Abstract
A dysregulated overexpression of inflammatory mediators by microglia may facilitate cognitive aging and neurodegeneration. Considerable evidence suggests the flavonoid luteolin has antiinflammatory effects, but its ability to inhibit microglia, reduce inflammatory mediators, and improve hippocampal-dependent learning and memory in aged mice is unknown. In initial studies, pretreatment of BV-2 microglia with luteolin inhibited the induction of inflammatory genes and the release of inflammatory mediators after lipopolysaccharide (LPS) stimulation. Supernatants from LPS-stimulated microglia caused discernible death in Neuro.2a cells. However, treating microglia with luteolin prior to LPS reduced neuronal cell death caused by conditioned supernatants, indicating luteolin was neuroprotective. In subsequent studies, adult (3–6 mo) and aged (22–24 mo) mice were fed control or luteolin (20 mg/d)-supplemented diet for 4 wk and spatial working memory was assessed as were several inflammatory markers in the hippocampus. Aged mice fed control diet exhibited deficits in spatial working memory and expression of inflammatory markers in the hippocampus indicative of increased microglial cell activity. Luteolin consumption improved spatial working memory and restored expression of inflammatory markers in the hippocampus compared with that of young adults. Luteolin did not affect either spatial working memory or inflammatory markers in young adults. Taken together, the current findings suggest dietary luteolin enhanced spatial working memory by mitigating microglial-associated inflammation in the hippocampus. Therefore, luteolin consumption may be beneficial in preventing or treating conditions involving increased microglial cell activity and inflammation.
Introduction
Age-related deterioration of brain function produces a variety of behavioral deficits that are independent of disease. Cognitive aging, e.g., is a term used to describe a pattern of age-related impairments in cognitive functions, including fluid reasoning, mental speed, memory, and spatial ability. Other changes such as decreased motivation for food and deficits in motor coordination also are common. Behavioral indications of brain aging are evident by middle-age in clinically healthy individuals, so deterioration of brain function precedes the acceleration in mortality rate that is typical in old age. According to the United Nations demography, the segment of the population over 60 y of age is rapidly growing and is anticipated to number 2 billion worldwide by the year 2050 (1). Therefore, more people than ever will encounter age-related neurobehavioral deficits and be at increased risk for developing neurodegenerative diseases like Alzheimer’s unless measures to slow or reverse brain aging can be envisaged.
An intrinsic factor that has emerged as a leading threat to successful cognitive aging is inflammation (2). The inflammatory cytokine, interleukin (IL)7-6, was initially found to be increased in blood of healthy aged humans (3) and mice (4). It was subsequently found to be increased in the brain of aged mice due to increased activity of nuclear factor κB (NFκB)7 in microglial cells (5). Consistent with NFκB’s role as a universal regulator of inflammatory genes (6), a microarray study that compared steady-state gene expression in brain of young adult and aged mice discovered 38 genes associated with inflammation that were differentially expressed: 34 were upregulated and only 4 were downregulated in the aged (7). Inflammation is speculated to be a predisposing factor in the development of neurodegenerative diseases and to play a role in cognitive aging.
Although serum IL-6 is the only inflammatory cytokine thus far to be positively correlated with age-related cognitive deficits (8, 9), some evidence suggests it has a permissive effect in the brain (10) for another cytokine, IL-1β. IL-1β is directly implicated in reduced hippocampal neurogenesis and inhibition of the consolidation of hippocampal-dependent memories (11). The hippocampus is densely populated with IL-1 receptors (12) and central administration of IL-1 receptor antagonist has been shown to inhibit neurobehavioral deficits caused by inflammatory stimuli and stress (13, 14). The constitutive expression of IL-1β in the hippocampus of the elderly is typically higher than in young cohorts (13, 15) and IL-1β gene expression in the hippocampus is positively correlated with deficits in hippocampal-dependent tasks (16). Further, increased IL-1β was associated with impaired long-term potentiation (a proposed biological substrate for learning and memory) in the hippocampus of old rats (17). Therefore, inhibiting the age-associated increase in hippocampal IL-1β may be useful for preventing cognitive deficits, particularly those mediated by the hippocampus (e.g. spatial learning and memory).
Flavonoids, plant polyphenolic compounds abundant in fruits and vegetables, exhibit a wide array of biological effects, including antioxidant, free-radical scavenging, and antiinflammatory properties (18). The flavonoid luteolin (3′,4′,5,7-tetrahydroxyflavone) is abundant in celery, green pepper, parsley, perilla leaf, and chamomile tea (19), and has been shown to decrease lipopolysaccharide (LPS)-induced production of proinflammatory molecules such as tumor necrosis factor (TNF)α in macrophages (20). Several studies also demonstrated an antiinflammatory effect of luteolin in vivo. For instance, intraperitoneal injection of luteolin in mice reduced LPS-induced lethal toxicity and attenuated LPS-induced intercellular adhesion molecule-1 in the liver as well as TNFα in serum (21). Moreover, luteolin substantially suppressed clinical symptoms of experimental allergic encephalomyelitis in mice and reduced inflammation and axonal damage in the brain by preventing monocyte migration across the brain endothelium (22).
