Abstract
Background: Vitamin D sufficiency may be determined by the serum concentration of 25-hydroxyvitamin D [25(OH)D] that results in maximal intestinal calcium absorption efficiency. However, some investigators questioned whether 25(OH)D concentrations above the concentrations associated with rickets or osteomalacia influence calcium absorption.
Objective: We determined whether calcium absorption efficiency is related to serum 25(OH)D or serum 1,25-dihydroxyvitamin D [1,25(OH)2D] concentrations.
Design: We measured calcium absorption efficiency in 492 black and white healthy women (age range: 20–80 y) by the single-isotope method with 45Ca. Serum 25(OH)D concentrations were measured by a radioreceptor assay. Other relevant measurements included concentrations of serum 1,25(OH)2D, serum parathyroid hormone, serum creatinine, and serum estradiol, calcium intake, and bone mineral density.
Results: There was no relation between serum 25(OH)D concentrations and calcium absorption efficiency. In development of a multivariate model, the 4 major determinants of calcium absorption efficiency were menopausal status, calcium intake, and serum estradiol and serum 1,25(OH)2D concentrations. There was an interaction between serum 25(OH)D and 1,25(OH)2D concentrations on calcium absorption efficiency. The relation between calcium absorption and 1,25(OH)2D was positive, and this relation was stronger for lower concentrations of 25(OH)D than for higher concentrations of 25(OH)D.
Conclusion: The relation of serum 25(OH)D to calcium absorption is not useful as an indicator of vitamin D sufficiency.
INTRODUCTION
See corresponding editorial on page 673.
Serum 25-hydroxyvitamin D [25(OH)D] is the accepted biomarker of vitamin D exposure. One approach to the determination of the sufficiency of vitamin D for bone health outcomes has been to seek the serum concentration of 25(OH)D that would prevent a rise in the parathyroid hormone (PTH) concentration (1, 2). Another approach has been to seek the concentration of serum 25(OH)D that maximizes intestinal calcium absorption (3, 4). These approaches are based on the inferred prevention of bone loss from secondary hyperparathyroidism in the first case and on maximizing intestinal absorption of calcium in the second case.
Malabsorption of calcium in rickets was reported >80 y ago and was confirmed in several recent studies at serum 25(OH)D concentrations below 10–12 nmol/L (5, 6). Optimal serum 25(OH)D concentrations to maintain bone health and prevent osteoporosis are believed to be higher than the low concentrations needed to prevent rickets and osteomalacia. There is active debate over what these concentrations should be, and expert opinion has suggested that they should be either 50–65 or >75 nmol/L (5, 7). The attainment of each of these 25(OH)D concentrations in the general population requires substantially different intakes of vitamin D.
There is controversy over the concentration of serum 25(OH)D that suppresses PTH (8, 9). There has also been recent controversy concerning whether the relation between serum 25(OH)D concentrations and maximal calcium absorption efficiency may be useful as an indicator of vitamin D sufficiency. Heaney (10) reviewed evidence that a concentration of serum 25(OH)D of 75–80 nmol/L reflects the inflection for maximal calcium absorption efficiency in response to increasing serum 25(OH)D concentrations. However, Need and Nordin (11) challenged the concept that calcium absorption is related to serum 25(OH)D at concentrations above those associated with osteomalacia.
Because the relation of calcium absorption to serum 25(OH)D concentrations has been used to propose optimal vitamin D intake, we reanalyzed the data from a previously reported study in which we measured concentrations of serum 25(OH)D, 1,25(OH)2D and PTH and calcium absorption efficiency in almost 500 healthy women (12). We wished to explore whether intestinal calcium absorption was related to either serum 25(OH)D or 1,25(OH)2D concentrations and whether there was an interaction between the concentrations of these vitamin D metabolites.
SUBJECTS AND METHODS
The data reported in the present study analyses are from a previously reported study of body composition and calcium metabolism in 503 healthy black and white women aged 20–80 y (12). A total of 492 participants had calcium absorption measured. The study was carried out between February 1991 and November 1994.
