Abstract
Background: Eating in the absence of hunger (EAH) is typically assessed by measuring youths’ intake of palatable snack foods after a standard meal designed to reduce hunger. Because energy intake required to reach satiety varies among individuals, a standard meal may not ensure the absence of hunger among participants of all weight strata.
Objective: The objective of this study was to compare adolescents’ EAH observed after access to a very large food array with EAH observed after a standardized meal.
Design: Seventy-eight adolescents participated in a randomized crossover study during which EAH was measured as intake of palatable snacks after ad libitum access to a very large array of lunch-type foods (>10,000 kcal) and after a lunch meal standardized to provide 50% of the daily estimated energy requirements.
Results: The adolescents consumed more energy and reported less hunger after the large-array meal than after the standardized meal (P values < 0.001). They consumed ≈70 kcal less EAH after the large-array meal than after the standardized meal (295 ± 18 compared with 365 ± 20 kcal; P < 0.001), but EAH intakes after the large-array meal and after the standardized meal were positively correlated (P values < 0.001). The body mass index z score and overweight were positively associated with EAH in both paradigms after age, sex, race, pubertal stage, and meal intake were controlled for (P values ≤ 0.05).
Conclusion: EAH is observable and positively related to body weight regardless of whether youth eat in the absence of hunger from a very large-array meal or from a standardized meal. This trial was registered at clinicaltrials.gov as NCT00631644.
INTRODUCTION
Eating in the absence of hunger (EAH) refers to eating in response to the presence of palatable foods in the absence of perceived hunger (1). Pediatric EAH was first examined objectively in the laboratory by observing young children's snack intakes after a standard meal intended to reduce hunger (2, 3). Five- and 7-y-old white girls who consumed large amounts of snack foods after a standard lunch were found to have a nearly 5-fold greater likelihood of being overweight [body mass index (BMI; in kg/m) ≥85th percentile standard for age and sex (4)] than did their counterparts who had low levels of snack intake (3). In a slightly modified paradigm, Hispanic girls and boys aged 5–18 y (mean ± SD: 11.8 ± 0.2 y) who had high levels of snack food intake after a standardized dinner meal (providing 50% of daily estimated energy needs based on age, sex, and weight) similarly had a 50% greater odds of being obese (BMI ≥95th percentile) (5).
The propensity to engage in EAH, evaluated after a standardized meal, appears to represent a potential vulnerability for childhood overweight (6). However, because the original EAH paradigm was designed to reduce, rather than to eliminate, hunger, it is unknown whether the youth of all weight ranges in prior studies were equally satiated. Overweight youth have the capacity to consume more than their nonoverweight peers at meals (7–9) and, thus, may require greater energy intakes, relative to energy requirements, to achieve satiation or sustain satiety. Thus, it is not entirely certain whether overweight youth simply eat more overall or truly eat more in the absence of hunger than do nonoverweight youth. Such differences might be anticipated to be magnified among adolescents because EAH appears to increase with age (2, 5).
We therefore tested a modified laboratory paradigm of EAH, designed to produce a more complete absence of hunger among youth of all weight strata, against the usual approach for measuring EAH. By using a randomized crossover design, we compared adolescents’ EAH as determined after ad libitum intake from a very large food array with EAH as assessed after a standardized meal. We predicted that EAH would be significantly lower when measured after a fully satiating large multi-item food array than when estimated after a standardized meal. Consistent with the notion that EAH represents a valid behavioral endophenotype associated with overweight among youth (1), we also hypothesized that EAH would nevertheless be observed after access to a large food array and, furthermore, that it would be related to body weight.
SUBJECTS AND METHODS
Subjects
The participants were healthy adolescent volunteers recruited for the study through flyers posted at the National Institutes of Health and at local libraries, supermarkets, and school-parent e-mail listservs in Washington, DC, and the greater metropolitan area. The study was advertised as an investigation of eating behaviors in adolescents and indicated that no treatment would be provided. The youth were financially compensated for their participation. Individuals were eligible to participate if they were between 13 and 17 y of age and were in good general health. Exclusion criteria included chronic illnesses, use of medications likely to affect energy intake, pregnancy, ongoing weight-loss treatment, or a psychiatric condition that would impede adherence to study procedures. The adolescents provided written assent and their parents or guardians gave written consent for participation. The study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board.
Procedures
The participants completed 3 outpatient appointments on 3 separate days at the National Institutes of Health Hatfield Clinical Research Center. The adolescents were instructed to adhere to a fast (only water) initiated at 2200 on the night before all visits.
