Abstract
Purpose
To examine an immortalized mouse retinal cell line (661W) for markers characteristic of photoreceptor cells and validate its photoreceptor origin.
Methods
The 661W cells were cloned from retinal tumors of a transgenic mouse line that expresses the simian virus (SV) 40 T antigen under control of the human interphotoreceptor retinol-binding protein (IRBP) promoter. Morphologic, immunocytochemical, and immunoblot analyses were performed to characterize these cells. Total cellular protein was used for immunoblot analysis of various photoreceptor-specific proteins.
Results
661W cells grew as a monolayer and exhibited processes characteristic of neuronal cells. Immunoblot analysis showed that 661W cells expressed SV40 T antigen, blue and green cone pigments, transducin, and cone arrestin. Immunocytochemical detection of blue and green opsins showed distribution throughout the cell, the nucleus included. However, these cells did not express rod-specific antigens, such as opsin and arrestin or rod- and cone-specific proteins such as phosducin, peripherin/rds, and ROM1. Furthermore, the cells did not express RPE65, a cone- and RPE-cell–specific protein.
Conclusions
661W cells demonstrate cellular and biochemical characteristics exhibited by cone photoreceptor cells. These cells also resemble neuronal cells with their spindlelike processes and should serve as a useful alternative in vitro model for the study of cone photoreceptor cell biology and associated diseases.
Retinal cell culture has been a useful tool for ocular research. Although it does not replace the intact eye, retinal cell cultures provide convenient experimental systems for the assessment of numerous retinal processes. As with any in vitro systems, cell culture offers great advantages, but not without potential limitations. Advantages include controllable conditions that allow for the assessment of isolated cellular functions, a more affordable system when compared with the more costly animal research, and time course flexibility. Potential limitations include loss of native tissue architecture, lack of functional feedback from other retinal cell types, and a questionable correlation between in vitro and in vivo findings. However, for a great number of research applications, the advantages offered by in vitro systems outweigh the limitations.
Retinal cell culture can be routinely used to determine the cell specificity of promoter sequences,1 the effect of mutations on the structure and function of retinal proteins,2 or the role of multiple domains on the function of retinal proteins.3 Furthermore, retinal cell culture has been applied in studies of cell growth, death, differentiation, and cytotoxicity (for review, see Ref. 4).
Photoreceptor cells are terminally differentiated, specialized neuronal cells with a limited capacity for cell division. Therefore, to establish a line of photoreceptor cells, it is essential to transform them, possibly with a virus. Immortalized cell lines of several ocular cell types currently exist, including Müller,5 ganglion,6 corneal endothelial,7 and RPE cells.8 A cell line expressing retina-specific genes, including interphotoreceptor retinol-binding protein (IRBP) and cone transducin, has been isolated from a mouse ocular tumor.9 In addition, Y-7910 and WERI-Rb11 are immortalized human retinoblastoma cell lines available for the study of photoreceptors. Initially, it was thought that the Y-79 cells were of cone cell origin,12 but more recently these cells have been shown to express rod-specific antigens, such as opsin, transducin, phosphodiesterase, and recoverin.13,14 Primary retinal cultures have been created from several vertebrate retinas, including those of humans.15 These types of cultures, in addition to being tedious to prepare, are not adequate for some types of studies, because of their heterogeneity, limited cell division, and special conditions for growth as monolayers. Thus, there is a need for additional photoreceptor cell models that are homogeneous, passageable, and easily grown as a monolayer by using standard tissue culture techniques.
Herein, we describe a mouse photoreceptor-derived cell line (661W) immortalized by the expression of simian virus (SV)40 T antigen (T-ag) under control of the human IRBP promoter.16 Cellular, and molecular analyses show that these cells express cone but not rod photoreceptor markers, which suggests that the cells arise from a cone photoreceptor lineage. For this reason, the 661W cell line should contribute significantly to the study of cone photoreceptor cell function and of diseases affecting cone photoreceptor cells, including mechanisms of photoreceptor cell death in various retinal dystrophies.