Participants
Participants were recruited through advertisements in the local media and a direct mail campaign. Exclusion characteristics consisted of any chronic illness, medication know to affect bone metabolism, use of oral contraceptives, hormonal replacement therapy, or hysterectomy. The study was approved by the Institutional Review Board of Winthrop University Hospital, and written informed consent was obtained from each participant. A 3-d diet history was completed and reviewed with the study dietitian. A body mass index (in kg/m2) of 18–33 was considered acceptable for inclusion in the study.
Calcium absorption
Calcium absorption efficiency was based on a single measurement of serum specific activity after administration of an oral calcium tracer by using the method of Heaney and Recker (13). Participants fasted overnight and drank 170 mL orange juice containing 5 μCi of 45Ca and 200 mg calcium as CaCl2. Five hours later, an aliquot of blood was drawn, and the serum calcium concentration and radioactivity were determined.
Laboratory studies
Serum PTH concentrations were measured with the Allegro intact PTH immunoassay (Nichols Institute, San Juan Capistrano, CA) (14). The intraassay CV was 5.2%, and the interassay CV was 9.0%. Serum 1,25-dihydroxyvitamin D [1,25(OH)2D] concentrations were measured by a radio-receptor binding assay with a calf thymus receptor (INCSTAR, Stillwater, MN). Serum was extracted by a single column that contained a C18OH-activated matrix before the receptor assay (15). The intraassay CV was 8.5%, and the interassay CV was 17.3%. Serum 25(OH)D concentrations were measured by a radioreceptor assay (INCSTAR). The intraassay CV was 4.1%, and the interassay CV was 7.0%. Estradiol (E2) was measured by radioimmunoassay (Diagnostic Products Corp, Los Angeles, CA). Serum calcium concentrations were measured by atomic absorption spectrophotometry (model 560; Perkin-Elmer Corp, Norwalk, CT) Serum creatinine concentrations were measured by using the method of Heinegard and Tiderstrom (16). Bone mineral density was measured with a Lunar Radiation densitometer (model DPX-L; Lunar Radiation, Madison, WI).
Statistical analyses
Correlation analyses were used to estimate associations among relevant variables. Multiple linear regression analyses were used to model the calcium absorption to predictive factors. A regression model that included all significant predictors was chosen as the main effect model. The interaction between 25(OH)D and 1,25(OH)2D was tested by adding an interaction term of 25(OH)D × 1,25(OH)2D to the main effect model (17). We standardized all variables (all variables had a standardized score of 0 ± 1 [mean ± SD]), which avoided multicollinearity on testing interactions and produced estimates of effects of predictors that could be compared directly and most readily interpreted as standardized mean differences (17).
The sample was based on 492 participants who had calcium absorption measured. Seven variables had missing data. Missing values among potential predictors were addressed by using multiple imputation (18) to avoid the possible estimate bias and to maximize our sample size and power for interaction testing (17). Multiple imputation was applied to the 7 variables that had incomplete data by using the other observed variables to impute the missing data. Missing data were replaced by random draws from a distribution of plausible values with SAS Proc MI (SAS Institute, Cary, NC) (19) to create 5 imputed data sets. Each imputed data set was analyzed separately. The findings from these analyses were summarized with SAS Proc MIANALYZE (SAS Institute) to combine the uncertainty in the estimated variables within and across the 5 imputed data sets (19).
On the basis of a total sample size of 492, we had 3, 4, 5, 9, 9, and 37 cases with missing values for concentrations of serum creatinine, PTH, 1,25(OH)2D, serum estradiol, and 25(OH)D and calcium intake, respectively. We only had n = 430 with complete data when we put all of these variables in the same model for selecting the final model. Analyses that compared the imputed dataset with the complete dataset showed no significant difference in regression coefficients. Moreover, the SEs for imputed data were smaller than the SEs for the completer data, which indicated that the imputation procedure was successful in recovering part of the missing information. We report findings from both complete data and imputed data.
RESULTS
Demographic and biochemical variables
Demographic characteristics and the distribution (mean ± SD) of biochemical variables are given in Table 1. There were 146 black and 126 white premenopausal participants and 84 black and 136 white postmenopausal participants. Serum calcium concentrations were in the reference range for all participants.