Visit 1: screening
The participants were screened for eligibility at an initial visit that included a medical history and a physical examination performed by an endocrinologist or a nurse practitioner. Testicular volume (mL) was measured by using a set of orchidometer beads as standards according to Prader (10), and breast development was assigned according to the 5 stages of Tanner (11, 12). Testicular volume for males and Tanner breast staging for females were used to categorize youth as in prepuberty or early/midpuberty (boys: testes <15 mL; girls: Tanner stages 1–3) or in late puberty (boys: testes ≥15 mL; girls: Tanner stages 4–5). Height was measured 3 times to the nearest millimeter by using a stadiometer (Holtain, Crymmych, United Kingdom) calibrated before each participant's measurement. Fasting weight was measured to the nearest 0.1 kg with a calibrated digital scale (Scale-Tronix, Wheaton, IL). Height and weight were used to compute BMI, calculated as weight (kg) divided by the square of height (m). BMI SD (BMI z) scores for sex and age were calculated according to the Centers for Disease Control and Prevention 2000 standards (4). Overweight status was dichotomized into nonoverweight (BMI <85th percentile) and overweight or obese (BMI ≥85th percentile). Fat-free mass (kg) and percentage body fat were assessed with air-displacement plethysmography (Life Measurement Inc, Concord, CA). These body-composition measurements were obtained as recommended, ie, after the participants had fasted and while they were wearing underclothes only (13).
To acclimate the participants to the test meal condition, each adolescent was served a breakfast shake (33% of daily estimated energy needs based on age, sex, and BMI: 52% carbohydrate, 11% protein, 37% fat) in a private room. They were given a tape-recorded instruction: "Please drink all of the shake. Try to drink it all within 5 minutes." Consumption of <50% of the shake was considered an index of inability to acclimate to laboratory test meal conditions and was a criterion for exclusion.
At the screening appointment, the participants completed a food-preference questionnaire on which they rated how much they liked or disliked each food to be served at the test meals on a 10-point Likert scale from 1 = "I hate the food" to 10 = "I love the food." Any adolescent who disliked (defined as a rating <6) >50% of the food items that were to be used in the test meals was excluded.
Participants who completed the screening visit were entered into the randomized crossover phase of the study, for which they returned on separate days (mean ± SD: 22.5 ± 29.2 d apart) for EAH measurements that differed only in the type of meal provided (a large-array meal compared with standardized meal) before EAH was assessed.
Visits 2 and 3: test meals and EAH assessments
Each participant was served a lunch-type meal at 1100 in a private room and given the tape-recorded instruction to "Please eat until you are no longer hungry. Take as much time as you need and open the door when you're done." In the large-array condition, the meal consisted of a 10,934 -kcal multi-item buffet test meal with individual items that varied in macronutrient composition (overall: 54% carbohydrate, 12% protein, 33% fat). Items included a wide assortment of foods that most youth like (Table 1). In the standardized meal condition (Table 1), a subset of foods from the large-array meal (chicken nuggets, grapes, baby carrots, tortilla chips, sandwich cookies, ketchup, 2%-fat milk, and lemonade) were served in amounts adjusted for each participant such that the meal provided 50% of the adolescent's total daily estimated energy needs based on age, sex, and BMI (14). On average, the standardized meal provided 1364.9 ± 273.8 (mean ± SD) kcal. The participants were played the identical tape-recorded instruction to eat until no longer hungry. The standardized meal was designed to approximate the macronutrient composition of the large-array meal (55.4 ± 2.4% carbohydrate, 11.6 ± 0.2% protein, 35.2 ± 0.4% fat). After completing the meal, the participants were escorted to a separate room, where they were invited to view magazines screened to be devoid of content related to food, eating, body shape, or weight. They were also asked to rate their subjective feelings of hunger and fullness on a visual analog scale (VAS) that ranged from 1 = "not at all" to 100 = "extremely."
TABLE 1.