Despite the number of studies that show luteolin has antiinflammatory properties and that inflammation is involved in age-associated decrements in cognition, there have been no studies to our knowledge in aged animals investigating the potential for dietary luteolin to mitigate inflammation and protect against deficits in cognition. Therefore, the purpose of the present study was to determine whether luteolin would inhibit production of inflammatory mediators by microglial cells in vitro and be neuroprotective and if luteolin dietary supplementation would inhibit inflammation in the hippocampus of aged mice and improve hippocampal-dependent learning and memory.
Materials and Methods
Reagents.
LPS from Escherichia coli (serotype 0127:B8) and retinoic acid were obtained from Sigma. Luteolin was obtained from Calbiochem. The antibodies and standards for the IL-1β and TNFα ELISA were purchased from Pharmingen. The MTS cell proliferation assay was purchased from Promega. DMEM and HBSS were purchased from Bio-Whittaker. Fetal bovine serum (FBS) was purchased from Hyclone. Penicillin and streptomycin were purchased from Invitrogen.
BV-2 microglial cell and Neuro.2a neuronal cell culture.
The immortalized murine microglia cell line, BV-2 (a gift from Dr. Linda Van Eldik, Feinberg School of Medicine, Department of Cell and Molecular Biology, Northwestern University, Evanston, IL), has been used as a model to investigate the neuroimmune system (23). Murine Neuro.2a neuronal cells from American Type Culture Collection have been used to investigate neuronal damage and death (24) and are inherently capable of undergoing differentiation toward a neuronal phenotype given the appropriate conditions (25). BV-2 and Neuro.2a cells were maintained in 150-cm2 tissue culture flasks (BD) in DMEM supplemented with 10% FBS and 100 kU/L penicillin/streptomycin at 37°C in a humidified incubator under 5% CO2. Confluent cultures were passed by trypsinization. Cells were centrifuged at 250 × g for 5 min at 4°C and culture medium was removed. In all experiments, cells were resuspended in DMEM supplemented with 10% FBS and seeded in 6-well plates (BD) before being subjected to treatments. To differentiate the Neuro.2a cells, the medium was changed to DMEM containing 2% FBS and 20 μmol/L retinoic acid, and cells were cultured for 48 h. Neurites were observed using a ZEISS inverted phase-contrast microscope (data not shown).
Cytokine production.
BV-2 cells were pretreated with vehicle (0.05% dimethylsulfoxide, v:v) or luteolin (0–50 μmol/L) for 1 h and incubated with LPS for 4 h or 24 h for measurement of IL-1β and TNFα, respectively. IL-1β and TNFα protein levels were determined using in-house sandwich ELISA that have been previously described in detail (26). The IL-1β assay was sensitive to 4 ng/L and the TNFα assay was sensitive to 15 ng/L. For both IL-1β and TNFα, inter-and intra-assay CV were <10%. Luteolin was introduced at the 0–50 μmol/L range, because the physiologically relevant concentration of flavonoids is reportedly 10 μmol/L (27, 28) and 1 study showed that plasma luteolin increased up to 14 μmol/L after oral gavage with 50 μmol/kg of luteolin administration (19).
Quantitative real-time PCR.
BV-2 cells were pretreated with vehicle (0.05% dimethyl sulfoxide, v:v) or luteolin (0–50 μmol/L) for 1 h and incubated with LPS for 8 h. Expression of IL-1β, TNF-α, inducible nitric oxide (NO) synthase (iNOS), and cyclooxygenase (COX)-2 mRNA was determined by RT-PCR. A preliminary study that examined mRNA at various times after introduction of LPS (0, 2, 4, 8, 16, and 24 h) revealed maximal induction of mRNA at this time point. In mouse studies, the hippocampus was dissected and placed in RNAlater and stored (−80°C). Expression of IL-1β, TNFα, IL-6, and major histocompatibility complex (MHC) class II mRNA was determined by RT-PCR. Quantitative real-time PCR was performed using the Applied Biosystems Assay-on Demand Gene Expression protocol as previously described (7). Data were analyzed using the comparative threshold cycle method and results are expressed as fold difference.
Nitrite and prostaglandin E2 assay.
To examine NO and prostaglandin (PG) E2 production, BV-2 cells were pretreated with luteolin (0–50 μmol/L) for 1 h and incubated with 10 μg/L LPS for 24 h. The supernatant from the culture was collected and used for the following experiments. Measurement of nitrite production as an assay of NO release was performed. Accumulation of nitrite in the medium was determined by colorimetric assay with Griess reagent. Nitrite concentrations were determined by comparison with the OD550 using standard solutions of sodium nitrite prepared in cell culture medium. The level of PGE2 in the media was measured by enzyme immunoassay (no. 513010; Cayman) according to the manufacturer’s instructions.
Neuronal cell death assay.