TABLE 1.
Demographic and biochemical variables1
| Variable | Total subjects (n = 492) | Black subjects (n = 230) | White subjects (n = 262) |
| Calcium absorption (efficiency percentage) | 30.02 ± 7.78 | 30.99 ± 8.07 | 29.16 ± 7.43 |
| Calcium intake (mg) | 662.07 ± 309.25 | 575.73 ± 300.12 | 744.13 ± 292.07 |
| Serum estradiol (pg/mL) | 50.17 ± 57.37 | 56.74 ± 55.69 | 45.28 ± 58.39 |
| Serum parathyroid hormone (pg/mL) | 37.28 ± 13.12 | 38.91 ± 13.50 | 35.87 ± 12.62 |
| Serum creatinine (mg/dL) | 0.94 ± 0.11 | 0.96 ± 0.11 | 0.93 ± 0.11 |
| Bone mineral density (g/cm2) | 1.15 ± 0.11 | 1.21 ± 0.09 | 1.09 ± 0.10 |
| BMI (kg/m2) | 24.96 ± 3.87 | 26.23 ± 4.11 | 23.85 ± 3.26 |
| 1,25(OH)2D (pmol/L) | 81.28 ± 19.95 | 84.31 ± 21.00 | 78.65 ± 18.62 |
| 25(OH)D (nmol/L) | 51.62 ± 33.67 | 32.87 ± 21.20 | 67.73 ± 34.11 |
All values are means ± SDs. 25(OH)D , 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Model selection for calcium absorption using relevant covariates for selecting the best predictive model
The data were used to build a predictive model to relate calcium absorption to other measured covariates (Tables 2 and 3). A multiple regression approach was used for this modeling, and a stepwise model selection was used to statistically select the best predictors. The most significant variables related to calcium absorption were menopausal status, calcium intake, and serum estradiol and serum 1,25(OH)2D concentrations. There was an interaction between 25(OH)D and 1,25(OH)2D (Table 4).
TABLE 2.
Final linear regression model with significant main effects1
| Imputed data (n = 492) |
Complete data (n = 430) |
|||
| Variable | Values | P | Values | P |
| For calcium absorption | ||||
| Menopause status2 | −0.471 ± 0.107 | <0.001 | −0.501 ± 0.111 | <0.001 |
| Calcium intake (mg) | −0.129 ± 0.046 | 0.005 | −0.146 ± 0.050 | 0.004 |
| Serum estradiol (pg/mL) | 0.130 ± 0.052 | 0.012 | 0.120 ± 0.056 | 0.035 |
| 1,25(OH)2D (pmol/L) | 0.147 ± 0.042 | <0.001 | 0.133 ± 0.044 | 0.003 |
| For PTH | ||||
| BMI | 0.240 ± 0.044 | <0.001 | 0.248 ± 0.048 | <0.001 |
| 25(OH)D (nmol/L) | −0.093 ± 0.044 | 0.035 | −0.090 ± 0.050 | 0.074 |
| 1,25(OH)2D (pmol/L) | 0.121 ± 0.043 | 0.005 | 0.123 ± 0.045 | 0.006 |
| For 1,25(OH)2D | ||||
| PTH (pg/mL) | 0.125 ± 0.044 | 0.005 | 0.135 ± 0.045 | 0.003 |
| Calcium absorption (%) | 0.134 ± 0.044 | 0.003 | 0.123 ± 0.046 | 0.008 |
| Serum creatinine (mg/dL) | −0.105 ± 0.044 | 0.018 | −0.103 ± 0.046 | 0.025 |
| Bone mineral density (g/cm2) | −0.184 ± 0.053 | 0.001 | −0.185 ± 0.054 | 0.001 |
| Race3 | −0.443 ± 0.106 | <0.001 | −0.473 ± 0.111 | <0.001 |
All values are estimates ± SEs. PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Premenopausal was coded 0, and postmenopausal was coded 1.