Food items presented at the large-array meal, standardized meal, and snack array
| Large-array meal | Standardized meal1 | Snack array | |
| 6 Slices white bread | Chicken nuggets (270 g) | Chicken nuggets (121 ± 26 g)2 | Popcorn (65 g) |
| 6 Slices wheat bread | Tortilla chips (120 g) | Tortilla chips (31 ± 3 g) | Potato chips (70 g) |
| 3 Kaiser rolls | Grapes (250 g) | Grapes (178 ± 55 g) | Pretzel twists (70 g) |
| Ham (180 g) | Chocolate candy (120 g)3 | Baby carrots (91 ± 28 g) | Fig bars (120 g) |
| 3 Bananas | 12 Sandwich cookies4 | 2–8 Sandwich cookies4 | Chocolate chip cookies (90 g) |
| Turkey (180 g) | 2%-Fat milk (850 g) | 2%-Fat milk (370 ± 66 g) | Fruit chew candy5 (150 g) |
| American cheese (240 g) | Lemonade (850 g) | Lemonade (371 ± 67 g) | Chocolate malt balls (120 g) |
| Jelly beans (120 g) | Mayonnaise (90 g) | Ketchup (30 g) | Chocolate ice cream (150 g) |
| Peanut butter (120 g) | Mustard (90 g) | Strawberry ice cream (150 g) | |
| Grape jelly (120 g) | Barbecue sauce (90 g) | Vanilla ice cream (150 g) | |
| Tomatoes (200 g) | Mild salsa (250 g) | Cherry Italian ice (200 g) | |
| Lettuce (50 g) | Ranch dressing (90 g) | Lemon Italian ice (200 g) | |
| Baby carrots (200 g) | Water (850 g) | ||
| 3 Oranges | Apple juice (850 g) | ||
| Pretzel sticks (150 g) | 12 Vanilla wafers | ||
Foods were served in amounts adjusted for each participant such that the meal provided 50% of each adolescent's total daily estimated energy needs based on age, sex, and BMI (14).
Mean ± SD (all such values).
M&M's (Mars Inc, Hackettstown, NJ).
Oreos (Kraft Foods, Northfield, IL).
Starbursts (Mars Inc).
One half-hour after meal termination, the participants were escorted back to the test meal room where they were provided with a 4055-kcal array of novel, highly palatable snack food items in generous portions (Table 1). The adolescents were played a tape-recorded instruction that stated, "Please taste each of the foods. Rate your preferences for how much you like or dislike the foods on this rating form. Try to take at least 2 bites of each food. When you're done, feel free to use any of the activities in the room, and eat as much of the foods as you like. The investigator will return in 15 minutes." Participants were provided with a food-preference rating form that listed each snack food. Ratings were completed on a 10-point Likert scale from 1 = "I hate the food" to 10 = "I love the food." Nonfood activities were available on a separate side table, including hand-held computer games, playing cards, magazines, word and drawing games, paper, and crayons/markers. The adolescents were left alone for a 15-min period. At the conclusion of the EAH assessment period, the participants were escorted to a separate room to again rate their hunger and fullness.
The amounts of each food and beverage consumed from the meals (large-array and standardized meals) and from the snack array (EAH after the large-array meal and EAH after the standardized meal) were measured by using the difference in weight (g) of each item before and after the meal. Energy (kcal) intakes were calculated with data from the US Department of Agriculture (USDA) National Nutrient Database for Standard Reference (USDA, Agricultural Research Service, Beltsville, MD) and food manufacturer nutrient information obtained from food labels. An exclusion criterion was energy intake during the lunch meals that was markedly less than could be expected for a standard, nonfasting meal (<300 kcal) (15); however, no participant met this criterion.
Statistical methods
All analyses were performed with SPSS 16.0 (SPSS Inc, Chicago, IL). Procedures advocated by Behrens (16) were used to examine study variables to determine whether the assumptions of univariate and multivariate analyses were met. The skew and kurtosis were satisfactory on all variables, and outliers were adjusted to fall 1.5 times the interquartile range below or above the 25th or 75th percentile. This strategy was used because it minimizes the influence of outliers on the characteristics of the distribution, minimally changes the distribution overall, and avoids potential bias associated with the elimination of outliers altogether. This correction did not significantly alter the direction or magnitude of any result. Pearson's correlations were used to examine the relatedness of energy intake at the large-array and standardized meals and the relatedness of EAH (ie, snack intake) after the large-array and standardized meals. Paired-samples t tests were conducted to compare: 1) adolescents’ observed intake at the large-array and standardized meals, 2) adolescents’ EAH after the large-array and standardized meals, 3) perceived pleasantness ratings of snack foods during the EAH snack arrays, and 4) adolescents’ subjective feelings of hunger and fullness before and after measurement of EAH. The univariate relations of demographic and anthropometric characteristics with observed meal and EAH intake were assessed with Pearson's correlations. Linear mixed models with repeated measures were used to examine whether BMI z scores, weight status (nonoverweight compared with overweight or obese), and body composition [fat-free mass (kg), percentage fat mass, and height (cm)] were associated with meal and EAH intakes (kcal) after adjustment for covariates. The fixed main effects in all models were age (y), sex (male compared with female), race (white, non-Hispanic compared with other), puberty (prepuberty or early/midpuberty compared with late pubserty), and meal type (large-array meal compared with standardized meal). In the models predicting EAH, meal intake (kcal) was included as a covariate. Separate models were run for BMI z score, weight status, and body composition to avoid issues of multicollinearity. After the main effects were calculated, the interaction of BMI z score, overweight status, fat-free mass, or fat mass with meal type was entered into the respective models to investigate meal type as a moderator. This permitted us to test whether the observed associations of BMI z score, overweight status, and body composition with meal and EAH intakes varied as a function of the type of meal served.
