortical/striatal stroke we observed doublecortin-positive neuroblasts migrating from the SVZ toward the infarct region in the striatum (Figure 1D). In contrast, after dMCAO, we did not observe doublecortin-positive neuroblasts exiting the SVZ (Figure 1C).
Figure 1.
Neuroblast activation in the SVZ after cortical/striatal stroke, but not after focal cortical stroke, 3 days after surgery. (A, B) Cresyl violet staining of cortical (A) and cortical/striatal (B) strokes. (C, C’; D, D’) Migrating neuroblasts (Doublecortin, Green) and reactive astrocytes (Nestin, Red) after cortical (C, C’) and cortical/striatal stroke (D, D’). Arrows: migrating neuroblasts. (E, E’; F, F’) Radial glial-like cells (RC2, Red) and (BLBP, Green) after cortical (E, E’) and cortical striatal stroke (F, F’). Note: radial glial-like cells extend into the striatum after cortical/striatal stroke. Arrows: radial glial-like cells.
With cortical/striatal stroke, we observed that RC2-positive and/or BLBP-positive radial glial-like cells extended their processes into the striatum from the SVZ, suggesting that the radial glial-like cells might function as scaffolds for migrating neuroblasts (Figure 1F). However, after focal cortical stroke, we did not observe any RC2-positive radial glial-like cells in the SVZ (Fig. 1E).
To delineate the peri-infarct area (which is typically flanked by reactive astrocytes), we localized reactive astrocytes with antisera against Nestin. We did not observe any PSANCAM-positive or doublecortin-positive neuroblasts that were migrating from the SVZ to the peri-infarct or the infarct areas at 3, 14 or 28 days after focal cortical stroke (Figure 2 A-B). Furthermore, we did not see any differences in doublecortin staining between the ipsilateral and contralateral SVZ at 3, 14, or 28 days after focal cortical stroke (Figure 2C and Supplemental Figure 1).
Lack of neuroblast migration from the SVZ to the cortex after focal cortical stroke
To confirm that neuroblasts from the SVZ had not migrated to the peri-infarct or infarct areas at 3 or 14 days after focal cortical stroke, cells at the SVZ were labeled by DiI injection into the lateral ventricle immediately following dMCAO surgery. As a positive control, successful DiI labeling was confirmed by localization of DiI-positive cells in the rostral migratory stream (RMS) (Figure 2D, E). We did not observe doublecortin or PSANCAM-positive DiI-positive cells in the peri-infarct area at both 3 and 14 days after stroke (Figure 2B). Interestingly, however, we detected a few DiI-labeled cells in the peri-infarct area at both 3 and 14 days after stroke. The DiI-labeled cells were positive for the pan-hematopoietic marker CD45, indicating that they were likely to be either macrophages or microglial cells (Supplemental Figure 1).
Reactive astrocytes expressing radial glial cell markers 3 days after stroke
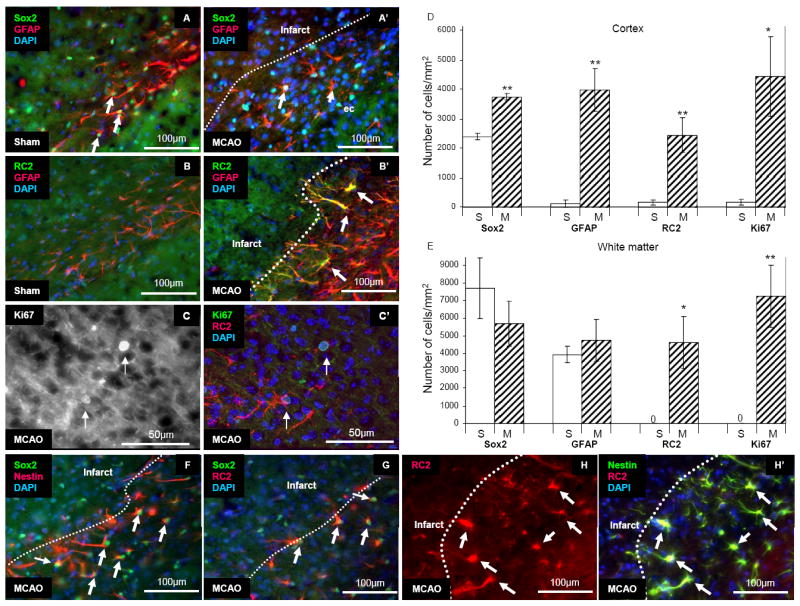