Methods
Immortalization, Culture Conditions, and Morphology of 661W Cells
Immortalization and growth conditions of 661W cells has been described before.17 Cells grown in culture were photographed with Nomarski optics on a microscope (Axioscope; Carl Zeiss Meditec, Oberkochen, Germany). To perform the described experiments, cells were harvested while in the logarithmic growth phase.17 Data presented herein were generated from 661W cells at passages between 15 and 22, except for the immunoblot for GαT1, where cells at passage 67 were used.
Treatment with medium supplements was started 24 hours after the cells were seeded at 106 cells/75 cm2. Sodium butyrate was added at a final concentration of 0.5 mM, hydrocortisone sodium succinate at 0.1 mM, retinoic acid at 10 mM in dimethylsulfoxide (DMSO), and DMSO at 0.5%. After incubation for 48 hours in the presence of supplements, cells were harvested by mild trypsinization and either counted before use or used directly.
Immunoblot Analysis
Cells were collected by centrifugation, used fresh or immediately frozen in liquid nitrogen, and stored at −70°C until use. Protein extracts and protein concentration determinations, from 661W cells and mouse retinas, were prepared as described elsewhere.18 Aliquots of each sample were resolved by SDS-PAGE, and transferred to a polyvinylidene difluoride (PVDF) membrane (Immunblot; Bio-Rad, Hercules, CA) as described elsewhere.18 Primary antibody incubations (see Table 1 for specific antibodies and concentrations used) were performed in 5% milk and Tween-Tris-buffered saline (TTBS) for 16 hours at 4°C. Membranes were then washed five times at room temperature (5 minutes each time) in TTBS and incubated in a horseradish-peroxidase–linked goat anti-mouse IgG (Pierce, Rockford, IL) for 1 hour at room temperature, at a dilution of 1:20,000 in 5% milk/TTBS. Membranes were then washed again as described. Blots were incubated in an enhanced chemiluminescent detection system (SuperSignal; Pierce) for 5 minutes and coupled to autoradiograph film (XAR; Eastman-Kodak, Rochester, NY).
Table 1.
Antibodies Used in the Analysis and Their Sources
| Antibody/Antigen | Source | Dilution* | Comments | References |
|---|---|---|---|---|
| 1D4/rod opsin (M) | R. S. Molday (University British Columbia) | 1:1,000 | IB | 19 |
| JH492/cone red/green (P) | J. Nathans (John Hopkins University School of Medicine) | 1:1,000 | IB, IC | 20 |
| JH455/cone blue (P) | J. Nathans (John Hopkins University School of Medicine) | 1:1,000 | IB, IC | 20 |
| Phosducin (P) | R. Lee (UCLA School of Medicine) | 1:2,000 | IB | 21 |
| GαT1/transducin SC-389 (P) | Santa Cruz Biotechnology (Santa Cruz, CA) | 1:1,000 | IB | 22 |
| GαT/transducin, 4A (M)† | H. E. Hamm (Vanderbilt University Medical Center) | 1:10,000 | IB | 23 |
| Rod H11A2/arrestin (M) | L. Donoso (Wills Eye Hospital) | 1:1,000 | IB | 24 |
| 5C6.47/rod/cone arrestin (M) | L. Smith (Alcon Laboratories) | 1:2,000 | IB | 24 |
| MAb8B11/RPE65 (M) | D. Thompson (University of Michigan) | 1:500 | IB | ‡ |
| T antigen (P) | J. Butel (Baylor College of Medicine) | 1:1000 | IB | 25 |
P, polyclonal; M, monoclonal; IB, immunoblot (Western) analysis; IC, immunocytochemistry by light and deconvolution microscopy.
All secondary antibodies were used at 1:20,000 dilutions.
Shown to cross react with α subunit of other G-proteins.23
Thompson DA, et al. IOVS 2003; 44; ARVO E-Abstract 402.
Quantification of cone opsin modulation with the different treatments was performed by densitometry of bands on immunoblots (Western) and then normalization to either the densitometric reading of a specific protein band on a Coomassie-stained sister gel or to the cell count.