Black race was coded 0, and white race was coded 1.
TABLE 3.
Main effects for black, white, and premenopausal (PRE) and postmenopausal (POST) women1
| Black (n = 230) | White (n = 262) | PRE (n = 272) | POST (n = 220) | |
| For calcium absorption | ||||
| Calcium intake (mg) | −0.197** | −0.036 | −0.109 | −0.115 |
| Serum estradiol (pg/mL) | 0.292** | 0.234** | 0.128* | 0.122 |
| 1,25(OH)2D (pmol/L) | 0.180** | 0.090 | 0.121* | 0.174** |
| For PTH | ||||
| BMI | 0.060** | 0.070** | 0.071** | 0.061** |
| 25(OH)D (nmol/L) | −0.194 | −0.077 | −0.183** | 0.015 |
| 1,25(OH)2D (pmol/L) | 0.099 | 0.158** | 0.147* | 0.099 |
| For 1,25(OH)2D | ||||
| PTH (pg/mL) | 0.113 | 0.141** | 0.158** | 0.122 |
| Calcium absorption (%) | 0.182** | 0.083 | 0.110 | 0.273** |
| Serum creatinine (mg/dL) | −0.122* | −0.096 | −0.110 | −0.098 |
| Bone mineral density (g/cm2) | −0.205* | −0.162* | −0.036 | −0.018 |
PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D. *P < 0.05 for the regression coefficient; **P < 0.01 for the regression coefficient.
TABLE 4.
Interaction model of vitamin D metabolites and calcium absorption1
| Imputed data (n = 492) |
Complete data (n = 430) |
|||
| Variable | Values | P | Values | P |
| 1,25(OH)2D (pmol/L) | 0.147 ± 0.042 | <0.001 | 0.129 ± 0.047 | 0.007 |
| 25(OH)D (nmol/L) | 0.014 ± 0.044 | 0.743 | 0.017 ± 0.046 | 0.718 |
| 1,25(OH)2D × 25(OH)D | −0.074 ± 0.037 | 0.048 | −0.070 ± 0.041 | 0.089 |
All values are estimates ± SEs. 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D. The interaction model was based on the main-effects model of calcium absorption (Table 2).
Model selection for PTH
The following variables significantly related to serum PTH concentrations in our final model (Table 2): body mass index, 25(OH)D concentrations, and 1,25(OH)2D concentrations.
Model selection for 1,25(OH)2D
The following variables were significantly related to 1,25(OH)2D concentrations in our final model (Table 2): serum PTH concentrations, calcium absorption, serum creatinine concentrations, bone mineral density, and race.
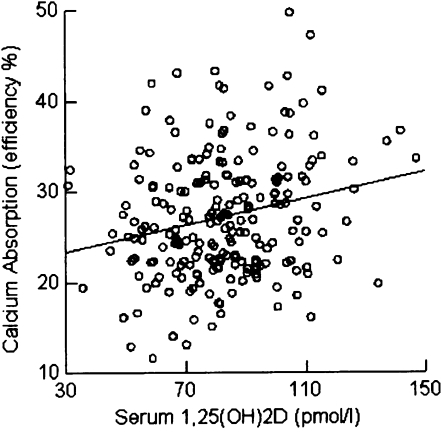
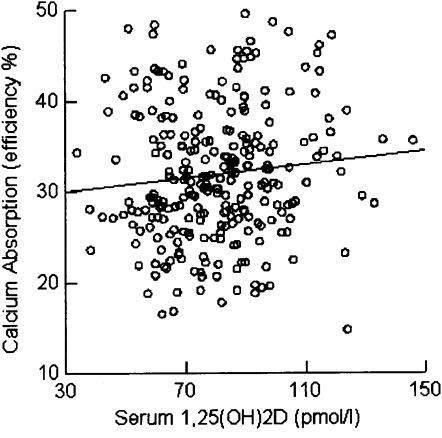
Calcium absorption as a function of serum 1,25(OH)2D concentrations had a lower intercept (z score) in postmenopausal women [−0.369 (P < 0.0001)] than in premenopausal women [0.295 (P < 0.0001)]. The slopes were 0.121 (P < 0.05) and 0.174 (P < 0.01) for premenopausal and postmenopausal women after adjusting for other significant predictors (Figures 1 and 2). The regression coefficients of age on PTH, 1,25(OH)2D, and calcium absorption were −0.044 (P = 0.335), 0.056 (P = 0.213), and −0.318 (P < 0.001), respectively. The regression coefficient of menopausal status on calcium absorption was −0.647 (P < 0.0001). For the final model of calcium absorption, we only used menopausal status to avoid collinearity between age and menopausal status because r between them was >0.8.
