Abstract
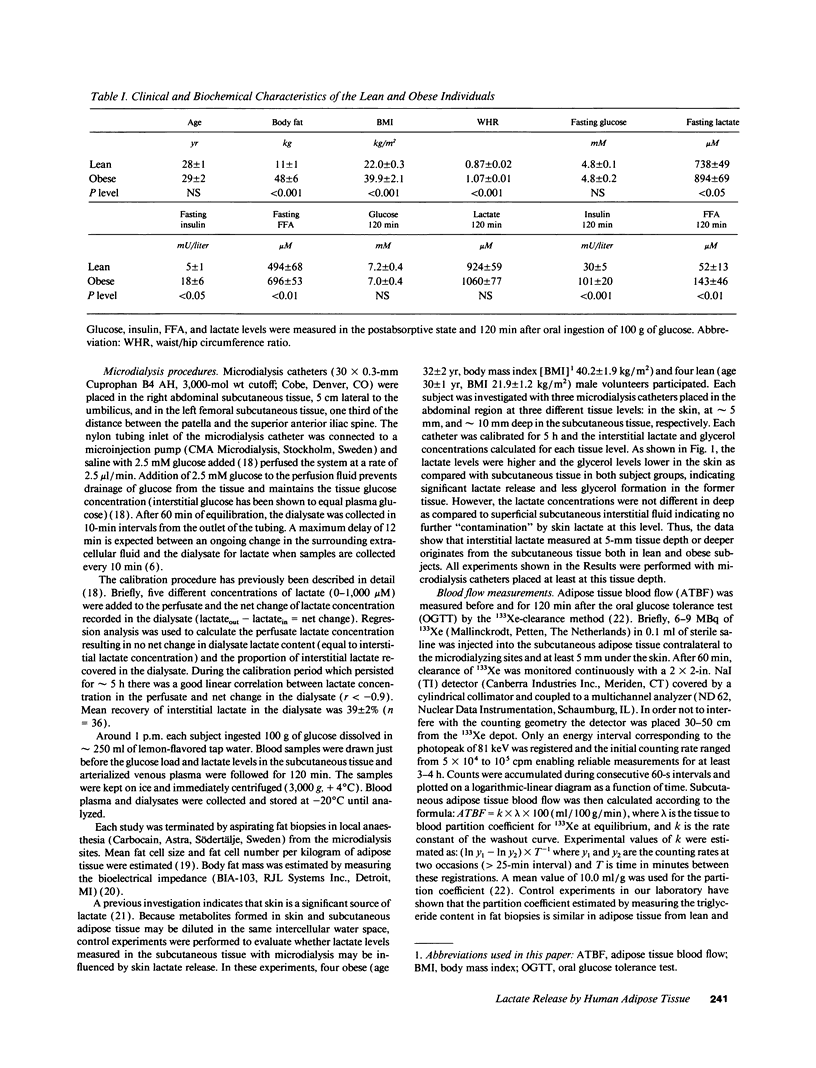
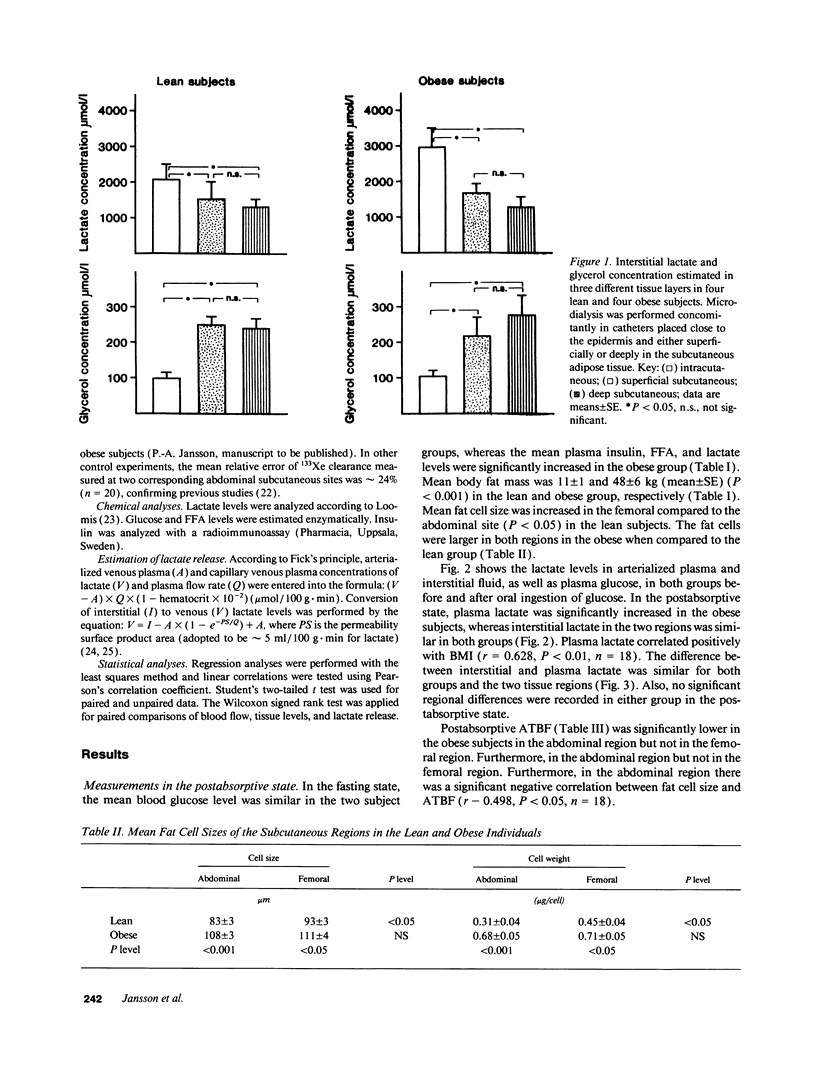
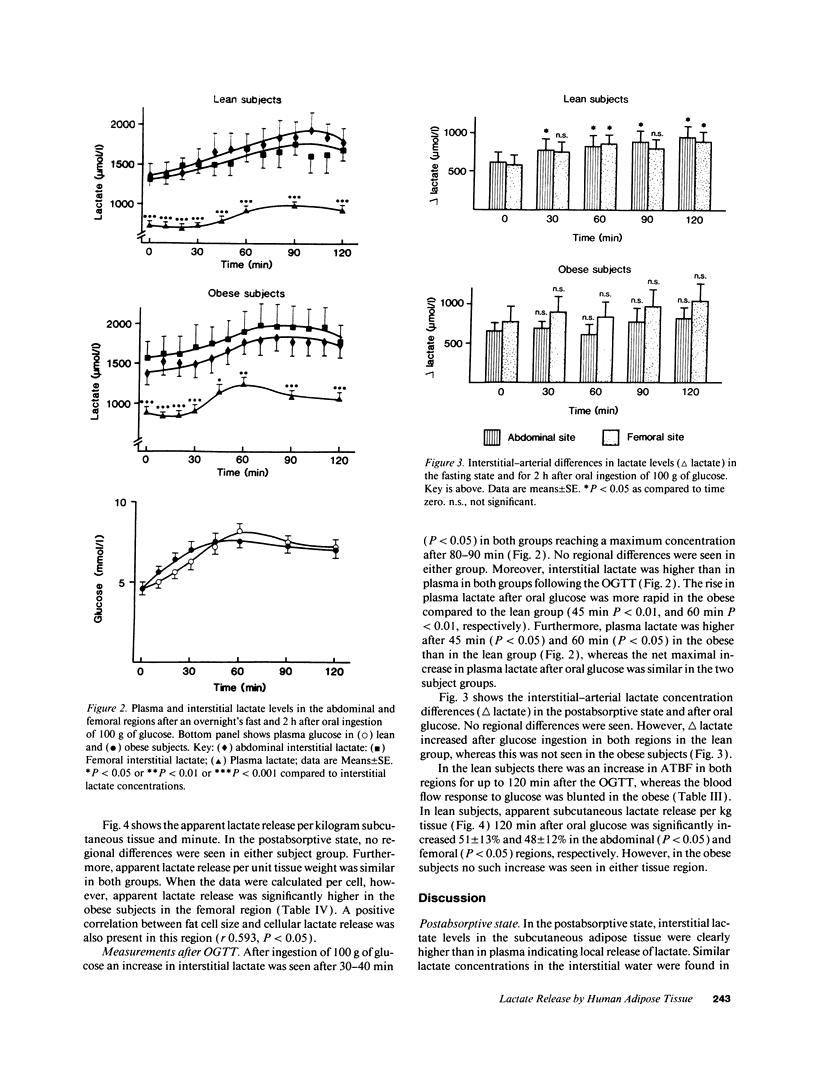
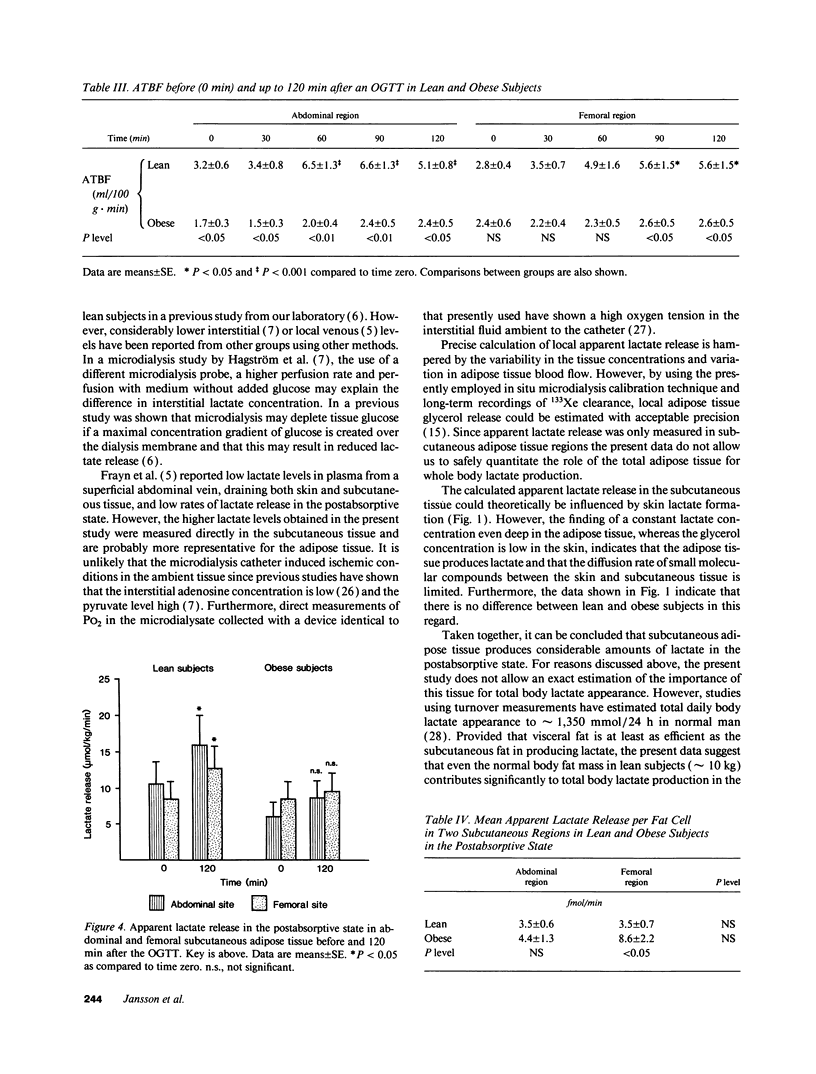
Lactate concentration in the subcutaneous interstitial fluid and adipose tissue blood flow (ATBF, ml/100 g.min) were simultaneously measured with the microdialysis technique combined with 133Xe clearance in the abdominal and femoral subcutaneous adipose tissue in nine lean and nine obese men. The studies were performed both in the postabsorptive state and 2 h after an oral glucose load and the results compared to the lactate levels in arterialized venous plasma. After an overnight's fast, arterial lactate was 738 +/- 49 and 894 +/- 69 microM (mean +/- SE) (P < 0.05) in the lean and obese subjects, respectively. The interstitial lactate levels were significantly higher than blood lactate in both subject groups without any regional differences. Abdominal and femoral ATBF was 3.2 +/- 0.6 vs. 2.8 +/- 0.4 and 1.7 +/- 0.3 vs. 2.4 +/- 0.4 ml/100 g.min (P < 0.05) in lean and obese subjects, respectively. Mean apparent lactate release from the abdominal vs. femoral adipose tissue in the fasting state was 10.5 +/- 3.1 vs. 8.6 +/- 2.3 and 6.0 +/- 2.3 vs. 8.5 +/- 2.3 mumol/kg.min (NS) in lean and obese subjects, respectively. Both plasma and interstitial lactate levels increased significantly after an oral glucose load in both subject groups. However, apparent lactate release increased significantly only in the lean group. It is concluded that subcutaneous adipose tissue is a significant source of whole-body lactate release in the postabsorptive state and that this is further enhanced in obese subjects due to their large adipose mass.