Immunocytochemical Localization of Cone Opsins by Light and Deconvolution Microscopy
661W cells were processed for fluorescent immunocytochemical localization of cone-specific proteins using anti-blue or -red/green opsin antibody (Table 1). Cells were seeded onto 12-mm circular noncoated coverslips (Fisher Scientific, Pittsburgh, PA) and fixed with cold acetone for 2 minutes.
Before immunocytochemistry, the cultured cells were permeabilized with 0.3% Triton X-100 in PBS for 2 minutes. The cultured cells were blocked with a 1:10 dilution of serum and primary and secondary antibodies were diluted in 1% bovine serum albumin (fraction V; Roche Diagnostics, Indianapolis, IN). Anti-rabbit FITC– or rhodamine-labeled secondary antibodies at 1:100 (Sigma-Aldrich, St. Louis, MO) were applied after primary antibody incubations. Between primary and secondary incubations, the cells were washed three times for 5 minutes each with 1% Triton X-100 in PBS. After a final series of washes in PBS, the cultured cells were mounted with antifade medium (Vectashield with 4′,6′-diamino-2-phenylindole [DAPI]; Vector Laboratories, Burlingame, CA) containing mounting medium for fluorescent secondary antibodies. The cells were photographed using either an FITC or rhodamine filter on a microscope (Axioscope; Carl Zeiss Meditec). For deconvolution, a microscope with a z-drive was used (Carl Zeiss Meditec).
Results
Expression of Photoreceptor-Specific Proteins in 661W Cells
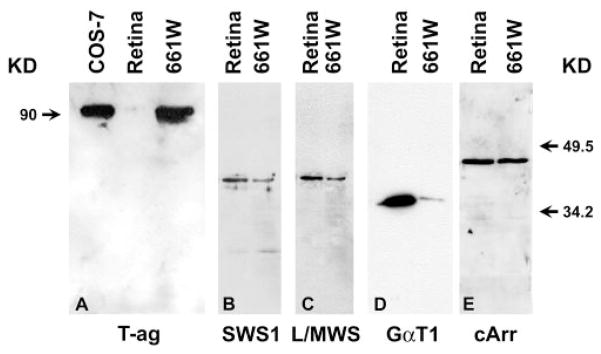
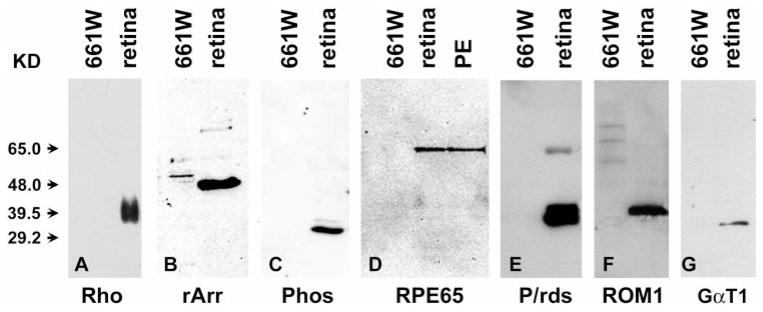
This cell line was originally established from retinal tumors arising from the expression of SV40 T-ag.16 Therefore, immunoblot analysis (Fig. 1A) revealed that while T-ag is absent in the normal retina, its expression in the 661W cells is as abundant as in COS-7 cells, the monkey kidney cells transformed by T-ag.26 In terms of photoreceptor-specific proteins, these cells were found to express cone blue opsin (SWS1, Fig. 1B), cone red or green opsin (L/MWS, Fig. 1C), transducin (GαT1, Fig. 1D), and cone arrestin (cArr, Fig. 1E). Because mouse cone photoreceptors express only green opsin,27 the cone pigment detected in 661W cells was probably green opsin. However, 661W cells did not express rod-photoreceptor–specific proteins, such as rod opsin (Rho, Fig. 2A) or rod arrestin (rArr, Fig. 2B). Furthermore, 661W cells do not express RPE65 (Fig. 2D), which has been shown to be expressed in RPE and cone photoreceptors.28 Finally, these cells were devoid of other proteins that are common to both rods and cones. Proteins such as phosducin (Phos, Fig. 2C), peripherin/rds (P/rds, Fig. 2E), and ROM1 (Fig. 2F) were not detected in 661W cells.