FIGURE 1.
Relation between calcium absorption [efficiency percentage (%)] and 1,25-dihydroxyvitamin D [1,25(OH)2D] (pmol/L) for premenopausal women.
FIGURE 2.
Relation between calcium absorption [efficiency percentage (%)] and 1,25-dihydroxyvitamin D [1,25(OH)2D] (pmol/L) for postmenopausal women.
Interaction of 25(OH)D and 1,25(OH)2D with calcium absorption
We observed that there was no statistically significant relation (r = −0.085; P = 0.06) between serum 25(OH)D and calcium absorption efficiency in the entire sample. The correlation between serum 25(OH)D and 1,25(OH)2D concentrations was −0.048 (P = 0.29), which was also not significant. The interaction (regression coefficient = −0.074; P = 0.048) between 25(OH)D and 1,25(OH)2D was significant for imputed data (regression coefficient = −0.074; P = 0.048) and marginally significant for complete data (regression coefficient = −0.070; P = 0.089) (Table 4). The relation between calcium absorption and 1,25(OH)2D was positive, and this relation was stronger for low concentrations of 25(OH)D than it was for high concentrations of 25(OH)D.
DISCUSSION
We observed no relation between serum 25(OH)D concentrations and calcium absorption efficiency in a large sample of healthy women over a wide age range. On the basis of this finding, calcium absorption efficiency was not useful as an indicator for vitamin D sufficiency.
A review article by Heaney (10) presented a widely cited graph that purported to show that calcium absorption rose progressively with an increasing serum 25(OH)D concentration until a plateau (threshold) was reached at 80 nmol/L. The conclusions in this article have been promulgated to suggest that serum 25(OH)D concentrations influence calcium absorption and optimal 25(OH)D concentrations are above this threshold. However, analyses of the individual studies that composed the graph and the construction of the graph made it clear that these conclusions were not justified. The lowest point on the graph was derived from a study that did not measure calcium absorption by either calcium loading or the use of radioisotopes. Indeed, the value was based on urinary calcium (5, 20). Two of the other points (50 and 86 nmol/L were taken from studies (21, 22) that used the calcemic response to a 500-mg calcium load rather than the measurement of fractional calcium absorption. The remaining 2 points in the graph were from studies that did measure fractional calcium absorption by using the single-isotope technique. The difference between these 2 points (74 and 122 nmol/L) was not significant (23). Thus, by using the 2 graph points that were not questionable, there is little evidence for a threshold. Therefore, this analysis of published studies may be considered hypothesis-generating.
The measurement of calcium absorption by using isotopes may be influenced by several methodologic issues. A low-carrier load is more sensitive to early transcellular transport and is favored by some investigators (24). Other investigators give the tracer with a small meal at breakfast. Double-tracer methods allow for correction of calcium body pools. In single-tracer studies, either stool is collected or mathematical corrections are made for determinations of calcium body pools. The dual-isotope method is considered the gold standard. The protocol used for this study (a high carrier and single isotope) is useful for large studies of groups (25).
A large study conducted by Need and Nordin (11) that used a low calcium carrier and single isotope also showed no relation between serum 25(OH)D concentrations and calcium absorption. The investigators proposed that vitamin D deficiency does not reduce serum 1,25(OH)2D concentrations or calcium absorption until the serum 25(OH)D concentration falls to 10–12 nmol/L, which results in substrate deficiency.