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen T., McNair P., Fogh-Andersen N., Nielsen T. T., Hyldstrup L., Transbøl I. Increased parathyroid hormone as a consequence of changed complex binding of plasma calcium in morbid obesity. Metabolism. 1986 Feb;35(2):147–151. doi: 10.1016/0026-0495(86)90116-2. [DOI] [PubMed] [Google Scholar]
- Attvall S., Fowelin J., von Schenck H., Smith U., Lager I. Insulin-antagonistic effects of pulsatile and continuous glucagon infusions in man--a comparison with the effect of adrenaline. J Clin Endocrinol Metab. 1992 May;74(5):1110–1115. doi: 10.1210/jcem.74.5.1569157. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Sjöström L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism. 1978 Dec;27(12 Suppl 2):1853–1865. doi: 10.1016/s0026-0495(78)80004-3. [DOI] [PubMed] [Google Scholar]
- Buchalter S. E., Crain M. R., Kreisberg R. Regulation of lactate metabolism in vivo. Diabetes Metab Rev. 1989 Jun;5(4):379–391. doi: 10.1002/dmr.5610050405. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Capani F., Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989 May;38(5):550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Reilly J. J., Jr, Bier D. M., Gerich J. E. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990 Dec;86(6):2038–2045. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppack S. W., Fisher R. M., Gibbons G. F., Humphreys S. M., McDonough M. J., Potts J. L., Frayn K. N. Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci (Lond) 1990 Oct;79(4):339–348. doi: 10.1042/cs0790339. [DOI] [PubMed] [Google Scholar]