RESULTS
Eighty-four youth were screened for eligibility. Six youth were excluded before participating in the meal studies, including 3 whose questionnaire scores indicated that they did not like enough foods offered at the test meals, 1 who reported psychiatric symptoms that were judged to potentially impede study compliance, and 2 who could not be scheduled for test meal appointments within 6 mo of their screening visits. The final sample consisted of 78 youth (43.6% female). Descriptive characteristics are shown in Table 2.
TABLE 2.
Demographic and anthropometric characteristics and their univariate correlations with observed energy intakes1
| Correlation with observed energy intake |
|||||
| Characteristic | Sample (n = 78) | Large-array meal | Standardized meal | EAH after array | EAH after meal |
| Age (y) | 15.2 ± 1.4 (13.1–17.9)2 | −0.03 | 0.05 | −0.10 | −0.11 |
| Sex: female (%) | 43.6 | −0.363 | −0.543 | −0.14 | 0.08 |
| Race: white, non-Hispanic (%) | 47.4 | −0.04 | 0.13 | 0.18 | 0.01 |
| Pubertal status: late puberty (%) | 82.1 | 0.07 | 0.07 | 0.234 | 0.21 |
| BMI z score | 0.7 ± 1.1 (−1.5–2.6) | 0.17 | 0.21 | 0.274 | 0.244 |
| Overweight status (%) | 35.9 | 0.06 | 0.15 | 0.285 | 0.14 |
| Fat-free mass (kg) | 49.8 ± 9.5 (30.9–70.2) | 0.264 | 0.523 | 0.09 | 0.10 |
| Percentage fat mass | 22.9 ± 11.8 (6.2–52.1) | −0.10 | −0.15 | 0.234 | 0.09 |
For the correlation analyses: Sex = 0 for males and 1 for females, Race = 0 for whites and non-Hispanics and 1 for other, Pubertal status = 0 for pre- or early/midpuberty (boys: testes <15 mL; girls: Tanner stages 1–3) and 1 for late puberty (boys: testes ≥15 mL; girls: Tanner stages 4–5), and Overweight status = 0 for BMI <85th percentile and 1 for BMI ≥85th percentile. EAH, eating in the absence of hunger.
Mean ± SD; range in parentheses (all such values).
P < 0.001.
P < 0.05.
P < 0.01.
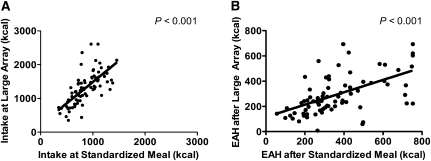
Participants consumed, on average (±SE), 861 ± 30 kcal (range: 345–1458 kcal) at the standardized meal (63.1 ± 1.7% of energy offered) and 1309 ± 55 kcal (range: 352–2610 kcal) at the large-array meal (12.0 ± 0.5% of energy offered). Energy intakes at the large-array and standardized meals were significantly related (P < 0.001; Figure 1A). Likewise, EAH assessed after the large-array meal and EAH assessed after the standardized meal were significantly correlated (P < 0.001; Figure 1B). As shown in Figure 2A, the adolescents consumed, on average, 448 kcal (95% CI: 367, 529 kcal) more at the large-array meal than at the standardized meal (P < 0.001). The adolescents reported less hunger after the large-array meal (mean ± SE: 8.4 ± 1.1; range: 0–31) than after the standardized meal (15.2 ± 1.7; 0–48) on a 1–100 VAS (t = −4.50, P < 0.001). Likewise, they reported greater fullness after the large-array meal (76.5 ± 1.9; 39–100) than after the standardized meal (64.5 ± 2.4; 8–99) on a 1–100 VAS (t = 4.52, P < 0.001).
FIGURE 1.
A: Relation between the adolescents’ standardized meal intake and large-array meal intake (Pearson's r = 0.69, P < 0.001); n = 78. B: Relation between the adolescents’ eating in the absence of hunger (EAH) assessed after a standardized meal and EAH assessed after a large-array meal (Pearson's r = 0.55, P < 0.001); n = 78.