A previous report demonstrated that reactive astrocytes isolated from the brain after stab injury became multipotent neural stem cells in culture 6. Sox2 is a marker of neural stem cells in the hippocampus and lateral ventricle and also expressed by astrocytes in the uninjured cortex 19, 20. We found that GFAP-positive cells expressed Sox2 in the brains of sham-operated mice and those with stroke (Figure 3A). The number of GFAP and Sox2-positive cells was increased significantly in the cortex but not in the white matter at 3 days following stroke (GFAP: cortex, 71±71vs. 3512±293 cells/mm2, p < 0.01; white matter, 3929±481 vs. 4731±1193 cells/mm2, p = 0.5, sham [n = 3] and dMCAO [n = 3]; Sox2: cortex, 2397±159 vs. 3753±105 cells/mm2, p < 0.01; white matter, 7678±1729 vs. 5671±1261 cells/mm2, p = 0.4, sham [n = 3] and dMCAO [n = 3]) (Figure 3D, E).
Figure 3.
Activation and proliferation of RC2-positive reactive astrocytes in the peri-infarct area 3 days following dMCAO. (A, A’) Sox2–positive cells (Green) in the white matter of sham-operated mice and 3 days following stroke. Some also expressed GFAP (Red). Arrows: Sox2-and GFAP-positive cells. Arrow heads: Sox2-positive but GFAP-negative cells. (B, B’) RC2–positive cells (Green) in the white matter 3 days following stroke. Interestingly, only the inner layer of GFAP cells (Red) was positive for RC2. Arrows: double-positive RC2 reactive astrocytes. (C, C’). RC2 reactive astrocytes (RC2, Red) localized at the border of the cortex and white matter 3 days following dMCAO. Arrows: Ki67-positive cells (White or Green). (D) Increased number of cortical Sox2-, GFAP-, RC2-, and Ki67-positive cells in stroke brains compared with sham brains. (E) Increased number of cortical RC2- and Ki67-positive cells in stroke brains compared with sham brains. (F) Most Nestin-positive cells (Red) expressed Sox2 (Green) in the white matter. Arrows indicate double-positive radial glial-like cells. (G) Most RC2-positive cells (Red) expressed Sox2 (Green) in the white matter. Arrows: double-positive RC2 reactive astrocytes. (H) All RC2-positive cells (Red) expressed Nestin (Green) in the white matter (Arrows) 3 days following stroke. ec: External capsule S: Sham, M; dMCAO * p ≤ 0.05, ** p ≤ 0.01
GFAP-positive cells also expressed Nestin, a marker of radial glial cells, neural progenitor cells, and reactive astrocytes, and/or the RC2 antigen (Figure 3B). The RC2 antigen is the most commonly used radial glial cell marker 21. Interestingly, only the most inner layer of GFAP-positive reactive astrocytes at the edge of the peri-infarct area labeled with the RC2 antibody. All of the RC2-positive cells co-expressed Nestin (Figure 3H). We did not observe any RC2-positive cells or Nestin-positive cells in the cortex or white matter in sham-operated brains (Figure 3B and data not shown).
To determine whether the RC2-positive reactive astrocytes were proliferating, we performed double immunohistochemistry against RC2 and Ki67 (Figure 3C). The number of RC2-positive cells and Ki67-positive cells significantly increased in the cortex and in the white matter 3 days following stroke (RC2: cortex, 159±86 vs. 2450±597 cells/mm2, p≤0.01; Ki67: cortex, 172±96 vs. 4438±1343 cells/mm2, p<0.05; RC2/Ki67 double positive: cortex, 65±44 vs. 982±185 cells/mm2; sham [n=3] and dMCAO [n=5]; RC2: white matter, 0±0 vs. 4607±1497 cells/mm2 p<0.05; Ki67: white matter, 0±0 vs. 7246±1755 cells/mm2, p≤0.01; RC2/Ki67 double positive: white matter, 0±0 vs. 1691±558 cells/mm2, p<0.05; sham [n=3] and dMCAO [n=5]) (Figure 3D, E).