- Crandall D. L., Fried S. K., Francendese A. A., Nickel M., DiGirolamo M. Lactate release from isolated rat adipocytes: influence of cell size, glucose concentration, insulin and epinephrine. Horm Metab Res. 1983 Jul;15(7):326–329. doi: 10.1055/s-2007-1018710. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Skinner N. S., Jr, Hanley H. G., Sachs R. G. Relationship of adipose tissue blood flow to fat cell size and number. Am J Physiol. 1971 Apr;220(4):932–937. doi: 10.1152/ajplegacy.1971.220.4.932. [DOI] [PubMed] [Google Scholar]
- Frayn K. N., Coppack S. W., Humphreys S. M., Whyte P. L. Metabolic characteristics of human adipose tissue in vivo. Clin Sci (Lond) 1989 May;76(5):509–516. doi: 10.1042/cs0760509. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B. Vascular and metabolic effects of theophylline, dibuturyl cyclic AMP and dibuturyl cyclic GMP in canine subcutaneous adipose tissue in situ. Acta Physiol Scand. 1974 Jan;90(1):226–236. doi: 10.1111/j.1748-1716.1974.tb05581.x. [DOI] [PubMed] [Google Scholar]
- Hagström E., Arner P., Ungerstedt U., Bolinder J. Subcutaneous adipose tissue: a source of lactate production after glucose ingestion in humans. Am J Physiol. 1990 May;258(5 Pt 1):E888–E893. doi: 10.1152/ajpendo.1990.258.5.E888. [DOI] [PubMed] [Google Scholar]
- Jansson P. A., Larsson A., Smith U., Lönnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest. 1992 May;89(5):1610–1617. doi: 10.1172/JCI115756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. A., Smith U., Lönnroth P. Evidence for lactate production by human adipose tissue in vivo. Diabetologia. 1990 Apr;33(4):253–256. doi: 10.1007/BF00404805. [DOI] [PubMed] [Google Scholar]