Several studies that used double isotopes also showed no relation between 25(OH)D concentrations and calcium absorption in children and adolescents (26, 27). Recently, Hansen et al (28) measured fractional calcium absorption in 18 postmenopausal women before and after repletion. The mean 25(OH)D concentration at baseline was 52.5 nmol/L, and all women had postergocalciferol concentrations >87.5 nmol/L (mean: 160 nmol/L). The increase in fractional calcium absorption was only 3%, which may not be clinically significant. Furthermore, a recent randomized controlled trial with an intervention of 1000 IU vitamin D2/d (and an achieved mean serum 25(OH)D >100 nmol/L) showed no effect on calcium absorption (29). Importantly, this was a long-term study that performed low- and high-carrier absorption studies.
The interactions we observed in our population were consistent with the known physiology of the vitamin D–endocrine system. We observed the previously reported inverse relation of 25(OH)D with PTH, and s positive relation of PTH with 1,25(OH)2D. When serum 25(OH)D concentrations were low, the relation of serum 1,25(OH)2D concentrations with calcium absorption was enhanced, but with higher 25(OH)D concentrations, the relation was flat. This interaction of serum 25(OH)D concentrations is consistent with the interpretation of Need et al (30) that serum 25(OH)D concentrations influence calcium absorption only when 25(OH)D concentrations are low enough to lead to substrate deficiency (10–12 nmol/L).
Menopausal status (and estradiol concentrations) was a major influence on calcium absorption in this study (r = −0.647; P < 0.0001), which obscured the effect of age (r = −0.318; P < 0.001). Estrogen deficiency (and, therefore, menopause) lowers intestinal calcium absorption and is corrected by the administration of exogenous estrogen (31–33). The suggestion that there is intestinal resistance to calcitriol in estrogen deficiency is supported by our finding that calcium absorption was a function of serum calcitriol in pre-and postmenopausal groups but had a lower intercept in the postmenopausal women (34).
A single isotope with a test breakfast was used in our study, in accordance with the protocol described by Heaney and Recker (13). This protocol is a modification of a single-isotope method that is conducted with a low-carrier load (24). The high carrier moves more slowly throughout the small intestine and may be conceived as measuring a succession of isotope blood curves. Heaney and Recker (13) chose the 5-h point to reduce the variation in completeness of absorption, but this was a compromise with the variation in the pool turnover rate. Despite these limitations, the single isotope method in our study showed a relation to calcium absorption for 1,25(OH)2D but not for 25(OH)D.
In conclusion, we did not find a relation between serum 25(OH)D concentrations and calcium absorption in a multiethnic cross-sectional study conducted in almost 500 healthy women over a wide age range. We conclude that the relation of serum 25(OH)D concentrations to calcium absorption cannot be used as a biomarker for vitamin D sufficiency.
Acknowledgments
The authors’ responsibilities were as follows—JFA: conceived and designed the research and was principally responsible for writing the manuscript; D-GC and HC: were responsible for the statistical analyses and contributed to the writing of the manuscript; and JKY: was responsible for the biochemical assays. None of the authors had a conflict of interest.