Figure 1.
Comparison of pattern of expression of cone-specific antigens in 661W cells to that of normal retina. Aliquots of 20 μg protein in extracts from retinas and 661W cells were electrophoresed, transferred to PVDF membrane, and probed with antibodies against SV40 T-ag (T-ag, A), blue cone opsin (SWS1, B), red-green cone opsin (L/MWS, C), transducin (GαT1, D), and cone-rod arrestin (cArr, E). Left: molecular mass of T-ag; right: migration of two protein molecular mass markers.
Figure 2.
Immunoblot analysis of various photoreceptor markers in normal retinas, PE, and 661W cells. Twenty-microgram aliquots from 661W cells and C57BL/6 retinal or RPE lysates were separated on 12% polyacrylamide gels and blotted onto PVDF membranes and immunoreacted with anti-rod opsin (Rho, A), rod arrestin (rArr, B), phosducin (Phos, C), RPE65 (D), peripherin/rds (P/rds, E), ROM1 (F), and rod transducin (GαT1, G). The results show a positive immunoreaction for all antigens in normal retinal extracts (and PE in RPE65) but an absence of reactivity in extracts from 661W cells.
The mAb 5c6.47 recognizes both rod and cone arrestin24 and recognized an antigen in extracts from 661W and normal retinas (Fig. 1E). However, the antibody H11A2, which only recognizes rod arrestin,24 did not identify a band on immunoblots containing 661W extracts (Fig. 2B). Therefore, it is reasonable to assume that the identified arrestin in 661W is of cone origin.
The anti-GαT1 mAb 4A was shown to cross-react with α subunits of other G-proteins,23 whereas the antibody SC-389 recognized only the rod transducin α subunit. Because mAb 4A did (Fig. 1D) and SC-389 did not (Fig. 2G) recognize a protein in extracts of 661W cells, it is likely that the band that was identified in 661W extracts was cone transducin.
Effects of Differentiation Inducers on 661W Cells
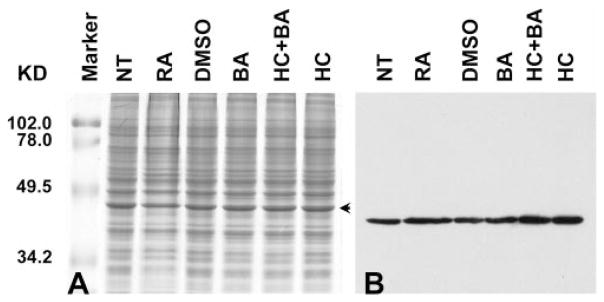
It has been shown that different factors can modulate the growth pattern and promote differentiation of retinoblastoma cells in culture.29,30 Treatment with sodium butyrate causes Y79 cells to undergo growth inhibition followed by either differentiation or cell death.31 Another study showed that retinoic acid treatment of WERI-Rb-1 cells leads to either differentiation toward a cone cell lineage or apoptosis.32 To determine whether treatment with some of these agents could force 661W cells to differentiate, cultures were individually treated with retinoic acid (Fig. 3, RA, in DMSO), DMSO, sodium butyrate (Fig. 3, BA), and hydrocortisone sodium succinate (Fig. 3, HC) for 48 hours. The effect of drug treatment was assessed by immunoblot analysis using anti red/green (Fig. 3B) and blue opsin (data not shown) antibodies. Visual examination of the blot presented in Figure 3B may suggest modulation of the amount of cone opsin with different treatments. However, quantification and normalization to the densitometric value of the specific band (Fig. 3A, arrow) demonstrated no significant increase in the expressed amount of cone pigments (data not shown). Furthermore, probing a comparable blot for cone arrestin showed no significant change in response to treatment of 661W cultures with different agents (data not shown). Similar observations were made whether equal amounts of protein extracts or an amount of protein equivalent to that in a similar number of cells was loaded on the gel for SDS-PAGE.
Figure 3.