- Johnson J. A., Fusaro R. M. The role of the skin in carbohydrate metabolism. Adv Metab Disord. 1972;60:1–55. doi: 10.1016/b978-0-12-027306-5.50006-1. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A., Verso M. A., Andrews J., Vasquez B., Reaven G., Foley J. E. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983 Oct;72(4):1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg R. A. Glucose-lactate inter-relations in man. N Engl J Med. 1972 Jul 20;287(3):132–137. doi: 10.1056/NEJM197207202870307. [DOI] [PubMed] [Google Scholar]
- LOOMIS M. E. An enzymatic fluorometric method for the determination of lactic acid in serum. J Lab Clin Med. 1961 Jun;57:966–969. [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Brechtel G., Baron A. D. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990 Jun;85(6):1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen O. A., Lassen N. A., Quaade F. Blood flow through human adipose tissue determined with radioactive xenon. Acta Physiol Scand. 1966 Mar;66(3):337–345. doi: 10.1111/j.1748-1716.1966.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Lassen N. A. Capillary diffusion capacity of sodium studied by the clearances of Na-24 and Xe-133 from hyperemic skeletal muscle in man. Scand J Clin Lab Invest Suppl. 1967;99:24–26. [PubMed] [Google Scholar]
- Lovejoy J., Mellen B., Digirolamo M. Lactate generation following glucose ingestion: relation to obesity, carbohydrate tolerance and insulin sensitivity. Int J Obes. 1990 Oct;14(10):843–855. [PubMed] [Google Scholar]