To determine whether luteolin is neuroprotective, BV-2 cells were treated with luteolin (0–50 μmol/L) for 1 h and incubated with 100 μg/L of LPS for 48 h. Supernatant from the microglia culture was collected and added to differentiated Neuro.2a cells for 24 h. In other studies, Neuro.2a cells were incubated with luteolin and then supernatants from LPS-stimulated BV-2 cells. To assess neuronal cell death, the viability of Neuro.2a cells was measured by MTS assay.
Mice and diet.
Adult (3–6 mo) and aged (22–24 mo) male Balb/c mice were obtained from our in-house, specific-pathogen–free colony. Mice were housed in polypropylene cages and maintained at 23°C under a reverse phase 12-h-light/-dark cycle with ad libitum access to water and rodent diet. Before the start of each experiment, mice were provided a standard AIN-93M (29) diet (Research Diets) for a 1-wk acclimation period. Mice receiving AIN-93M either continued on the standard diet or were switched to AIN-93M containing 6 g luteolin/kg diet. Luteolin was purchased from Shaanxi Sciphar Biotechnology and its purity (99%) was verified by HPLC. Diets were stored at 4°C and fresh food was provided every week. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
Mouse study design.
Adult (n = 26) and aged (n = 26) mice were provided an AIN-93M standard diet or AIN-93M plus 0.6% luteolin diet for 4 wk. Food intake and body weight were measured on a weekly basis for the duration of the study. Body weight changes were similar for the 4 treatment groups and average daily intake of luteolin over the course of the study was 19.9 ± 0.4 mg. During wk 4 of diet supplementation, a version of the Morris water maze was used to determine hippocampal-dependent learning and memory. After assessment of learning and memory, animals were maintained on their respective diets for 3 additional days and killed by CO2 asphyxiation 2 h after the onset of the dark period (i.e. after the initial burst of feeding activity) to verify the absorption of luteolin. Plasma was collected, stored (−80°C), and later analyzed for luteolin concentration. Brain tissue was dissected and hippocampus was placed in RNAlater and stored (−80°C).
Spatial working memory.
To evaluate hippocampal-dependent memory, a water maze was constructed as previously described (30). During wk 4 of diet supplementation, mice were trained in a 6-d acquisition phase with 3 massed trials administered each day. The platform remained in a constant location during the acquisition phase. Mice were allowed a 1-d rest period and on d 8, to evaluate working memory, they were subjected to a reversal test in which the platform was moved to the opposite quadrant of the pool but all distal visual cues remained constant. Performance parameters that were determined included swim speed, latency to platform, and distance swam.
Plasma luteolin.
An HPLC procedure was developed to determine whether luteolin was present in plasma after mice received control or luteolin-supplemented diets (0 or 19.9 ± 0.4 mg/d dietary luteolin, respectively) for 4 wk. Plasma samples were deproteinated by adding 100 μL of 6% perchloric acid to 100 μL of blank or sample plasma. Luteolin was extracted by adding 1.5 mL of ethyl acetate and vortexing for 5 min. After centrifugation for 10 min at 2000 × g, 1.0 mL of supernatant was transferred to a 1.5-mL microcentrifuge tube and dried under an argon stream. The remaining residue was reconstituted in 100 μL of mobile phase eluent, methanol:0.2% phosphoric acid (60:40, v:v) and centrifuged at 22,000 × g for 10 min before injection. Pure luteolin standard was reconstituted in mobile phase eluent at 43.75 (limit of detection), 546.88, and 1093.75 (limit of quantitation) μg/L to produce a standard curve previously determined to be appropriate for the expected concentration of plasma luteolin; a single luteolin peak eluted between 8.05 and 8.25 min. Recovery rate of luteolin when added to plasma from naïve animals (pooled plasma sample from mice fed commercial nonpurified diet) was 90.6%. The sample content of luteolin was calculated based on peak-area ratios of analyte determined using the standard curve procedure and data are expressed as μmol luteolin/L of plasma.
Statistical analysis.
All data were analyzed using SAS. For in vitro studies, data were subjected to 1-way ANOVA and differences between treatment means were determined by t tests using the least significant difference procedure of SAS. For reference memory (acquisition test), data were subjected to repeated-measures ANOVA in which day was a within-subjects measure (i.e. repeated measure) and diet (control or luteolin) and age (adult and aged) were between-subjects measures. All other data were subjected to 2-way ANOVA to determine the effects of diet (control or luteolin), age (adult or aged), and the age × diet interaction. Differences between treatment means were determined by t tests using the least significant difference procedure of SAS in instances when significant interactions were observed. Main effects and interactions were considered significant at P < 0.05 and P < 0.15, respectively. Cytokine data exhibiting unequal variance were corrected using appropriate transformations and these data are presented as nontransformed means ± SE values, but the associated significance values were derived from transformed data. All data are expressed as the treatment means ± SEM.
Results
Luteolin inhibited production of proinflammatory mediators by LPS-stimulated microglia.