We found that about half of the RC2-positive cells were positive for Ki67 (48.1±1.9 % n=5), suggesting that RC2-positive cells from the cortex and white matter were proliferating as observed for radial glial cells during brain development. Since we observed RC2-positive and RC2-negative GFAP-positive reactive astrocytes in the white matter, to distinguish them we called the RC2-positive cells “RC2 reactive astrocytes” (RC2/GFAP double positive cells: 66.7±10.8 %, n=3). The RC2-postive cells were not likely to derive from OPCs as they did not express the NG2 proteoglycan (data not shown).
Isolation of locally-derived stem/progenitor cells after stroke
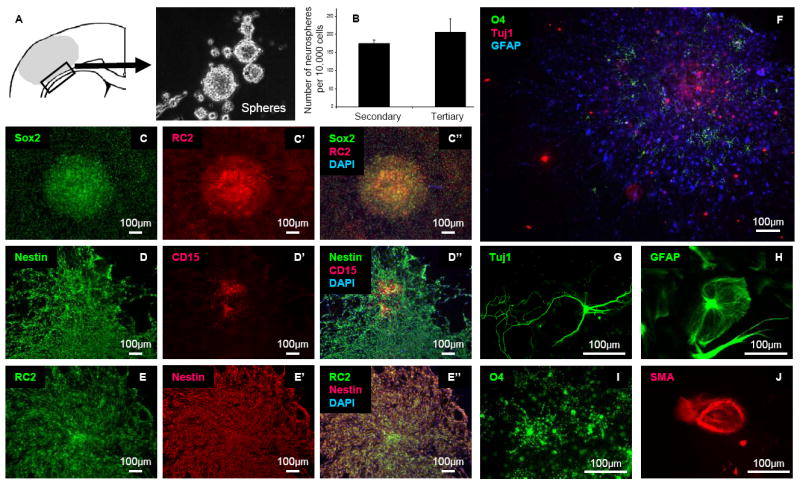
We isolated local stem/progenitor cells from the peri-infarct area (black box in Figure 4A) of the cortex and white matter 1 and 3 days following dMCAO and cultured them as spheres in NSC growth medium. As a negative control, tissues from the contralateral side of the brain were also harvested. Spheres formed from tissue derived from the ipsilateral peri-infarct region, but not from the analogous contralateral region after 1-2 weeks in culture (n=5). To examine self-renewal capacity, we performed sphere-formation assays. Primary spheres derived from the tissue were dissociated and the resulting cells were diluted and plated into the wells of a 24 well dish (20 cells/μl in 500 μl medium). Seven days after plating, the number of spheres was counted under microscopy (Secondary spheres 174.7±10.3 spheres/10,000 cells, Tertiary spheres 205.7±37.0 spheres/10,000 cells [n=3 wells counted per donor, 2 donors]) (Figure 4B). In NSC growth medium, the stem/progenitor cells expressed markers such as Sox2, CD15 (Lex/ssea-1), Nestin, and RC2, but not NG2 (Figure 4C-E and data not shown). To test for mutipotency, we differentiated clonally-isolated spheres. The clonal spheres differentiated into neurons in addition to astrocytes, oligodendrocytes and smooth muscle cells (Figure 4 F-J).
Figure 4.
Multipotency of cultured cells from the peri-infarct area. (A) Schematic illustration of cell isolation. The black box is the area of harvest. (B) Self-renewal of locally-derived spheres isolated 3 days after stroke. The data are expressed as the mean number of spheres generated per 10,000 dissociated cells of primary spheres and secondary spheres (n=3). The number of spheres was counted after 7 days of culture. (C, C’, C”) Expression of Sox2 (Green) and RC2 (Red), a radial glial cell marker. (D, D’, D”) Expression of Nestin (Green) and CD15 (Red), a cortical stem cell marker. Recently attached spheres expressed CD15. Cells that migrated peripherally from the attached spheres did not express CD15. (E, E’, E”) Expression of RC2 (Green) and Nestin (Red). (F) Clonal differentiation into oligodendrocytes (O4, Green), neurons (Tuj1, Red) and astrocytes (GFAP, Blue). Clones isolated from the peri-infarct area at both day 1 and day 3 after stroke all differentiated into (G) neurons (Tuj1, Green) and (H) astrocytes (GFAP, Green), (I) oligodendrocytes (O4, Green), and (J) smooth muscle cells (SMA, Red).
