Abstract
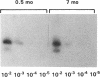
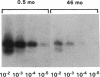
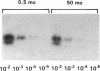
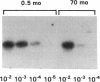
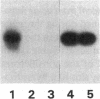
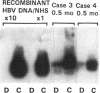
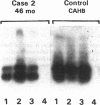
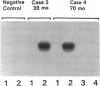
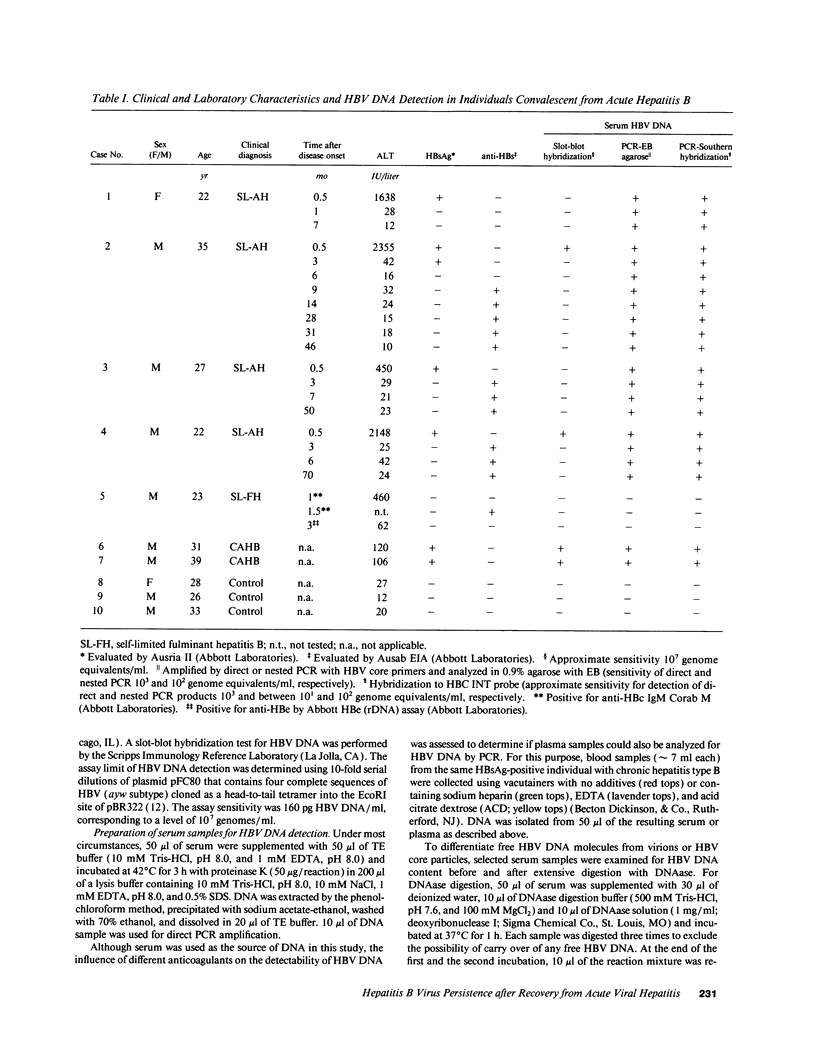
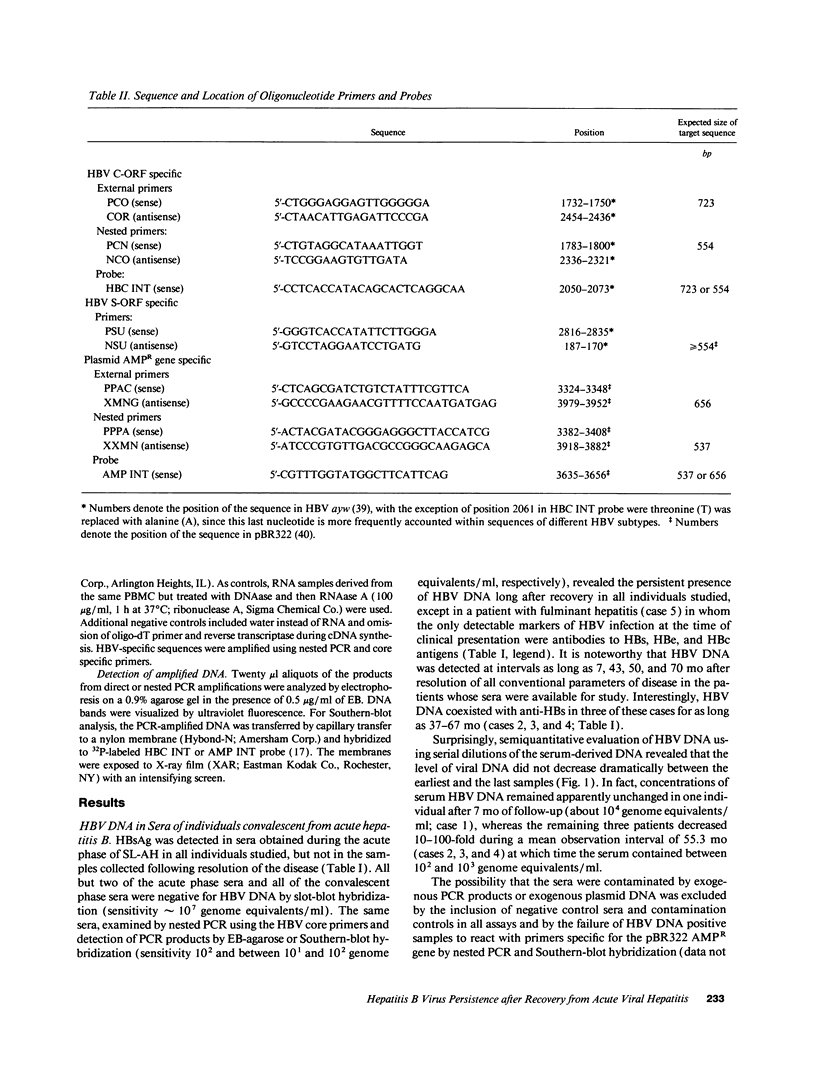
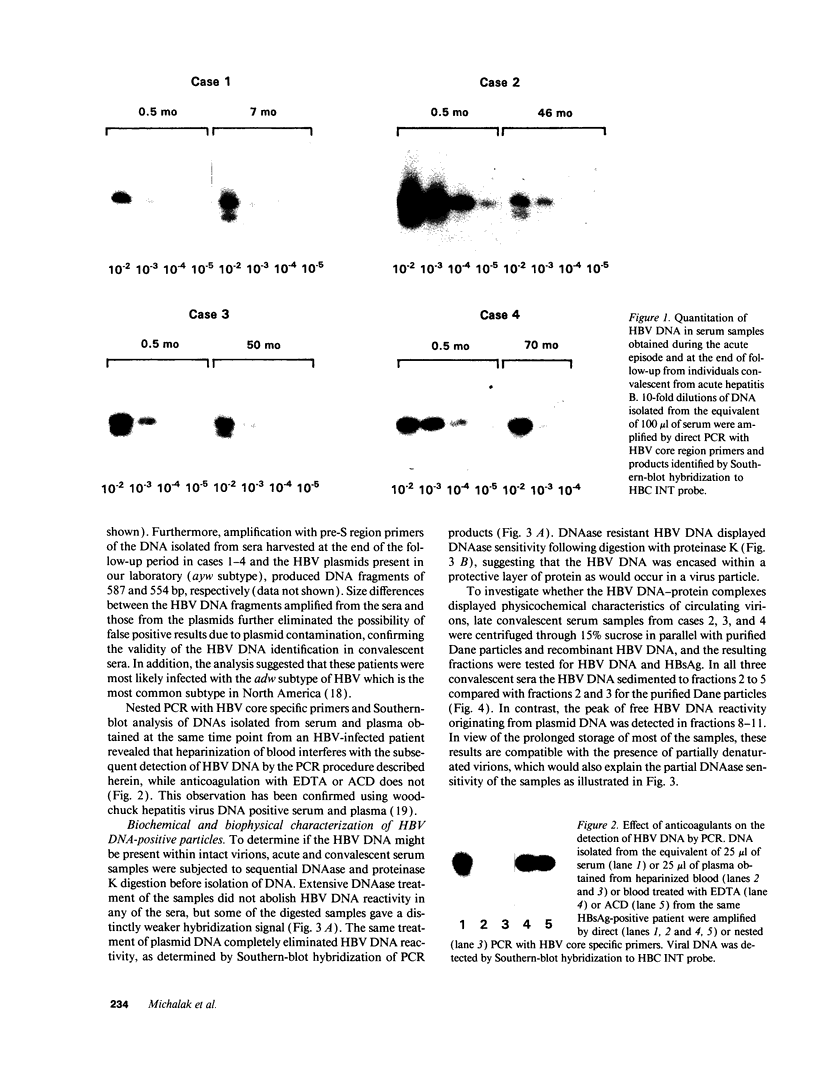
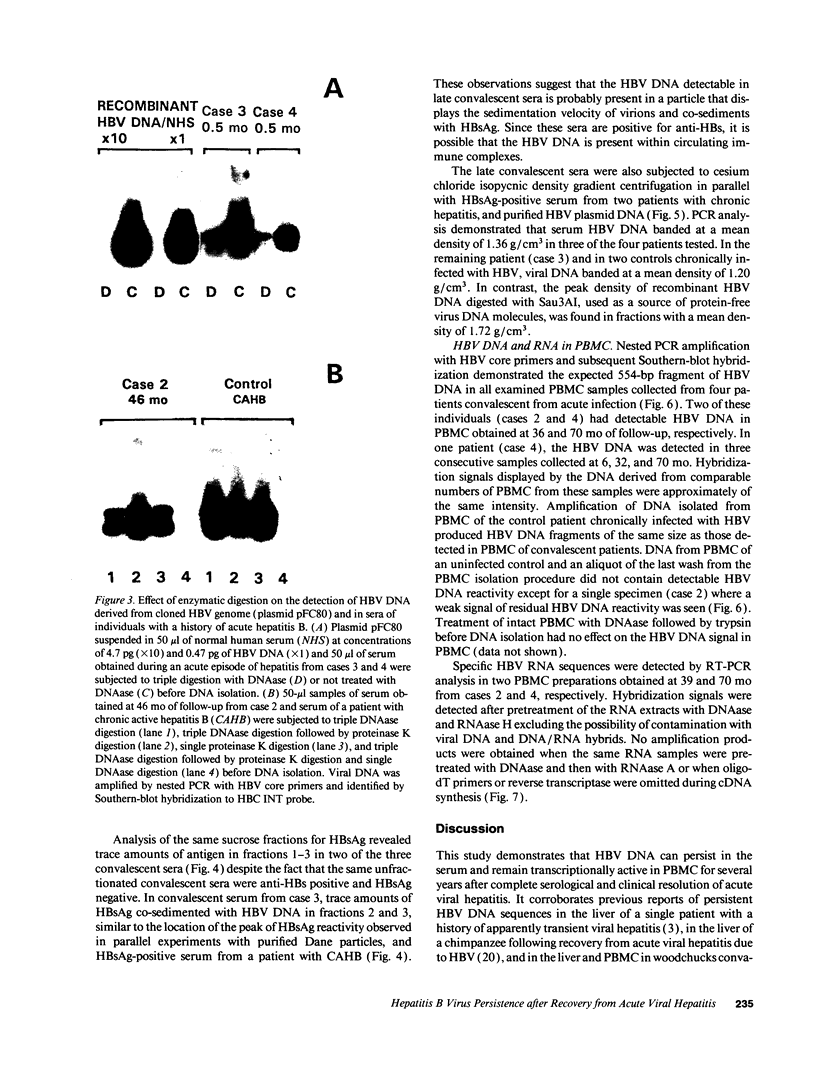
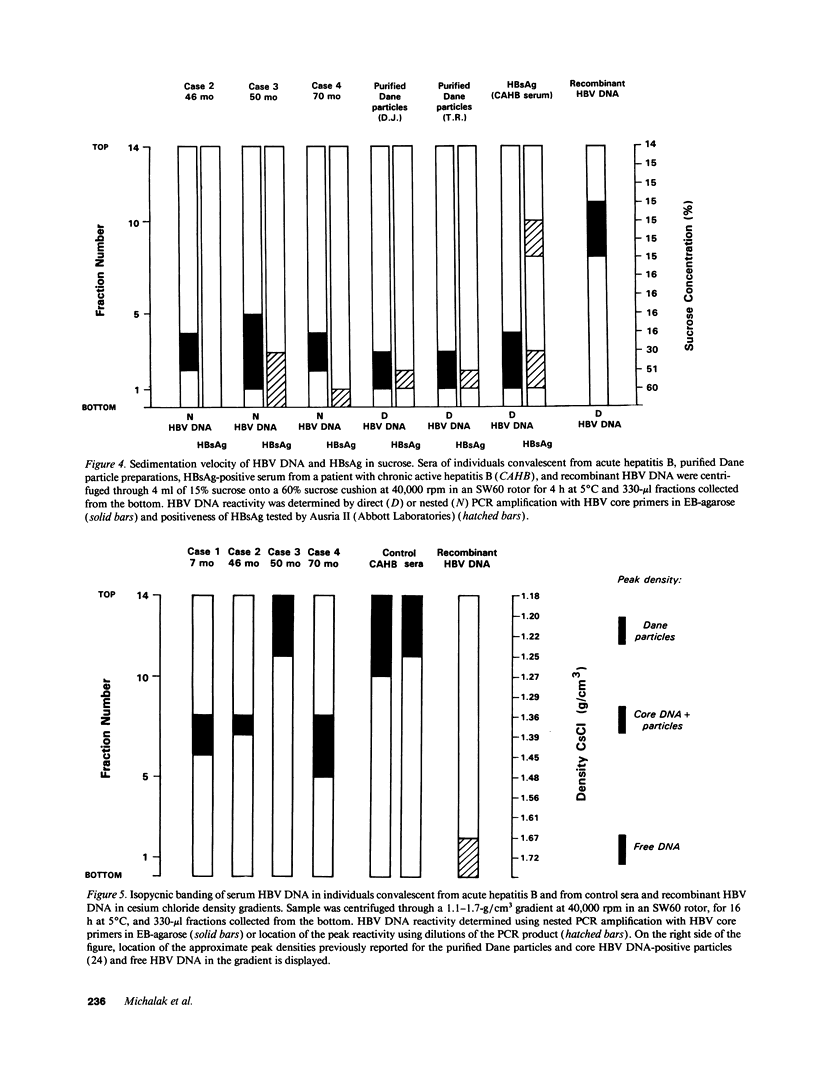
Contrary to current opinion, the disappearance of hepatitis B surface antigen (HBsAg) from the serum, the development of anti-HBs antibodies, and normalization of liver function may not reflect complete virological recovery from acute hepatitis B virus (HBV) infection. By using the polymerase chain reaction (PCR), in the current study we demonstrate long-term persistence of HBV DNA in the serum and peripheral blood mononuclear cells (PBMC) of four patients for up to 70 mo after complete clinical, biochemical, and serological recovery from acute viral hepatitis. Serum HBV DNA reactivity co-sedimented with HBsAg in sucrose gradients, and it displayed the size and density characteristics of naked core particles and intact HBV virions, presumably contained within circulating immune complexes in these anti-HBs antibody-positive sera. HBV DNA was also present in PBMC in late convalescent samples from all four patients, and HBV RNA was detected in late convalescent phase PBMC in two of these patients. These results suggest that HBV DNA, and possibly HBV virions, can be present