FIGURE 2.
A: Paired t test comparison of the adolescents’ large-array meal intake (mean ± SE: 1309.3 ± 54.9 kcal) and standardized meal intake (861.1 ± 29.9 kcal; t = 11.05, P < 0.001); n = 78. B: Paired t test comparison of adolescents’ eating in the absence of hunger (EAH) after a large-array meal (294.9 ± 18.2 kcal) and EAH after the standardized meal (365.2 ± 20.3 kcal; t = −3.82, P < 0.001); n = 78.
Greater intake at the large- array meal was associated with a small but significant attenuation in the amount of observed EAH. The adolescents ate an average of 70 kcal (95% CI: 34, 107 kcal) less from snack foods after the large-array meal than after the standardized meal (Figure 2B; P < 0.001). Nevertheless, EAH intakes after both meals were considerable: 295 ± 18 kcal after the large-array meal and 365 ± 20 kcal after the standardized meal. Pleasantness ratings of the EAH snacks were not significantly different between the large-array condition (6.9 ± 0.1; 4–9) and the standardized lunch condition (6.8 ± 0.1; 4–9) on a 1–10 scale (P = 0.61). After access to the EAH array, feelings of hunger remained lower after the large-array meal (5.2 ± 0.7; 0–18) than after the standardized meal (7.2 ± 0.9; 0–22) on a 1–100 VAS (t = −2.70, P = 0.009); feelings of fullness after access to snacks did not differ significantly between conditions (P = 0.11).
The univariate, unadjusted relations of demographic and anthropometric characteristics with meal and EAH intakes are described in Table 2. The BMI z score was positively correlated with EAH as assessed after both meal types (P values < 0.05), but was not related to meal intake in univariate analyses. Overweight status was significantly related to greater observed EAH after the large-array meal (P < 0.01). With respect to body composition, the adolescents’ fat-free mass was positively correlated with their meal energy intake in both conditions (P values < 0.05), and percentage fat mass was related to a greater EAH after the large-array meal (P < 0.05).
The results from linear mixed modeling analyses with repeated measures that examined whether BMI z score, weight status, and body composition remained associated with meal and EAH intakes after adjustment for covariates are shown inTable 3. After age, sex, race, puberty, and meal type were controlled for, BMI z score was positively associated with meal energy intake (P = 0.02). Overweight status showed a similar pattern; accounting for covariates, overweight and obese youth tended to have greater meal intake (P = 0.06). When fat-free mass, percentage fat mass, and height were modeled together, none of these variables predicted meal intake. Similar results were found for the models predicting EAH. BMI z score was related to EAH energy intake after adjustment for all covariates, including meal intake (P = 0.02). Overweight status showed the same pattern: overweight and obese youth had a significantly greater EAH than did nonoverweight youth (P = 0.05). Height, fat-free mass, and percentage fat mass were not significant predictors of EAH energy intake. None of the analyses that examined the effect of body size on meal intake or EAH intake showed a significant interaction with meal type (all P values > 0.10).
TABLE 3.
Linear mixed modeling repeated-measures analyses of the associations of BMI z scores, overweight status, and body composition with meal and eating in the absence of hunger (EAH) intakes (n = 78)1
| Meal intake (kcal) |
EAH intake (kcal) |
|||||
| Variable entered | Estimate | SE | P | Estimate | SE | P |
| Age (y) | −11.5 | 26.6 | 0.67 | −24.4 | 12.0 | 0.05 |
| Sex | −349.4 | 72.6 | <0.001 | −6.5 | 35.2 | 0.85 |
| Race | 18.9 | 70.9 | 0.79 | −38.6 | 32.0 | 0.23 |
| Pubertal status | 178.3 | 98.9 | 0.08 | 130.3 | 45.1 | 0.005 |
| Meal intake (kcal) | 0.0 | 0.0 | 0.96 | |||
| Meal type | −448.2 | 40.6 | <0.001 | 66.5 | 24.3 | 0.007 |
| Model with BMI z score | ||||||
| BMI z score | 85.7 | 34.6 | 0.02 | 38.0 | 16.1 | 0.02 |
| Meal type × BMI z score | −24.8 | 38.3 | 0.52 | −2.3 | 16.9 | 0.89 |
| Model with overweight status | ||||||
| Overweight status | 146.8 | 77.5 | 0.06 | 70.1 | 35.5 | 0.05 |
| Meal type × overweight | −18.3 | 85.1 | 0.83 | 45.2 | 37.1 | 0.23 |
| Model with body composition | ||||||
| Height (cm) | 0.3 | 7.8 | 0.97 | −3.9 | 3.4 | 0.26 |
| Fat-free mass (kg) | 5.6 | 7.3 | 0.45 | 4.4 | 3.3 | 0.18 |
| Percentage fat mass | 5.5 | 4.5 | 0.22 | 1.0 | 2.0 | 0.64 |
| Meal type × fat-free mass | 1.3 | 4.4 | 0.77 | 0.1 | 1.9 | 0.94 |
| Meal type × percentage fat mass | 1.0 | 3.6 | 0.77 | −2.6 | 1.5 | 0.10 |
For the correlation analyses: Sex = 0 for males and 1 for females, Race = 0 for whites and non-Hispanics and 1 for other, Pubertal status = 0 for pre- or early/midpuberty (boys: testes <15 mL; girls: Tanner stages 1–3) and 1 for late puberty (boys: testes ≥15 mL; girls: Tanner stages 4–5), and Overweight status = 0 for BMI <85th percentile and 1 for BMI ≥85th percentile.