- Lovejoy J., Newby F. D., Gebhart S. S., DiGirolamo M. Insulin resistance in obesity is associated with elevated basal lactate levels and diminished lactate appearance following intravenous glucose and insulin. Metabolism. 1992 Jan;41(1):22–27. doi: 10.1016/0026-0495(92)90185-d. [DOI] [PubMed] [Google Scholar]
- Lukaski H. C., Bolonchuk W. W., Hall C. B., Siders W. A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol (1985) 1986 Apr;60(4):1327–1332. doi: 10.1152/jappl.1986.60.4.1327. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Jansson P. A., Fredholm B. B., Smith U. Microdialysis of intercellular adenosine concentration in subcutaneous tissue in humans. Am J Physiol. 1989 Feb;256(2 Pt 1):E250–E255. doi: 10.1152/ajpendo.1989.256.2.E250. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Jansson P. A., Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987 Aug;253(2 Pt 1):E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Mårin P., Rebuffé-Scrive M., Smith U., Björntorp P. Glucose uptake in human adipose tissue. Metabolism. 1987 Dec;36(12):1154–1160. doi: 10.1016/0026-0495(87)90242-3. [DOI] [PubMed] [Google Scholar]
- Newby F. D., Sykes M. N., DiGirolamo M. Regional differences in adipocyte lactate production from glucose. Am J Physiol. 1988 Nov;255(5 Pt 1):E716–E722. doi: 10.1152/ajpendo.1988.255.5.E716. [DOI] [PubMed] [Google Scholar]
- Paaske W. P. Absence of restricted diffusion in adipose tissue capillaries. Acta Physiol Scand. 1977 Aug;100(4):430–436. doi: 10.1111/j.1748-1716.1977.tb05967.x. [DOI] [PubMed] [Google Scholar]
- Smith U., Sjöström L., Björnstorp P. Comparison of two methods for determining human adipose cell size. J Lipid Res. 1972 Nov;13(6):822–824. [PubMed] [Google Scholar]