in the serum, and that the viral genome can persist in a transcriptionally active form in PBMC for > 5 yr after complete clinical and serological recovery from acute viral hepatitis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Kaneko S., Matsushita E., Inagaki Y., Unoura M., Kobayashi K. Clearance of HBsAg in seven patients with chronic hepatitis B. Hepatology. 1992 Dec;16(6):1334–1337. doi: 10.1002/hep.1840160605. [DOI] [PubMed] [Google Scholar]
- Baginski I., Chemin I., Bouffard P., Hantz O., Trepo C. Detection of polyadenylated RNA in hepatitis B virus-infected peripheral blood mononuclear cells by polymerase chain reaction. J Infect Dis. 1991 May;163(5):996–1000. doi: 10.1093/infdis/163.5.996. [DOI] [PubMed] [Google Scholar]
- Blum H. E., Liang T. J., Galun E., Wands J. R. Persistence of hepatitis B viral DNA after serological recovery from hepatitis B virus infection. Hepatology. 1991 Jul;14(1):56–63. doi: 10.1002/hep.1840140110. [DOI] [PubMed] [Google Scholar]
- Blum H. E., Offensperger W. B., Walter E., Offensperger S., Gerok W. Latent hepatitis B virus infection with full-length viral genome in a patient serologically immune to hepatitis B virus infection. Liver. 1988 Oct;8(5):307–316. doi: 10.1111/j.1600-0676.1988.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Carman W. F., Dourakis S., Karayiannis P., Crossey M., Drobner R., Thomas H. C. Incidence of hepatitis B viraemia, detected using the polymerase chain reaction, after successful therapy of hepatitis B virus carriers with interferon-alpha. J Med Virol. 1991 Jun;34(2):114–118. doi: 10.1002/jmv.1890340208. [DOI] [PubMed] [Google Scholar]