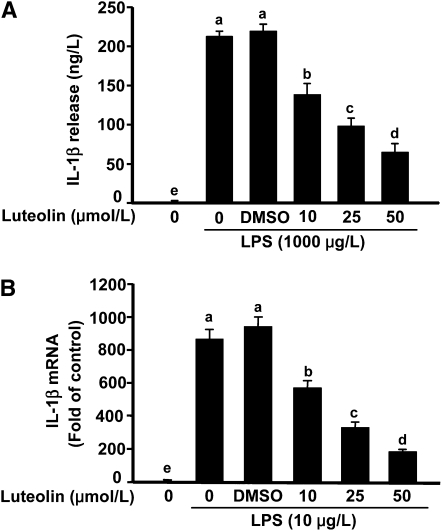
The IL-1β concentration in supernatant of microglial cells stimulated with LPS increased to >200 ng/L (Fig. 1A). Pretreatment of BV-2 cells with 10 and 25 μmol/L luteolin reduced LPS-induced IL-1β production by 25 and 50%, respectively. When luteolin was increased to 50 μmol/L, IL-1β secretion by LPS-stimulated microglia was blocked by up to 70%. LPS also induced a marked increase in IL-1β mRNA (Fig. 1B). Pretreatment with luteolin reduced steady-state levels of IL-1β mRNA in a dose-dependent manner. The effects of luteolin treatment on several other proinflammatory mediators produced by LPS-stimulated microglia are summarized in Table 1. In brief, luteolin inhibited the induction of inflammatory genes (i.e. TNFα, iNOS, and COX-2) and the release of proinflammatory mediators (i.e. TNFα, nitrite, and PGE2) by LPS-stimulated microglia. Neither LPS nor luteolin affected cell survival or proliferation. Taken together, these data indicate that luteolin is a potent, broad spectrum inhibitor of the proinflammatory response triggered by LPS in microglial cells.
FIGURE 1.
Luteolin inhibited LPS-induced IL-1β production (A) and mRNA expression (B) in BV-2 cells. Bars represent means ± SEM, n = 3. Means without a common letter differ, P < 0.05.
TABLE 1.
Effects of luteolin on LPS-stimulated proinflammatory mediators in microglial cells1
| Luteolin, μmol/L |
|||
| Proinflammatory mediators | 0 | 25 | 50 |
| TNFα, ng/L | 3164 ± 154a | 1834 ± 60b | 923 ± 143c |
| PGE2, ng/L | 220.0 ± 6.44a | 10.2 ± 2.50b | 7.6 ± 0.96b |
| Nitrite, μmol/L | 48.29 ± 2.64a | 6.51 ± 0.88b | 0.28 ± 0.33c |
| TNFα mRNA,2fold of control | 39.6 ± 2.55a | 33.2 ± 2.95b | 24.1 ± 2.83c |
| COX-2 mRNA, fold of control | 110.9 ± 7.89a | 96.0 ± 3.59b | 63.1 ± 2.08c |
| iNOS mRNA, fold of control | 132.5 ± 8.49a | 89.1 ± 6.74b | 45.2 ± 2.61c |
All microglia were stimulated with 10 μg/L LPS. Data are means ± SEM, = 3. Means in a row without a common letter differ, P < 0.05.
The control was untreated microglia.
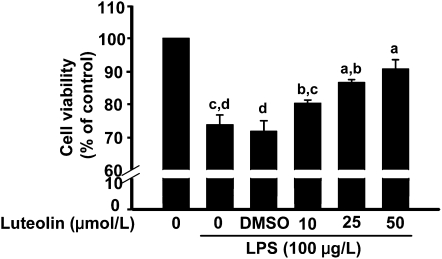
Luteolin inhibited neuronal cell death caused by activated microglia.
Conditioned supernatants from LPS-stimulated microglia caused discernible neuronal cell death (Fig. 2). However, treating microglia with luteolin prior to LPS reduced neuronal cell death caused by the conditioned supernatants (Fig. 2). Neither LPS nor luteolin induced cell death when added directly to differentiated Neuro.2a cells. Moreover, pretreating differentiated Neuro.2a cells with luteolin did not prevent cell death caused by supernatants from LPS-stimulated microglia (data not shown). Thus, the neuroprotective properties of luteolin were mediated through its effects on microglia.
FIGURE 2.
Treatment of microglia with luteolin prior to LPS stimulation inhibited neuronal cell death caused by microglial supernatants. Bars represent means ± SEM, n = 3. Means without a common letter differ, P < 0.05.
Dietary luteolin improved working memory and reduced neuroinflammation in aged mice.
Inflammatory cytokines are upregulated in the hippocampus of old animals due to increased microglial cell activity (31) and are considered to play a role in several behavioral deficits associated with aging (2). Therefore, we investigated if feeding aged mice a diet containing luteolin would reduce signs of inflammation in the hippocampus and improve performance in a hippocampal-dependent task. As expected, luteolin was not detectable in plasma of mice fed the control diet (Supplemental Fig. 1A). However, in mice fed the supplemented diet, the plasma luteolin concentration was estimated to be 2.29 ± 0.53 μmol/L (Supplemental Fig. 1B). Interestingly, in addition to luteolin, several unidentifiable peaks were detected in plasma from mice fed luteolin, suggesting either a portion of the absorbed luteolin was bound to another compound (e.g. fatty acid or cholesterol) or the presence of luteolin metabolites such as luteolin monoglucuronide, the main metabolite of luteolin found in rat and human plasma (19).