Treatment of 661W cells with growth-differentiation modulators. 661W cells were either grown without treatment (NT) or treated individually with retinoic acid (RA), DMSO, sodium salt of butyric acid (BA), hydrocortisone succinate alone (HC), or HC+BA. (A) A Coomassie-blue–stained 12% SDS-polyacrylamide gel; (B) a sister gel that was transferred to a PVDF membrane and immunoreacted with anti-red/green opsin antibodies. Each lane contained 20 μg of total protein extract. Although there is potential visual variation in the amount of cone opsins, quantification by densitometry and normalization to the specific band (A, arrow) failed to show significant alteration in the expressed amount of cone pigments (data not shown).
Growth Characteristics and Morphology of 661W Cells
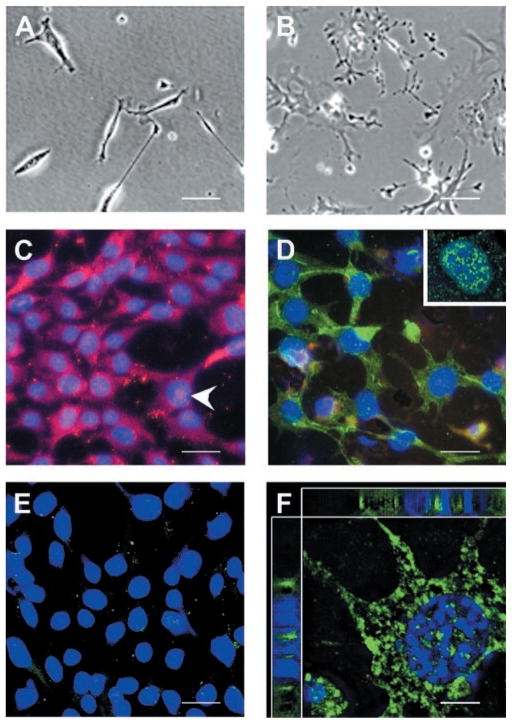
On attachment to the growth surface, 661W cells appeared elongated (Fig. 4A), with cytoplasm size equivalent to that of the nucleus. Shortly thereafter, they begin to project elongated extensions and, once established on the growth surface, the cytoplasm increased in size and the cells flattened out (Fig. 4B). At this stage, cells also start to contact each other through their projected extensions, and often multiple cells are connected to each other (Fig. 4B).
Figure 4.
(A) Morphology of 661W cells. Cultured 661W cells initially appeared spindlelike. (B) As they grew, 661W cells increased in cytoplasm size, appeared flattened with prominent nuclei and began to project neuronal processes. The processes became more extensive and cells touched one another. (C–F) Immunocytochemical localization of cone pigments in 661W cells. Cells were grown on glass coverslips, fixed with cold acetone, immunolabeled with primary antibodies against either green (C) or blue (D) opsins. (C, arrowhead) Labeling of green opsin in or over the nucleus. (D, inset) Distribution of blue opsin in or over the nucleus. (E) 661W cells incubated in absence of the primary antibodies serving as the control. (F) Deconvolved confocal images of 661W cells labeled with antibodies against blue opsin. DAPI (blue) was used to detect the nuclei. Notice the large cytoplasm and the presence of label in the cytoplasm and in or around the nucleus. Top and left: deconvolved stacks of images to show colocalization of DAPI and blue opsin stains in the nucleus. Scale bar: (A–E) 20 μm; (F) 3.5 μm.
Localization of Cone Opsin
Immunocytochemical detection of cone pigments in 661W cells demonstrated that both green (Fig. 4C) and blue (Fig. 4D) opsins were abundantly expressed. Elimination of the primary antibody abolished the signal (Fig. 4E).
Expression of both green and blue opsins was observed throughout the 661W cells, including in or around the nuclear body (Fig. 4C, arrowhead; 4D, inset). Deconvolution confocal microscopy was used to determine whether these pigments were actually localized in the nucleus or simply inserted in the membrane overlaying the nucleus. The pigment was present inside the nucleus, as demonstrated by the colocalization of DAPI and cone opsin staining (Fig. 4F, top and side panels).