- Chemin I., Baginski I., Vermot-Desroches C., Hantz O., Jacquet C., Rigal D., Trepo C. Demonstration of woodchuck hepatitis virus infection of peripheral blood mononuclear cells by flow cytometry and polymerase chain reaction. J Gen Virol. 1992 Jan;73(Pt 1):123–129. doi: 10.1099/0022-1317-73-1-123. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Couroucé-Pauty A. M., Plançon A., Soulier J. P. Distribution of HBsAg subtypes in the world. Vox Sang. 1983;44(4):197–211. doi: 10.1111/j.1423-0410.1983.tb01885.x. [DOI] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gilles P. N., Fey G., Chisari F. V. Tumor necrosis factor alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J Virol. 1992 Jun;66(6):3955–3960. doi: 10.1128/jvi.66.6.3955-3960.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadchouel M., Pasquinelli C., Fournier J. G., Hugon R. N., Scotto J., Bernard O., Brechot C. Detection of mononuclear cells expressing hepatitis B virus in peripheral blood from HBsAg positive and negative patients by in situ hybridisation. J Med Virol. 1988 Jan;24(1):27–32. doi: 10.1002/jmv.1890240105. [DOI] [PubMed] [Google Scholar]
- Holodniy M., Kim S., Katzenstein D., Konrad M., Groves E., Merigan T. C. Inhibition of human immunodeficiency virus gene amplification by heparin. J Clin Microbiol. 1991 Apr;29(4):676–679. doi: 10.1128/jcm.29.4.676-679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. M., Ford E. C., Purcell R. H., Gerin J. L. Demonstration of subpopulations of Dane particles. J Virol. 1976 Mar;17(3):885–893. doi: 10.1128/jvi.17.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Cote P. J., Gerin J. L. Mitogen-induced replication of woodchuck hepatitis virus in cultured peripheral blood lymphocytes. Science. 1988 Sep 2;241(4870):1213–1216. doi: 10.1126/science.3261887. [DOI] [PubMed] [Google Scholar]