We conducted 2 sets of follow-up analyses. First, because there were differences between conditions in subjective hunger ratings before the measurement of EAH, we examined the models predicting EAH intake when hunger was included as a predictor. After adjustment for all other covariates, postmeal hunger score was not significantly related to EAH intake (P > 0.30), and its inclusion did not alter the magnitude or significance of the BMI z score and overweight status effects. Second, an examination of analyses including meal order (ie, large-array meal first compared with standardized meal first) in the models predicting meal intake and EAH showed no significant main effects of meal order on meal intake (P = 0.98) or EAH (P = 0.10). An interaction effect of meal order by meal type on meal intake was observed (P = 0.05): the discrepancy between large-array meal intake and standardized meal intake was greater when the large-array meal was first (difference = 533 kcal) than when the standardized meal was first (difference = 372 kcal). There was no significant meal order by meal type interaction for EAH (P = 0.08). The inclusion of meal order and meal order by meal type did not alter the magnitude of any result, but the significance of overweight status on EAH intake was attenuated (P = 0.065).
DISCUSSION
In the current laboratory study of adolescent eating behavior, adolescents’ energy intakes were observed during and after 2 different meals designed to reduce hunger: a standardized meal and a very large food array expected to diminish hunger to an even greater extent. Energy intake at the standardized meal (861 kcal) was similar, on average, to that reported in prior studies that used standard meals and explored EAH (5); consumption from the larger array was, as expected, greater (1309 kcal). EAH was assessed as the postmeal intake of highly palatable snack foods. Adolescents showed EAH after both types of meals. The findings provide initial support for the use of a large food array paradigm to assess EAH and lend further credence to the validity of the EAH construct in youth.
Before measurement of EAH, youth were served a large array of lunch-type foods or a lunch meal standardized to provide approximately half of their daily estimated energy needs. Although each participant was given the identical instruction to "eat until you are no longer hungry" at each lunch meal, adolescents consumed 448 kcal more, on average, at the large lunch buffet array than at the standardized meal. Stated differently, average meal consumption at the large-array meal amounted to a 52% greater energy intake than at the standardized meal. This result is consistent with prior data showing that children (17, 18) and young adults (19) of all weight strata eat more when served greater portions sizes and greater variety. Adjustment for relevant covariates, adolescent BMI z score, and overweight status were significantly related to greater intake at both lunchtime meals and the EAH arrays. These findings add to the relatively small body of adolescent observational eating data showing that overweight and obese adolescents consume greater energy intakes than do their nonoverweight peers (9), which possibly contributes to a positive energy balance and excessive weight gain.
The difference in energy intake between the large-array and standardized meals was paralleled by discrepancies in subjective feelings of hunger and satiety. Adolescents reported minimal to no hunger after the large food array and less hunger and greater fullness than after eating from a standardized meal. Consequently, and consistent with our predictions, EAH observed after the large-array meal was somewhat less than EAH observed after the standardized meal. This pattern suggests that administration of a very large, free-access food array produces a more conservative measurement of EAH—one that ensures that intake largely occurs in the absence of hunger.