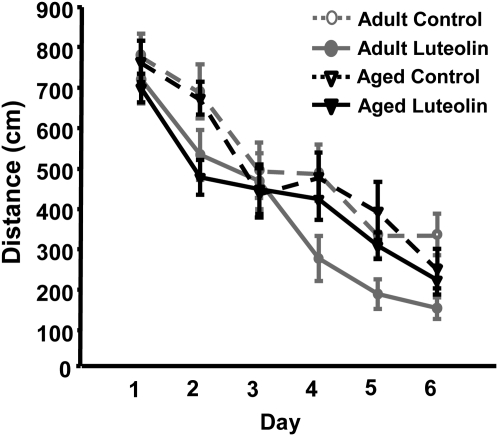
The distance swam to locate the platform is the most reliable indicator of learning and memory in the water maze, because latency to reach the platform can be affected by motor function and motivation. Repeated-measures ANOVA of distance swam to locate the platform in the acquisition phase revealed main effects of day (P < 0.001) and diet (P < 0.001), but none of the interactions were significant (P > 0.3). During the acquisition phase, the distance swam to reach the hidden platform decreased across sessions, indicating mice learned to utilize the extra maze visual cues to locate the platform (Fig. 3). An effect of diet on distance swam also was evident, where mice fed luteolin showed increased efficiency in locating the platform, irrespective of age (Fig. 3). These data indicate aged and adult mice used extra maze visual cues equally well to resolve the acquisition task and that luteolin improved spatial learning equally in both age groups.
FIGURE 3.
Effects of luteolin on performance of adult and aged mice during the 6-d acquisition phase of the Morris water maze. The distance swam to locate the static platform was measured. Values are means ± SEM, n = 12–14. Repeated-measures ANOVA: day, P < 0.001; diet, P < 0.001.
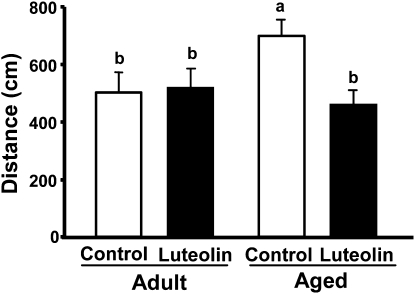
To assess spatial working memory, following the acquisition phase where the platform position was kept constant, the platform was moved to the opposite quadrant for a single session of reversal testing. This task is considered more difficult, because mice must integrate new information (i.e. new platform location) with existing memories (i.e. spatial qualities of the visual cues) to complete the hippocampal-dependent task. Two-way ANOVA of distance swam revealed an age × diet interaction (P < 0.05). Aged mice fed control diet performed poorer in this task than adults fed the same diet (Fig. 4). When aged mice received the luteolin-supplemented diet; however, the distance swam to locate the platform was similar to that of young adult controls. Luteolin did not affect spatial working memory in young adults. Analysis of latency to the platform revealed effects of age (P < 0.01) and diet (P < 0.01) (Supplemental Fig. 2). The shorter latency for adult mice fed luteolin, however, was due to improved motor function or motivation, because swim speed was faster than adult mice fed the control diet.
FIGURE 4.
Effects of luteolin on performance of adult and aged mice in a reversal test of spatial working memory. The distance swam to find the relocated platform was measured. Two-way ANOVA: age × diet, P < 0.05. Bars represent means ± SEM, n = 12–14. Means without a common letter differ, P < 0.05.
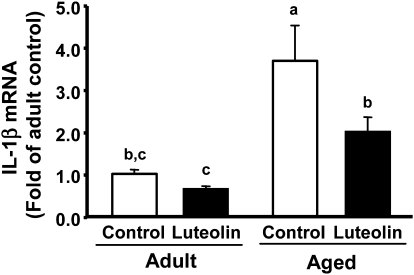
To determine whether consuming the luteolin-supplemented diet reduced age-associated brain inflammation, steady-state levels of IL-1β, IL-6, and TNFα mRNA were measured in the hippocampus. In addition, MHC class II mRNA was assessed to serve as a marker of activated microglia (32). Two-way ANOVA of IL-1β mRNA revealed main effects of age (P < 0.001) and diet (P < 0.03) and an age × diet interaction (P = 0.13) (Fig. 5). Consistent with previous studies (7, 33), steady-state levels of mRNA for IL-1β were increased in aged mice fed the control diet. Notably, when aged mice were fed diet containing luteolin, mRNA levels for IL-1β were reduced to levels in young adults fed the control diet. MHC class II, IL-6, and TNFα mRNA also increased in aged mice, suggesting microglial cell activation and increased constitutive expression of proinflammatory cytokines (Table 2). When aged mice were fed the diet containing luteolin, MHC class II mRNA was lower (P < 0.05) and that for IL-6 tended to be lower (P = 0.09) compared with aged mice fed the control diet. Luteolin consumption did not affect TNFα mRNA expression, which is consistent with what was observed with microglia in vitro (only the higher concentrations of luteolin inhibited LPS-induced TNFα production). These results indicate luteolin reduced constitutive microglial cell activation and proinflammatory cytokine production in the hippocampus.