Discussion
In a previous report, we described tumor formation in transgenic mice expressing SV40 T-ag in rod and cone photoreceptors under control of the human IRBP promoter.16 A cell line, designated 661W, was established from one of these tumors. The 661W cells grow to confluence in culture and proliferate rapidly, with a slight lag phase of approximately 2 days and a log phase extending from days 2 to 8.17 After logarithmic growth, the cells reach a plateau at day 9. Based on the growth curve,17 661W cells have been shown to have a doubling rate of 1.1 days. So far, these cells have been maintained in culture for more than 60 passages, with no apparent slowing of mitotic activity or loss of photoreceptor-specific markers.
The results presented herein show that these cells express cone but not rod specific antigens, which supports the cone origin of 661W. The patterns of expression of cone opsin and arrestin were not modulated by treatment with factors that stimulate differentiation, such as retinoic acid and hydrocortisone. This behavior is contrary to that of the human retinoblastoma (Y79) cells grown in culture, in which cone arrestin is upregulated in response to retinoic acid.30 The difference in behavior of 661W and Y79 in response to retinoic acid may reflect a more differentiated nature of the 661W cells. This is supported by the fact that 661W cells have larger cytoplasm than Y79 (data not shown), a characteristic usually used by pathologists to assess the differentiated status of tumor cells.
Although the human IRBP promoter was used to direct the expression of T-ag to both rods and cones, the 661W cells originating from the resultant tumors expressed only cone-specific antigens, perhaps because, in the mouse retina, cone genesis precedes that of rods.33 Alternatively, this could be due to an inherit characteristic in rods that makes them more difficult to transform by an oncogene.
The 661W cells do not exhibit cone photoreceptor morphology, such as formation of outer-segment–like membranes, and they do not express outer segment structural proteins such as P/rds and ROM1. A potential explanation for this behavior could be the need for RPE-produced factor or proper physical contact with the RPE to establish polarity and form outer segments. However, growing these cells in the presence of PE-conditioned medium did not alter their behavior (data not shown), a result that favors the physical contact as a potential explanation. Alternatively, because 661W cells were derived from tumors that had originated in utero before the onset of expression of P/rds and ROM1, it is possible that these cells had never expressed these proteins in the first place. This is supported by the finding that whereas the peak of cone genesis is between embryonic days 13 and 14,33 P/rds transcript is first detected in the mouse retina at postnatal day 1.34
Immunolocalization showed that cone opsins were distributed all over the cell, including the nucleus. Again, this may be explained by the lack of polarity in 661W cells. This phenomenon has also been observed in vivo associated with the expression of mutant opsin in transgenic models where opsin was localized to the inner segments, perinuclear space, and the synaptic terminals.35
We anticipate that these cells express most, if not all, of the proteins involved in cone phototransduction, because they respond to light and can undergo light-induced cell death.36 An intriguing question, though, is how these cells regenerate their pigment and respond to light stress in the absence of RPE65. Cones are thought to express their own RPE65.28 So unless cones, or 661W cells specifically, have an alternative pathway for pigment regeneration, it is difficult to understand how they can be light sensitive. One possibility is that the 661W cells express RGR, the protein that structurally resembles visual pigments and other G-protein–coupled receptors.37 RGR may play a role as a photoisomerase in the production of 11-cis-retinal, the chromophore of the visual pigments. We are currently investigating whether 661W and cone photoreceptor express RGR.
In conclusion, the 661W cell line characterized herein is of cone photoreceptor origin and can serve as a useful tool for investigating photoreceptor cell biology and function.
Acknowledgments
Supported by Grants R01 EY14052 from the National Eye Institute (MRA) and R01 EY10609 (MIN), Core Grant for Vision Research (EY12190), and Grants T-CB-0609-0190 (MRA) and T-GT-0900-0028 (MIN) from the Foundation Fighting Blindness.
The authors thank Laura Bucher for technical help, May Nour for critical evaluation of the manuscript, and the colleagues listed in Table 1 for providing the antibodies.