Mature neurons were not generated 28 days following dMCAO
To determine whether the local stem/progenitor cells produced new neurons in the cortex following focal stroke, we administered the thymidine analogue, BrDU, for 3 or 5 consecutive days starting one day after the dMCAO surgery and then euthanized the mice at 14 or 28 days following dMCAO (Figure 5A). We performed double immunohistochemistry against BrDU and NeuN, a mature neuronal marker, to track the fate of cells that had divided after dMCAO (n=3 animals). We observed no BrDU-labeled neurons in either the cortex or the white matter (Figure 5B, B’). In order to determine whether the local progenitor cells had differentiated into other cell types, we performed double immunohistochemistry against BrDU with the astrocyte marker, GFAP, or the oligodendrocyte marker, Glutathione S-transferase (GST)-π. We observed both BrDU-positive astrocytes and oligodendrocytes in the cortex and the white matter (Figure 5C-D’).
Figure 5.
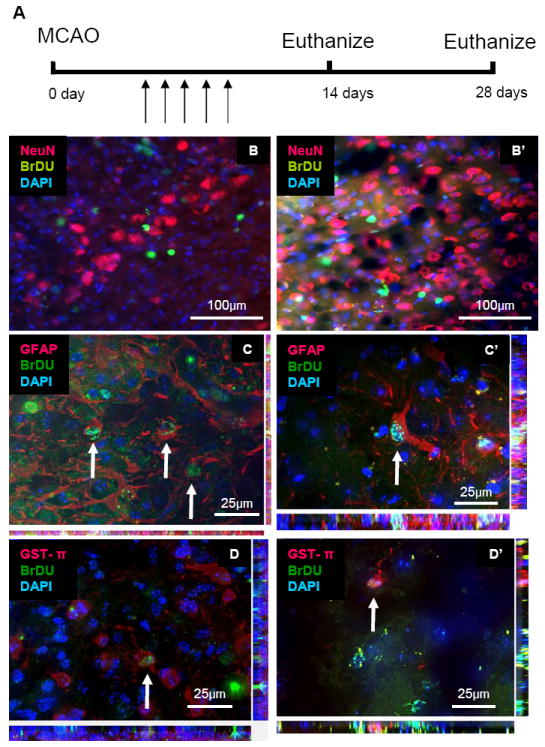
No new neurons were derived in the cortex 28 days following dMCAO. (A) Time schedule of dMCAO. BrDU was administered for 3 or 5 days (arrows) following dMCAO and mice were euthanized 14 or 28 days later. (B, B’) No BrDU-positive neurons were observed in the cortex 14(B) or 28 (B’) days following dMCAO. Colocalization was not observed (NeuN, Red; BrDU, Green). (C, C’) Newly-born astrocytes were observed in the cortex 14(C) or 28 (C’) days following dMCAO. Co-localizations: GFAP, Red; BrDU, Green. (D, D’) Newly-born astrocytes were observed in the cortex 14(D) or 28 (D’) days following dMCAO. Colocalizations: GST-pi, Red; BrDU, Green.
Discussion
Adult neurogenesis from neurogenic niches (SVZ and the SGZ) has been intensively studied in both normal and injured brains 2, 10, 11. However, whether or not mild cortical strokes induce neurogenesis from the SVZ is controversial 7, 22-25. So far, little evidence exists for surviving newly-born neurons in the peri-infarct area and cortex after mild cortical stroke models and other cortical injury models 7, 11, 26, 27. By BrDU tracing, we did not find any newly born neurons that survived in the peri-infarct area after focal cortical stroke. Furthermore, we found that focal cortical stroke did not induce neuroblast migration from the SVZ in the adult mouse brain as opposed to the neuroblast migration observed after cortical/striatal stroke.