FIGURE 5.
IL-1β mRNA in the hippocampus of adult and aged mice fed control or luteolin diet. Two-way ANOVA of IL-1β mRNA: age, P < 0.001; diet, P < 0.03; and age × diet , P = 0.13. Bars represent means ± SEM, n = 6–7. Means without a common letter differ, P < 0.05.
TABLE 2.
Effects of luteolin on expression of proinflammatory markers in the hippocampus of adult and aged mice1
| Adult |
Aged |
P-values |
|||||
| Control | Luteolin | Control | Luteolin | Age | Diet | Diet × Age | |
| mRNA, fold of adult control | |||||||
| IL-6 | 1.00 ± 0.12 | 0.96 ± 0.07 | 1.51 ± 0.14 | 1.19 ± 0.10 | 0.003 | 0.126 | 0.241 |
| TNFα | 1.00 ± 0.11 | 1.25 ± 0.20 | 2.56 ± 0.25 | 2.26 ± 0.35 | <0.001 | 0.911 | 0.289 |
| MHCII | 1.00 ± 0.16 | 0.62 ± 0.10 | 2.70 ± 0.60 | 1.51 ± 0.51 | 0.003 | 0.059 | 0.323 |
Data are means ± SEM, = 6–7.
Discussion
In this study, we showed that luteolin inhibited the production of inflammatory mediators by LPS-stimulated microglial cells and that treating microglial cells with luteolin prior to LPS reduced the neurotoxicity of conditioned supernatants when added to differentiated Neuro.2a cells. In a murine model, aging was confirmed to increase markers of inflammation in the hippocampus and inhibit hippocampal-dependent working memory. However, when aged mice received a luteolin-supplemented diet, signs of inflammation were reduced and performance in the hippocampal-dependent working memory task was restored to that in young adult cohorts. Thus, age-related conditions that are exacerbated by inflammation, including cognitive aging and neurodegenerative diseases, may be inhibited by increased dietary intake of luteolin or other plant-derived polyphenolic flavonoids.
The effects of luteolin consumption on age-related inflammation in the hippocampus as well as performance in a hippocampal-dependent cognitive task have not, to our knowledge, been studied previously. Nonetheless, our finding of reduced inflammation and improved working memory is consistent with several earlier reports. Letenneur et al. (34) reported an inverse relationship between flavonoid intake and cognitive decline in older persons. Commenges et al. (35) also reported that the relative risk of incident dementia was inversely related to the mean flavonoid intake in a cohort of 1367 participants older than 65 y. Beneficial effects of flavonoids on age-related cognitive deficits also have been studied in animal models. For example, aged rats fed a diet supplemented with spinach, blueberry, or strawberry extracts that were flavonoid-rich exhibited faster learning and better memory retention than unsupplemented controls (36). Furthermore, long-term administration of green tea catechins suppressed morphologic and functional regression of the brain in SAMP10 mice, a mouse strain that ages prematurely (37). Catechins were further shown to improve spatial learning in rats (38). The neuroprotective effect of flavonoids also was observed in a study in which chronic administration of apigenin-7-glucoside and quercetin reversed the age- and LPS-induced retention deficits in passive avoidance and elevated plus-maze tasks (39). These reports indicate that flavonoid intake may inhibit or slow cognitive aging. The fact that IL-1β in the hippocampus is closely related to deficits in hippocampal function led us to speculate flavonoids may improve learning and memory by reducing microglial cell activity.
The present study focused on luteolin, because previous reports comparing its antiinflammatory properties to other flavonoids such as quercetin, genistein, or hesperetin in peripheral macrophages found luteolin to be the most potent (40, 41). Luteolin was recently found to inhibit microglia in several in vitro models of inflammation (42, 43) and flavonoids have been speculated for some time to inhibit inflammation in the brain (44). These previous reports made the outcome of the in vitro studies with LPS-stimulated microglia rather predictable. Nonetheless, the current studies provide additional evidence of the specific effects of luteolin on IL-1β production by microglia and suggest that luteolin imparts its neuroprotective effects by direct action on microglia and not neurons. This observation is consistent with the well-documented role of inflammatory mediators in the progression of neurodegenerative diseases (45).