- Korba B. E., Cote P. J., Shapiro M., Purcell R. H., Gerin J. L. In vitro production of infectious woodchuck hepatitis virus by lipopolysaccharide-stimulated peripheral blood lymphocytes. J Infect Dis. 1989 Oct;160(4):572–576. doi: 10.1093/infdis/160.4.572. [DOI] [PubMed] [Google Scholar]
- Korba B. E., Wells F. V., Baldwin B., Cote P. J., Tennant B. C., Popper H., Gerin J. L. Hepatocellular carcinoma in woodchuck hepatitis virus-infected woodchucks: presence of viral DNA in tumor tissue from chronic carriers and animals serologically recovered from acute infections. Hepatology. 1989 Mar;9(3):461–470. doi: 10.1002/hep.1840090321. [DOI] [PubMed] [Google Scholar]
- Korba B. E., Wells F., Tennant B. C., Cote P. J., Gerin J. L. Lymphoid cells in the spleens of woodchuck hepatitis virus-infected woodchucks are a site of active viral replication. J Virol. 1987 May;61(5):1318–1324. doi: 10.1128/jvi.61.5.1318-1324.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Wells F., Tennant B. C., Yoakum G. H., Purcell R. H., Gerin J. L. Hepadnavirus infection of peripheral blood lymphocytes in vivo: woodchuck and chimpanzee models of viral hepatitis. J Virol. 1986 Apr;58(1):1–8. doi: 10.1128/jvi.58.1.1-8.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenman J., Baker B., Waggoner J., Everhart J. E., Di Bisceglie A. M., Hoofnagle J. H. Long-term remission of chronic hepatitis B after alpha-interferon therapy. Ann Intern Med. 1991 Apr 15;114(8):629–634. doi: 10.7326/0003-4819-114-8-629. [DOI] [PubMed] [Google Scholar]