REFERENCES
- 1.Heaney RP. Serum 25-hydroxyvitamin D and parathyroid hormone exhibit threshold behavior. J Endocrinol Invest 2005;28:180–2 [DOI] [PubMed] [Google Scholar]
- 2.Vieth R, El-Hajj Fuleihan G. There is no lower threshold level for parathyroid hormone as 25-hydroxyvitamin D concentrations increase. J Endocrinol Invest 2005;28:183–6 [DOI] [PubMed] [Google Scholar]
- 3.Heaney RP. Vitamin D endocrine physiology. J Bone Miner Res 2007;22(suppl 2):V25–7 [DOI] [PubMed] [Google Scholar]
- 4.Heaney RP. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol 2005;97:13–9 [DOI] [PubMed] [Google Scholar]
- 5.Lips P. Vitamin D physiology. Prog Biophys Mol Biol 2006;92:4–8 [DOI] [PubMed] [Google Scholar]
- 6.Telfer S. Studies of calcium and phosphate metabolism. Q J Med 1926;20:7–25 [Google Scholar]
- 7.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28 [DOI] [PubMed] [Google Scholar]
- 8.Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr 2006;84:602–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durazo-Arvizu RA, Dawson-Hughes B, Sempos CT, et al. Three-phase model harmonizes estimates of the maximal suppression of parathyroid hormone by 25-hydroxyvitamin D in persons 65 years of age and older. J Nutr 2010;140:595–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol 2008;3:1535–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Need AG, Nordin BE. Misconceptions – vitamin D insufficiency causes malabsorption of calcium. Bone 2008;42:1021–4 [DOI] [PubMed] [Google Scholar]
- 12.Aloia JF, Vaswani A, Yeh JK, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int 1996;59:415–23 [DOI] [PubMed] [Google Scholar]
- 13.Heaney RP, Recker RR. Estimation of true calcium absorption. Ann Intern Med 1985;103:516–21 [DOI] [PubMed] [Google Scholar]
- 14.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80 [DOI] [PubMed] [Google Scholar]
- 15.Hollis BW. Assay of circulating 1,25-dihydroxyvitamin D involving a novel single-cartridge extraction and purification procedure. Clin Chem 1986;32:2060–3 [PubMed] [Google Scholar]
- 16.Heinegard D, Tiderstrom G. Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta 1973;43:305–10 [DOI] [PubMed] [Google Scholar]
- 17.Cohen M, Cohen P, West SB, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed.Mahwah, NJ: Lawrence Erlbaum Associates, 2003 [Google Scholar]
- 18.Little R, Rubin D. Statistical analysis with missing data. 2nd ed.New York, NY: Wiley, 2002 [Google Scholar]
- 19.SAS Institute Statistical analysis system, version 9.1. Cary, NC: SAS Institute, 2007 [Google Scholar]
- 20.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res 2003;18:343–51 [DOI] [PubMed] [Google Scholar]
- 21.Heaney RP, Dowell MS, Bierman J, Hale CA, Bendich A. Absorbability and cost effectiveness in calcium supplementation. J Am Coll Nutr 2001;20:239–46 [DOI] [PubMed] [Google Scholar]
- 22.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 2003;22:142–6 [DOI] [PubMed] [Google Scholar]
- 23.Barger-Lux MJ, Heaney RP. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab 2002;87:4952–6 [DOI] [PubMed] [Google Scholar]
- 24.Nordin BE, Morris HA, Wishart JM, et al. Modification and validation of a single-isotope radiocalcium absorption test. J Nucl Med 1998;39:108–13 [PubMed] [Google Scholar]
- 25.Abrams SA. Setting dietary reference intakes with the use of bioavailability data: calcium. Am J Clin Nutr 2010;91:1474S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver CM, McCabe LD, McCabe GP, et al. Vitamin D status and calcium metabolism in adolescent black and white girls on a range of controlled calcium intakes. J Clin Endocrinol Metab 2008;93:3907–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrams SA, Griffin IJ, Hawthorne KM, et al. Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab 2005;90:5576–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen KE, Jones AN, Lindstrom MJ, et al. Vitamin D insufficiency: disease or no disease? J Bone Miner Res 2008;23:1052–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. J Clin Endocrinol Metab 2008;93:743–9 [DOI] [PubMed] [Google Scholar]
- 30.Need AG, O'Loughlin PD, Morris HA, et al. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res 2008;23:1859–63 [DOI] [PubMed] [Google Scholar]
- 31.Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab 2002;87:4914–23 [DOI] [PubMed] [Google Scholar]
- 32.Nordin BE, Wishart JM, Clifton PM, et al. A longitudinal study of bone-related biochemical changes at the menopause. Clin Endocrinol (Oxf) 2004;61:123–30 [DOI] [PubMed] [Google Scholar]
- 33.O'Loughlin PD, Morris HA. Oestrogen deficiency impairs intestinal calcium absorption in the rat. J Physiol 1998;511:313–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liel Y, Shany S, Smirnoff P, Schwartz B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology 1999;140:280–5 [DOI] [PubMed] [Google Scholar]