The proximity of stroke injury to the lateral ventricle wall may determine whether or not there is a stem/progenitor cell response from the SVZ. Alternatively, focal (mild) cortical stroke may not induce neuroblast migration from the SVZ because it does not directly injure the SVZ itself. The dMCAO model produces stroke by disruption of blood flow to cortical surface arteries. Other MCAO models are generated by occlusion of the proximal MCA that disrupts blood flow to areas including the lateral ventricle. Since the neurovascular relationship is a key factor in maintaining the neurogenic niche at the SVZ 28, reduced blood flow to the SVZ may impact neurogenesis. In proximal MCAO models, newly-born mature neurons in the striatum, radial glial-like cells at the SVZ and/or migration of neuroblasts to the infarct zone have been reported, suggesting that the disruption of blood flow in neurogenic niches may induce neurogenesis 14, 29, 30. In support of this concept, mild cortical stroke models that combine distal MCAO with transient proximal carotid artery occlusion do induce neurogenesis from the SVZ 14, 31.
Do actual multipotent stem/progenitor cells reside in the peri-infarct area after stroke or arise instead from reactive astrocytes that are activated by injury and acquire multipotency in culture, or both? WMPCs, OPCs and cortical stem/progenitor cells were all reported to possess the capacity for neuronal differentiation in culture 2, 3, 5, 32. In addition, reactive astrocytes isolated after CNS injuries were also shown to be multipotent in culture 6. The RC2 reactive astrocytes in vivo and RC2-positive neurosphere-forming cells in culture may arise from mature astrocytes that re-express proteins after injury that are associated with radial glial cells. Genetic lineage tracing studies will likely help to resolve issues regarding both the origin and differentiation capacity of local stem/progenitor cells in vivo after stroke.
Conclusion
In contrast with cortical/striatal stroke, focal cortical stroke did not induce neuroblast migration from the SVZ. Neural stem/progenitor cells isolated from the peri-infarct were multipotent and readily differentiated into neurons in culture. However, we detected no newly born neurons in the peri-infarct area after stroke. Strategies to enhance neurogenesis from local cortical stem/progenitor cell populations or RC2 reactive astrocytes rather than cells of the SVZ may improve repair after mild cortical stroke.
Supplementary Material
(A) Mouse IgG (Red). (B) Mouse IgM isotype (Red). (C) Rabbit IgG isotype (Red). (D, E) DiI-positive migrating cells (Red) reached the peri-infarct area from the posterior lateral ventricle (E), but not from the anterior lateral ventricle (D) 14 days following stroke. Arrows indicate migrating DiI-positive cells from the lateral ventricle. (F-I) DiI-positive migrating cells (Red) are CD45-positive microglia/macrophages (Green). Arrows: DiI-positive cells migrating from the lateral ventricle. (G-I) Magnified view of the yellow box in F. (J-M) Doublecortin-positive migrating cells (Green) did not reach the peri-infarct area from the lateral ventricle 14 days following stroke. Arrows: migrating Doublecortin-positive cells from the lateral ventricle. (N-P) Doublecortin-positive migrating cells (Green) did not reach the peri-infarct area from the lateral ventricle 28 days following stroke. Arrows: Doublecortin-positive cells.
Acknowledgments
We thank Dr. Nobuo Nagai, Nagahama Institute of Bio-Science and Technology, Shiga, Japan, for discussions regarding the dMCAO surgery. We thank Dr. Tomoya Nakamachi, Showa University School of Medicine, Tokyo, Japan for discussions regarding the intraluminal MCAO surgery. The RC2 antibody was obtained from the Developmental Studies Hybridoma Bank at University of Iowa. This work was supported by the National Institute of Health/National Center for Research Resources [grant number P20 RR016435]. Issei S. Shimada is a recipient of a Pre-doctoral Fellowship from the Founders Affiliate of the American Heart Association (09PRE2060107).
Footnotes
Author Contributions I.S.S and J.L.S: conception and design, collection of data, data analysis, manuscript writing; B.M.P: collection of data
Disclosure of Potential Conflicts of Interest The authors indicate no potential conflicts of interest.