Notably, even with such a conservative procedure to estimate EAH, systematic individual differences were observed in levels of EAH measured after the large-array meal. As expected, overweight and obese youth ate more in the absence of hunger than nonoverweight youth. Similarly, BMI z score and fat mass were positively associated with EAH after the large array. The relations of EAH with BMI z score and overweight status were robust after age, sex, race, puberty, and amount of meal intake were accounted for. Furthermore, the adjusted associations of body weight with EAH did not differ significantly across conditions. Thus, despite mean-level differences in EAH, BMI z score and overweight were associated with EAH after both the large-array and standardized meals. Similarities in the 2 measurements of EAH and evidence for their convergent validity were also evident in the significant correlation between EAH, as assessed in each condition. The current results add to a growing body of data consistent with a role for EAH in conferring risk of a positive energy balance in youth (2, 3, 5, 6, 20–22). EAH appears to be moderately heritable (5) and distinguishes children at risk of adult overweight by virtue of parent overweight (23, 24). The present findings bolster the notion of EAH as an obesity "endophenotype," observable whether youth first eat to satiation from a standardized meal or more conservatively from a very large array of foods. The careful delineation of such endophenotypes may ultimately help develop a more sophisticated nosology for pediatric obesity.
The limitations of this study include the cross-sectional nature of the data collection and the correlational analyses that precluded examination of the directionality of the link between body weight and EAH. The degree to which EAH might contribute to excessive weight gain in youth remains to be established (6), and further longitudinal studies of EAH and growth are required. Additionally, because the large-array lunch offered a greater variety of foods than did the standardized meal, including snacks that may have shared similar sensory properties with some of the food items presented at the snack array, the possibility that sensory-specific satiety (25, 26) partly contributed to observed differences in EAH between conditions cannot be ruled out. However, the participants reported no differences in perceived pleasantness of the food in each condition. Furthermore, although a 30-min interval approximated the time between the meal and the measurement of EAH in at least one prior study (23), it is possible that the delay used in the current study might have increased the observed EAH. However, given that the delay was the same regardless of meal type, we believe that the findings are still relevant in terms of the effect of a standardized compared with that of a large-array meal on EAH. Although intake during test meal studies has been shown to reflect eating behavior outside of the laboratory (27, 28), another limitation is the possibility that such studies may not yield the same eating patterns that occur in the natural environment. Nevertheless, the benefits of laboratory studies include the carefully controlled testing environment, direct observation of eating behaviors, and precise measurements of energy intake (29). Such methods are better than questionnaire measures of intake, on which individuals frequently underestimate food consumption (30–32).
In conclusion, the current study presents preliminary evidence of a modified laboratory paradigm assessing EAH. Adolescents, on average, consumed ≈300 kcal of highly palatable snack foods in the absence of hunger—even after ad libitum free access consumption from a very large food array—data that highlight the susceptibility of excessive intake from the palatable food choices often found in our current environment. In contemporary culture, easy access to highly palatable, energy-dense, inexpensive foods is the norm (33). The current data suggest that overweight and obese adolescents may be especially susceptible to intake in the absence of hunger when stimulated by external food cues. Further research is needed to investigate EAH as a prospective risk factor for weight gain and to identify the genetic and environmental underpinnings of this disinhibited eating behavior.
Acknowledgments
We thank the volunteers who participated for their help in completing these studies.
The authors’ responsibilities were as follows—LBS, MT-K, SZY, and JAY: conception and design of the study; and LBS, MT-K, JMZ, AC, MK, KMC, LEW, SMB, MKC, AHA, SZY, and JAY: analysis and interpretation of data and drafting of the article. All authors contributed to the collection and assembly of data, provided critical revision of the article for content, and approved the final version of the manuscript. All authors reported that there were no disclosures or conflicts of interest relevant to this publication. JAY and MK are Commissioned Officers in the US Public Health Service, DHHS.