- Krugman S., Overby L. R., Mushahwar I. K., Ling C. M., Frösner G. G., Deinhardt F. Viral hepatitis, type B. Studies on natural history and prevention re-examined. N Engl J Med. 1979 Jan 18;300(3):101–106. doi: 10.1056/NEJM197901183000301. [DOI] [PubMed] [Google Scholar]
- Kuhns M., McNamara A., Mason A., Campbell C., Perrillo R. Serum and liver hepatitis B virus DNA in chronic hepatitis B after sustained loss of surface antigen. Gastroenterology. 1992 Nov;103(5):1649–1656. doi: 10.1016/0016-5085(92)91191-6. [DOI] [PubMed] [Google Scholar]
- Liang T. J., Blum H. E., Wands J. R. Characterization and biological properties of a hepatitis B virus isolated from a patient without hepatitis B virus serologic markers. Hepatology. 1990 Aug;12(2):204–212. doi: 10.1002/hep.1840120205. [DOI] [PubMed] [Google Scholar]
- Lobbiani A., Lalatta F., Lugo F., Colucci G. Hepatitis B virus transcripts and surface antigen in human peripheral blood lymphocytes. J Med Virol. 1990 Jul;31(3):190–194. doi: 10.1002/jmv.1890310304. [DOI] [PubMed] [Google Scholar]
- Loriot M. A., Marcellin P., Bismuth E., Martinot-Peignoux M., Boyer N., Degott C., Erlinger S., Benhamou J. P. Demonstration of hepatitis B virus DNA by polymerase chain reaction in the serum and the liver after spontaneous or therapeutically induced HBeAg to anti-HBe or HBsAg to anti-HBs seroconversion in patients with chronic hepatitis B. Hepatology. 1992 Jan;15(1):32–36. doi: 10.1002/hep.1840150107. [DOI] [PubMed] [Google Scholar]