References
- 1.Capela A, Temple S. Lex/ssea-1 is expressed by adult mouse cns stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 2.Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 3.Seri B, Herrera DG, Gritti A, Ferron S, Collado L, Vescovi A, Garcia-Verdugo JM, Alvarez-Buylla A. Composition and organization of the scz: A large germinal layer containing neural stem cells in the adult mammalian brain. Cereb Cortex. 2006;16(Suppl 1):i103–111. doi: 10.1093/cercor/bhk027. [DOI] [PubMed] [Google Scholar]
- 4.Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 5.Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult cns. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotts JE, Chesselet MF. Migration and fate of newly born cells after focal cortical ischemia in adult rats. J Neurosci Res. 2005;80:160–171. doi: 10.1002/jnr.20434. [DOI] [PubMed] [Google Scholar]
- 8.Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB. Enriched environment after focal cortical ischemia enhances the generation of astroglia and ng2 positive polydendrocytes in adult rat neocortex. Exp Neurol. 2006;199:113–121. doi: 10.1016/j.expneurol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Biran V, Joly LM, Heron A, Vernet A, Vega C, Mariani J, Renolleau S, Charriaut-Marlangue C. Glial activation in white matter following ischemia in the neonatal p7 rat brain. Exp Neurol. 2006;199:103–112. doi: 10.1016/j.expneurol.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 11.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 14.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 16.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: A gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 17.Bakondi B, Shimada IS, Perry A, Munoz JR, Ylostalo J, Howard AB, Gregory CA, Spees JL. Cd133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009 doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- 19.Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of sox2(+) neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369:24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Misson JP, Edwards MA, Yamamoto M, Caviness VS., Jr Identification of radial glial cells within the developing murine central nervous system: Studies based upon a new immunohistochemical marker. Brain Res Dev Brain Res. 1988;44:95–108. doi: 10.1016/0165-3806(88)90121-6. [DOI] [PubMed] [Google Scholar]
- 22.Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- 23.Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus RT, Young WL, Yang GY, Chen Y. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke. 2008;39:1254–1261. doi: 10.1161/STROKEAHA.107.500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotts JE, Chesselet MF. Vascular changes in the subventricular zone after distal cortical lesions. Exp Neurol. 2005;194:139–150. doi: 10.1016/j.expneurol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Gotts JE, Chesselet MF. Mechanisms of subventricular zone expansion after focal cortical ischemic injury. J Comp Neurol. 2005;488:201–214. doi: 10.1002/cne.20609. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of cd34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatsumi K, Haga S, Matsuyoshi H, Inoue M, Manabe T, Makinodan M, Wanaka A. Characterization of cells with proliferative activity after a brain injury. Neurochem Int. 2005;46:381–389. doi: 10.1016/j.neuint.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult svz stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang RL, Zhang ZG, Wang Y, LeTourneau Y, Liu XS, Zhang X, Gregg SR, Wang L, Chopp M. Stroke induces ependymal cell transformation into radial glia in the subventricular zone of the adult rodent brain. J Cereb Blood Flow Metab. 2007;27:1201–1212. doi: 10.1038/sj.jcbfm.9600430. [DOI] [PubMed] [Google Scholar]
- 30.Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: A study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li WL, Yu SP, Ogle ME, Ding XS, Wei L. Enhanced neurogenesis and cell migration following focal ischemia and peripheral stimulation in mice. Dev Neurobiol. 2008;68:1474–1486. doi: 10.1002/dneu.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential cns stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Mouse IgG (Red). (B) Mouse IgM isotype (Red). (C) Rabbit IgG isotype (Red). (D, E) DiI-positive migrating cells (Red) reached the peri-infarct area from the posterior lateral ventricle (E), but not from the anterior lateral ventricle (D) 14 days following stroke. Arrows indicate migrating DiI-positive cells from the lateral ventricle. (F-I) DiI-positive migrating cells (Red) are CD45-positive microglia/macrophages (Green). Arrows: DiI-positive cells migrating from the lateral ventricle. (G-I) Magnified view of the yellow box in F. (J-M) Doublecortin-positive migrating cells (Green) did not reach the peri-infarct area from the lateral ventricle 14 days following stroke. Arrows: migrating Doublecortin-positive cells from the lateral ventricle. (N-P) Doublecortin-positive migrating cells (Green) did not reach the peri-infarct area from the lateral ventricle 28 days following stroke. Arrows: Doublecortin-positive cells.