To investigate the effects of luteolin on inflammatory markers in the hippocampus and performance in a hippocampal-dependent task, mice were fed control or luteolin-supplemented diets for 4 wk. When consumed as part of the normal diet, luteolin was present in plasma, indicating some portion was absorbed intact. The study was not designed to determine bioavailability but instead to measure what would be found in circulation after the feeding bout that takes place at the onset of the dark phase of the light:dark cycle. Although the presence of luteolin in the brain was not determined, evidence suggests that flavonoids generally can penetrate the blood-brain barrier (44) and a flavonoid quercetin that is structurally similar to luteolin is detectable in brain (46, 47). Thus, it is reasonable to predict that circulating luteolin had access to the microglial cell compartment. Nonetheless, if luteolin reduced immune-to-brain signaling by an undefined peripheral mechanism, this too could explain the reduced signs of inflammation in the brain (48).
Activated microglia can be identified by expression of MHC class II. Microglia isolated from brain of aged rodents and aged nonhuman primates exhibited increased MHC class II expression (49, 50). This is true not only in rodents but also in humans where increased HLA-DR, a MHC class II marker, was found in the hippocampus of older individuals (51). The increase in MHC class II-positive microglia in the hippocampus has been observed to be associated with age-related cognitive decline in rodents (52), a finding supported herein. Further, minocycline, a purported microglial inhibitor, attenuated the age-related increase in MHC class II as well as the age-related impairments in long-term potentiation (17). In light of these published studies, our findings can be interpreted to suggest that dietary luteolin enhanced spatial working memory by mitigating microglial cell activity. We further speculate this is related to inhibition of constitutive expression of IL-1β and perhaps other inflammatory cytokines. IL-1β is emphasized because the hippocampus is well populated with microglial cells and rich in IL-1 receptors (12). If IL-1β production exceeds normal physiological levels, hippocampal neurogenesis is reduced and consolidation of hippocampal-dependent memories is inhibited (11, 53). Further, intracerebroventricular injection of IL-1 receptor antagonist reduced excessive behavioral deficits in aged mice given LPS to mimic a peripheral infection (13).
The current findings thus implicate luteolin’s antiinflammatory action on microglial cells as the mechanism for improved cognition. This is also consistent with its well-documented role as an antioxidant (54), because NFκB and activated protein-1 are redox-sensitive transcription factors that regulate a number of inflammatory genes. We recently showed that luteolin inhibited IL-6 gene expression in microglia by inhibiting Jun N-terminal kinase phosphorylation and activation of activated protein-1 (48). Studies with other cell types suggest luteolin can also inhibit NFκB activity (40, 55). Therefore, luteolin consumption may be beneficial in preventing or treating conditions that involve increased microglial cell activity and inflammation.
Supplementary Material
Acknowledgments
S.J. carried out most of the experimental work and wrote the first draft of the manuscript; R.N.D. carried out some of the experimental work and revised the manuscript; R.W.J. had overall supervision of the project and final revision of the manuscript. All authors read and approved the final version.
Footnotes
Supported by NIH grants AG-016710 and MH-069148.
Supplemental Figures 1 and 2 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: COX, cyclooxygenase; FBS, fetal bovine serum; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MHC, major histocompatibility complex; NFκB, nuclear factor κB; NO, nitric oxide; PG, prostaglandin; TNF, tumor necrosis factor.
Literature Cited
- 1.Annan K. Demographics of older persons. New York; UN; 1998 [Google Scholar]
- 2.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition: the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–56 [DOI] [PubMed] [Google Scholar]
- 3.Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–6 [DOI] [PubMed] [Google Scholar]
- 4.Daynes RA, Araneo BA, Ershler WB, Maloney C, Li GZ, Ryu SY. Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative. J Immunol. 1993;150:5219–30 [PubMed] [Google Scholar]
- 5.Ye SM, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappaB. J Neuroimmunol. 2001;117:87–96 [DOI] [PubMed] [Google Scholar]
- 6.Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med (Maywood). 2008;233:21–31 [DOI] [PubMed] [Google Scholar]
- 7.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–31 [DOI] [PubMed] [Google Scholar]
- 8.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–8 [DOI] [PubMed] [Google Scholar]
- 9.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–62 [DOI] [PubMed] [Google Scholar]
- 12.Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987;139:459–63 [PubMed] [Google Scholar]
- 13.Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakawa H, Blandino P, Jr, Deak T. Central infusion of interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiol Behav. 2009;98:139–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–28 [DOI] [PubMed] [Google Scholar]
- 16.Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–72 [DOI] [PubMed] [Google Scholar]
- 18.Rice-Evans CPL. Flavonoids in health and diseases. New York: CRC Press; 2003. p. 329–95 [Google Scholar]