- Mason A., Yoffe B., Noonan C., Mearns M., Campbell C., Kelley A., Perrillo R. P. Hepatitis B virus DNA in peripheral-blood mononuclear cells in chronic hepatitis B after HBsAg clearance. Hepatology. 1992 Jul;16(1):36–41. doi: 10.1002/hep.1840160108. [DOI] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J., Bartolomé J., Moraleda G., Ruiz-Moreno M., Rua M. J., Moreno A., Carreño V. Persistence of hepatitis B virus DNA after reduction of viral replication in serum and liver. J Med Virol. 1992 Sep;38(1):11–15. doi: 10.1002/jmv.1890380104. [DOI] [PubMed] [Google Scholar]
- Noonan C. A., Yoffe B., Mansell P. W., Melnick J. L., Hollinger F. B. Extrachromosomal sequences of hepatitis B virus DNA in peripheral blood mononuclear cells of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5698–5702. doi: 10.1073/pnas.83.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli C., Melegari M., Villa E., Scaglioni P. P., Seidenari M., Mongiardo N., De Rienzo B., Manenti F. Hepatitis B virus infection of peripheral blood mononuclear cells is common in acute and chronic hepatitis. J Med Virol. 1990 Jun;31(2):135–140. doi: 10.1002/jmv.1890310211. [DOI] [PubMed] [Google Scholar]
- Pontisso P., Poon M. C., Tiollais P., Brechot C. Detection of hepatitis B virus DNA in mononuclear blood cells. Br Med J (Clin Res Ed) 1984 May 26;288(6430):1563–1566. doi: 10.1136/bmj.288.6430.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romet-Lemonne J. L., McLane M. F., Elfassi E., Haseltine W. A., Azocar J., Essex M. Hepatitis B virus infection in cultured human lymphoblastoid cells. Science. 