Footnotes
Disclosure: E. Tan, None; X.-Q. Ding, None; A. Saadi, None; N. Agarwal, None; M.I. Naash, None; M.R. Al-Ubaidi, None
References
- 1.Boatright JH, Buono R, Bruno J, et al. The 5′ flanking regions of IRBP and arrestin have promoter activity in primary embryonic chicken retina cell cultures. Exp Eye Res. 1997;64:269–277. doi: 10.1006/exer.1996.0222. [DOI] [PubMed] [Google Scholar]
- 2.Sung C-H, Davenport CM, Hennessey JC, et al. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 1991;88:6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anukanth A, Khorana HG. Structure and function in rhodopsin. J Biol Chem. 1994;31:19738–19744. [PubMed] [Google Scholar]
- 4.Seigel GM. The golden age of retinal cell culture. Mol Vis. 1999;5:4. [PubMed] [Google Scholar]
- 5.Roque RS, Agarwal N, Wordinger RJ, et al. Human papillomavirus-16 E6/E7 transfected retinal cell line expresses the Müller cell phenotype. Exp Eye Res. 1997;64:519–527. doi: 10.1006/exer.1996.0230. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 7.Araki K, Ohashi Y, Sasabe T, et al. Immortalization of rabbit corneal epithelial cells by a recombinant SV40-adenovirus vector. Invest Ophthalmol Vis Sci. 1993;34:2665–2671. [PubMed] [Google Scholar]
- 8.Nabi IR, Mathews AP, Cohen-Gould L, et al. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci. 1993;104:37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- 9.Bernstin SL, Kutty G, Wiggert B, et al. Expression of retina-specific genes by mouse retinoblastoma cells. Invest Ophthalmol Vis Sci. 1994;35:3931–3937. [PubMed] [Google Scholar]
- 10.Reid TW, Albert DM, Rabson AS, et al. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst. 1974;53:347–360. doi: 10.1093/jnci/53.2.347. [DOI] [PubMed] [Google Scholar]
- 11.McFall RC, Sery TW, Makadon M. Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res. 1977;37:1003–1010. [PubMed] [Google Scholar]
- 12.Bogenmann E, Lochrie MA, Simon MI. Cone cell-specific genes expressed in retinoblastoma. Science. 1988;240:76–78. doi: 10.1126/science.2451289. [DOI] [PubMed] [Google Scholar]
- 13.Di Polo A, Farber DB. Rod photoreceptor-specific gene expression in human retinoblastoma cells. Proc Natl Acad Sci USA. 1995;92:4016–4020. doi: 10.1073/pnas.92.9.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiechmann AF. Recoverin in cultured human retinoblastoma cells: enhanced expression during morphological differentiation. J Neurochem. 1996;67:105–110. doi: 10.1046/j.1471-4159.1996.67010105.x. [DOI] [PubMed] [Google Scholar]
- 15.Kelley MW, Turner JK, Reh TA. Regulation of proliferation and photoreceptor differentiation in fetal human retinal cell cultures. Invest Ophthalmol Vis Sci. 1995;36:1280–1289. [PubMed] [Google Scholar]
- 16.Al-Ubaidi MR, Font RL, Quiambao AB, et al. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J Cell Biol. 1992;119:1681–1687. doi: 10.1083/jcb.119.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford MJ, Krishnamoorthy RR, Rudick VL, et al. Bcl-2 overexpression protects photooxidative stress-induced apoptosis of photoreceptor cells via NF-kappaB preservation. Biochem Biophys Res Commun. 2001;281:1304–1312. doi: 10.1006/bbrc.2001.4501. [DOI] [PubMed] [Google Scholar]
- 18.Tan E, Wang Q, Quiambao AB, et al. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42:589–600. [PubMed] [Google Scholar]
- 19.Hicks D, Molday RS. Differential immunogold-dextran labeling of bovine and frog rod and cone cells using monoclonal antibodies against bovine rhodopsin. Exp Eye Res. 1986;42:55–71. doi: 10.1016/0014-4835(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Macke JP, Merbs SL, et al. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Lee RH. Phosducin and betagamma-transducin interaction I: effects of post-translational modifications. Biochem Biophys Res Commun. 1997;233:370–374. doi: 10.1006/bbrc.1997.6460. [DOI] [PubMed] [Google Scholar]