- 19.Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, Yamamoto H, Kinae N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998;438:220–4 [DOI] [PubMed] [Google Scholar]
- 20.Xagorari A, Roussos C, Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br J Pharmacol. 2002;136:1058–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotanidou A, Xagorari A, Bagli E, Kitsanta P, Fotsis T, Papapetropoulos A, Roussos C. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. Am J Respir Crit Care Med. 2002;165:818–23 [DOI] [PubMed] [Google Scholar]
- 22.Hendriks JJ, Alblas J, van der Pol SM, van Tol EA, Dijkstra CD, de Vries HE. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J Exp Med. 2004;200:1667–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Delta12,14-prostaglandin J2. Proc Natl Acad Sci USA. 1999;96:4668–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan XD, Chen XC, Zhu YG, Chen LM, Zhang J, Huang TW, Ye QY, Huang HP. Tripchlorolide protects neuronal cells from microglia-mediated beta-amyloid neurotoxicity through inhibiting NF-kappaB and JNK signaling. Glia. 2009;57:1227–38 [DOI] [PubMed] [Google Scholar]
- 25.Georgopoulou N, Hurel C, Politis PK, Gaitanou M, Matsas R, Thomaidou D. BM88 is a dual function molecule inducing cell cycle exit and neuronal differentiation of neuroblastoma cells via cyclin D1 down-regulation and retinoblastoma protein hypophosphorylation. J Biol Chem. 2006;281:33606–20 [DOI] [PubMed] [Google Scholar]
- 26.Godbout JP, Johnson RW. Interleukin-6 in the aging brain. J Neuroimmunol. 2004;147:141–4 [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa K, Uehara M, Yanagitani H, Hashimoto T. Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Arch Biochem Biophys. 2006;455:197–203 [DOI] [PubMed] [Google Scholar]
- 28.Lee-Hilz YY, Ter Borg S, van Berkel WJ, Rietjens IM, Aarts JM. Shifted concentration dependency of EpRE- and XRE-mediated gene expression points at monofunctional EpRE-mediated induction by flavonoids at physiologically relevant concentrations. Toxicol In Vitro. 2008;22:921–6 [DOI] [PubMed] [Google Scholar]
- 29.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51 [DOI] [PubMed] [Google Scholar]
- 30.Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12:445–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–22 [DOI] [PubMed] [Google Scholar]
- 32.Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–7 [DOI] [PubMed] [Google Scholar]
- 33.Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci. 2005;22:1751–6 [DOI] [PubMed] [Google Scholar]
- 34.Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165:1364–71 [DOI] [PubMed] [Google Scholar]
- 35.Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16:357–63 [DOI] [PubMed] [Google Scholar]
- 36.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unno K, Takabayashi F, Kishido T, Oku N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10). Exp Gerontol. 2004;39:1027–34 [DOI] [PubMed] [Google Scholar]
- 38.Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr. 2006;136:1043–7 [DOI] [PubMed] [Google Scholar]
- 39.Patil CS, Singh VP, Satyanarayan PS, Jain NK, Singh A, Kulkarni SK. Protective effect of flavonoids against aging- and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology. 2003;69:59–67 [DOI] [PubMed] [Google Scholar]
- 40.Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296:181–7 [PubMed] [Google Scholar]
- 41.Comalada M, Ballester I, Bailon E, Sierra S, Xaus J, Galvez J, de Medina FS, Zarzuelo A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem Pharmacol. 2006;72:1010–21 [DOI] [PubMed] [Google Scholar]
- 42.Chen HQ, Jin ZY, Wang XJ, Xu XM, Deng L, Zhao JW. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci Lett. 2008;448:175–9 [DOI] [PubMed] [Google Scholar]
- 43.Rezai-Zadeh K, Ehrhart J, Bai Y, Sanberg PR, Bickford P, Tan J, Shytle RD. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J Neuroinflammation. 2008;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med. 2004;36:592–604 [DOI] [PubMed] [Google Scholar]
- 45.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98 [DOI] [PubMed] [Google Scholar]
- 46.de Boer VC, Dihal AA, van der Woude H, Arts IC, Wolffram S, Alink GM, Rietjens IM, Keijer J, Hollman PC. Tissue distribution of quercetin in rats and pigs. J Nutr. 2005;135:1718–25 [DOI] [PubMed] [Google Scholar]
- 47.Paulke A, Schubert-Zsilavecz M, Wurglics M. Determination of St. John's wort flavonoid-metabolites in rat brain through high performance liquid chromatography coupled with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;832:109–13 [DOI] [PubMed] [Google Scholar]
- 48.Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA. 2008;105:7534–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55 [DOI] [PubMed] [Google Scholar]
- 51.Streit WJ, Sparks DL. Activation of microglia in the brains of humans with heart disease and hypercholesterolemic rabbits. J Mol Med. 1997;75:130–8 [DOI] [PubMed] [Google Scholar]
- 52.Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–31 [DOI] [PubMed] [Google Scholar]
- 53.Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–18 [DOI] [PubMed] [Google Scholar]
- 54.Harris GK, Qian Y, Leonard SS, Sbarra DC, Shi X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. J Nutr. 2006;136:1517–21 [DOI] [PubMed] [Google Scholar]
- 55.Kim SH, Shin KJ, Kim D, Kim YH, Han MS, Lee TG, Kim E, Ryu SH, Suh PG. Luteolin inhibits the nuclear factor-kappa B transcriptional activity in Rat-1 fibroblasts. Biochem Pharmacol. 2003;66:955–63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.