REFERENCES
- 1.Kral TV, Faith MS. Child eating patterns and weight regulation: a developmental behaviour genetics framework. Acta Paediatr Suppl 2007;96:29–34 [DOI] [PubMed] [Google Scholar]
- 2.Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr 2003;78:215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher JO, Birch L. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr 2002;76:226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000;8:1–27 [PubMed] [Google Scholar]
- 5.Fisher JO, Cai G, Jaramillo SJ, Cole SA, Comuzzie AG, Butte NF. Heritability of hyperphagic eating behavior and appetite-related hormones among Hispanic children. Obesity (Silver Spring) 2007;15:1484–95 [DOI] [PubMed] [Google Scholar]
- 6.Butte NF, Cai G, Cole SA, et al. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. Am J Clin Nutr 2007;85:1478–85 [DOI] [PubMed] [Google Scholar]
- 7.Jansen A, Theunissen N, Slechten K, et al. Overweight children overeat after exposure to food cues. Eat Behav 2003;4:197–209 [DOI] [PubMed] [Google Scholar]
- 8.Halford JC, Gillespie J, Brown V, Pontin EE, Dovey TM. Effect of television advertisements for foods on food consumption in children. Appetite 2004;42:221–5 [DOI] [PubMed] [Google Scholar]
- 9.Ebbeling CB, Sinclair KB, Pereira MA, Garcia-Lago E, Feldman HA, Ludwig DS. Compensation for energy intake from fast food among overweight and lean adolescents. JAMA 2004;291:2828–33 [DOI] [PubMed] [Google Scholar]
- 10.Tanner JM.Growth and maturation during adolescence. Nutr Rev 1981;39:43–55 [DOI] [PubMed] [Google Scholar]
- 11.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson JC, McDuffie JR, Bonat SH, et al. Estimation of body fatness by air displacement plethysmography in African American and white children. Pediatr Res 2001;50:467–73 [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine of the National Academies Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: IOM, 2005; [DOI] [PubMed] [Google Scholar]
- 15.Huang TTK, Howarth NC, Lin B-H, Roberts SB, McCrory MA. Energy intake and meal portions: associations with BMI percentile in U.S. children. Obes Res 2004;12:1875–85 [DOI] [PubMed] [Google Scholar]
- 16.Behrens JT.Principles and procedures of exploratory data analysis. Psychol Methods 1997;2:131–60 [Google Scholar]
- 17.Fisher JO, Arreola A, Birch LL, Rolls BJ. Portion size effects on daily energy intake in low-income Hispanic and African American children and their mothers. Am J Clin Nutr 2007;86:1709–16 [DOI] [PubMed] [Google Scholar]
- 18.Fisher JO, Liu Y, Birch LL, Rolls BJ. Effects of portion size and energy density on young children's intake at a meal. Am J Clin Nutr 2007;86:174–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levitsky DA, Youn T. The more food young adults are served, the more they overeat. J Nutr 2004;134:2546–9 [DOI] [PubMed] [Google Scholar]
- 20.Hill C, Llewellyn CH, Saxton J, et al. Adiposity and 'eating in the absence of hunger’ in children. Int J Obes (Lond) 2008;32:1499–505 [DOI] [PubMed] [Google Scholar]
- 21.Moens E, Braet C. Predictors of disinhibited eating in children with and without overweight. Behav Res Ther 2007;45:1357–68 [DOI] [PubMed] [Google Scholar]
- 22.Tanofsky-Kraff M, Ranzenhofer LM, Yanovski SZ, et al. Psychometric properties of a new questionnaire to assess eating in the absence of hunger in children and adolescents. Appetite 2008;51:148–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obesity (Silver Spring) 2006;14:131–8 [DOI] [PubMed] [Google Scholar]
- 24.Francis LA, Ventura AK, Marini M, Birch LL. Parent overweight predicts daughters’ increase in BMI and disinhibited overeating from 5 to 13 years. Obesity (Silver Spring) 2007;15:1544–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birch LL, Deysher M. Caloric compensation and sensory specific satiety: evidence for self regulation of food intake by young children. Appetite 1986;7:323–31 [DOI] [PubMed] [Google Scholar]
- 26.Rolls BJ.Sensory-specific satiety. Nutr Rev 1986;44:93–101 [DOI] [PubMed] [Google Scholar]
- 27.Hadigan CM, Kissileff HR, Walsh BT. Patterns of food selection during meals in women with bulimia. Am J Clin Nutr 1989;50:759–66 [DOI] [PubMed] [Google Scholar]
- 28.Walsh BT, Kissileff HR, Cassidy SM, Dantzic S. Eating behavior of women with bulimia. Arch Gen Psychiatry 1989;46:54–8 [DOI] [PubMed] [Google Scholar]
- 29.Tanofsky-Kraff M, Haynos AF, Kotler LA, Yanovski SZ, Yanovski JA. Laboratory-based studies of eating among children and adolescents. Curr Nutr Food Sci 2007;3:55–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Björntorp P.Abdominal fat distribution and the metabolic syndrome. J Cardiovasc Pharmacol 1992;20(suppl):26–8 [PubMed] [Google Scholar]
- 31.Fisher JO, Johnson RK, Lindquist C, Birch LL, Goran MI. Influence of body composition on the accuracy of reported energy intake in children. Obes Res 2000;8:597–603 [DOI] [PubMed] [Google Scholar]
- 32.Livingstone MB, Prentice AM, Coward WA, et al. Validation of estimates of energy intake by weighed dietary record and diet history in children and adolescents. Am J Clin Nutr 1992;56:29–35 [DOI] [PubMed] [Google Scholar]
- 33.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science 1998;280:1371–4 [DOI] [PubMed] [Google Scholar]