- 22.Weiler R, Schultz K, Pottek M, et al. Retinoic acid has light-adaptive effects on horizontal cells in the retina. Proc Natl Acad Sci USA. 1998;95:7139–7144. doi: 10.1073/pnas.95.12.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamm HE, Deretic D, Mazzoni MR, et al. A monoclonal antibody against the rod outer segment guanyl nucleotide-binding protein, transducin, blocks the stimulatory and inhibitory G proteins of adenylate cyclase. J Biol Chem. 1989;264:11475–11482. [PubMed] [Google Scholar]
- 24.Donoso LA, Gregerson DS, Smith L, et al. S-antigen: preparation and characterization of site specific monoclonal antibodies. Curr Eye Res. 1990;9:343–355. doi: 10.3109/02713689008999622. [DOI] [PubMed] [Google Scholar]
- 25.Lanford RE, Butel JS. Antigenic relationship of SV40 early proteins to purified large T polypeptides. Virology. 1979;97:295–306. doi: 10.1016/0042-6822(79)90341-6. [DOI] [PubMed] [Google Scholar]
- 26.Cattaneo R, Will H, Darai G, et al. Detection of an element of the SV40 late promoter in vectors used for expression studies in COS cells. EMBO J. 1983;2:511–514. doi: 10.1002/j.1460-2075.1983.tb01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smallwood PM, Olveczky BP, Williams GL, et al. Genetically engineered mice with an additional class of cone photoreceptors: Implications for the evolution of color vision. Proc Natl Acad Sci USA. 2003;100:11706–11711. doi: 10.1073/pnas.1934712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Znoiko SL, Crouch RK, Moiseyev G, et al. Identification of the RPE65 protein in mammalian cone photoreceptors. Invest Ophthalmol Vis Sci. 2002;43:1604–1609. [PubMed] [Google Scholar]
- 29.Howard MA, Wardwell S, Albert DM. Effect of butyrate and corticosteroids on retinoblastoma in vitro and in vivo. Invest Ophthalmol Vis Sci. 1991;32:1711–1713. [PubMed] [Google Scholar]
- 30.Li A, Zhu X, Craft CM. Retinoic acid upregulates cone arrestin expression in retinoblastoma cells through a Cis element in the distal promoter region. Invest Ophthalmol Vis Sci. 2002;43:1375–1383. [PubMed] [Google Scholar]
- 31.Kyritsis A, Joseph G, Chader GJ. Effects of butyrate, retinol, and retinoic acid on human Y-79 retinoblastoma cells growing in monolayer cultures. J Natl Cancer Inst. 1984;73:649–654. [PubMed] [Google Scholar]
- 32.Li A, Zhu X, Brown B, et al. Gene expression networks underlying retinoic acid-induced differentiation of human retinoblastoma cells. Invest Ophthalmol Vis Sci. 2003;44:996–1007. doi: 10.1167/iovs.02-0434. [DOI] [PubMed] [Google Scholar]
- 33.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 34.Cheng T, Al Ubaidi MR, Naash MI. Structural and developmental analysis of the mouse peripherin/rds gene. Somat Cell Mol Genet. 1997;23:165–183. doi: 10.1007/BF02721369. [DOI] [PubMed] [Google Scholar]
- 35.Wong F. Creating transgenic mouse models of photoreceptor degeneration caused by mutations in the rhodopsin gene. In: Hollyfield JG, Anderson RE, LaVail MM, editors. Retinal Degeneration: Clinical and Laboratory Applications. New York: Plenum Press; 1993. pp. 211–217. [Google Scholar]
- 36.Krishnamoorthy RR, Crawford MJ, Chaturvedi MM, et al. Photooxidative stress down-modulates the activity of nuclear factor-kappaB via involvement of caspase-1, leading to apoptosis of photoreceptor cells. J Biol Chem. 1999;274:3734–3743. doi: 10.1074/jbc.274.6.3734. [DOI] [PubMed] [Google Scholar]
- 37.Chen P, Hao W, Rife L, et al. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet. 2001;28:256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]