1983 Aug 12;221(4611):667–669. doi: 10.1126/science.6867736. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Lieberman H. M., Isselbacher K. J., Wands J. R. Monoclonal radioimmunoassays for hepatitis B surface antigen: demonstration of hepatitis B virus DNA or related sequences in serum and viral epitopes in immune complexes. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5675–5679. doi: 10.1073/pnas.79.18.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Wang J. T., Wang T. H., Sheu J. C., Lin S. M., Lin J. T., Chen D. S. Effects of anticoagulants and storage of blood samples on efficacy of the polymerase chain reaction assay for hepatitis C virus. J Clin Microbiol. 1992 Mar;30(3):750–753. doi: 10.1128/jcm.30.3.750-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. T., Wang T. H., Sheu J. C., Shih L. N., Lin J. T., Chen D. S. Detection of hepatitis B virus DNA by polymerase chain reaction in plasma of volunteer blood donors negative for hepatitis B surface antigen. J Infect Dis. 1991 Feb;163(2):397–399. doi: 10.1093/infdis/163.2.397. [DOI] [PubMed] [Google Scholar]
- Xu J., Brown D., Harrison T., Lin Y., Dusheiko G. Absence of hepatitis B virus precore mutants in patients with chronic hepatitis B responding to interferon-alpha. Hepatology. 1992 Jun;15(6):1002–1006. doi: 10.1002/hep.1840150605. [DOI] [PubMed] [Google Scholar]
- Yoffe B., Noonan C. A., Melnick J. L., Hollinger F. B. Hepatitis B virus DNA in mononuclear cells and analysis of cell subsets for the presence of replicative intermediates of viral DNA. J Infect Dis. 1986 Mar;153(3):471–477. doi: 10.1093/infdis/153.3.471. [DOI] [PubMed] [Google Scholar]