Abstract
Background: Poor maternal folate status has been associated with an increased risk of preterm birth. However, major gaps remain in our understanding of how individual folate species relate to preterm birth.
Objective: Our objective was to assess the association between maternal folate status as measured by 5-methyltetrahydrofolate (5MeTHF), 5-formyltetrahydrofolate (5FoTHF), and folic acid concentrations, which are the 3 primary folate species in serum, and the risk of preterm birth and spontaneous preterm birth (sPTB).
Design: A cohort of 313 pregnant women who received care at resident antepartum clinics at Magee-Womens Hospital (Pittsburgh, PA) (2003–2007) was enrolled at <16 wk gestation. We analyzed nonfasting blood samples that were drawn from subjects at enrollment for the 3 folate species by using HPLC–tandem mass spectrometry.
Results: Serum 5MeTHF and 5FoTHF concentrations comprised 65% and 33% of total folate concentrations, respectively. In confounder-adjusted, multivariable, log-binomial regression models, 1-SD increases in serum total folate and serum 5MeTHF concentrations were associated with significant reductions in the risk of sPTB (P < 0.05). There was a significant interaction between serum 5MeTHF and 5FoTHF concentrations and risk of preterm birth (P = 0.01). When serum 5MeTHF concentrations were low, there was a positive linear relation between 5FoTHF and risk of preterm birth. When 5MeTHF concentrations were high, there was a strong negative relation between 5FoTHF and preterm birth.
Conclusions: Our results imply that the relative concentrations of folate species may be more critical than total folate in preventing preterm birth. An improved understanding of folate metabolism during pregnancy may lead to targeted intervention strategies that decrease the rate of preterm birth.
INTRODUCTION
The prevention of preterm birth is a major public health priority (1). Infants born preterm (ie, <37 wk of gestation) are more likely than full-term infants to die as neonates or infants and to suffer from serious short- and long-term morbidities, including cerebral palsy, mental retardation, language, learning, and behavior problems, and poor health and growth (2). In the United States, 1 in 8 infants is born preterm, and in 2005, preterm births cost the United States >$26 billion (2). Unfortunately, interventions aimed at preventing preterm birth have been disappointing (2).
Maternal folate status is a modifiable factor that may be linked with preterm birth. Studies of dietary, supplemental, or biomarker folate in relation to preterm birth have produced conflicting results (3–14) that were likely due to varying ranges and timing of folate assessment, assessment methods of gestational age, and population characteristics. It is particularly critical to reexamine the association between folate intake and preterm birth after the nationwide 1998 implementation of the program to fortify enriched cereal-grain products with folic acid (15), which substantially improved the folate status of US childbearing-aged women (16). From 1988–1994 to 2003–2004, serum folate concentrations of women aged 15–45 y doubled, and the prevalence of deficiency dropped from 21% to <1% (16).
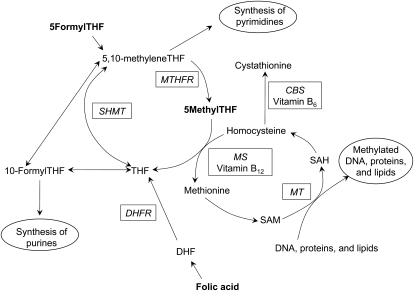
New investigations are needed to delineate the role of individual folate metabolites related to preterm birth. The folate metabolic pathway is critical for DNA synthesis and 1-carbon metabolism that leads to methylation (Figure 1). The 3 primary folate species in serum are 5-methyltetrahydrofolate (5MeTHF), 5-formyltetrahydrofolate (5FoTHF), and folic acid. The different folate species mediate the varied biological effects of folate. Studies that investigate specific folate species’ association with preterm birth can improve current nutrition intervention strategies and give insights into biological pathways that influence the risk of preterm birth. Our objective was to assess the independent association between first-trimester maternal folate status as measured by serum 5MeTHF, 5FoTHF, and folic acid concentrations and the risk of preterm birth.
FIGURE 1.
Folate metabolic pathway. THF, tetrahydrofolate; MTHFR, methylenetetrahydrofolate reductase; CBS, cystathionine β synthase; SHMT, serine hydroxymethyltransferase; MS, methionine synthase; SAH, S-adenosylhomocysteine; MT, methyltransferase; DHFR, dihydrofolate reductase; SAM, S-adenosylmethionine; DHF, dihydrofolate.
SUBJECTS AND METHODS
Data came from the Study of Nutrition and Pregnancy (SNAP), an ongoing prospective cohort study of pregnant women seeking care at resident antepartum clinics at Magee-Womens Hospital (Pittsburgh, PA). The antepartum clinics serve a predominantly publically insured, low-income population that is ≈55% black and 44% white. Eligible women had singleton pregnancies, were non-Hispanic whites or non-Hispanic blacks on the basis of self-reports, and had no known preexisting conditions, vaginal bleeding, fetal anomalies, or current or planned cervical cerclage. Enrollment took place at <16 wk of gestation (mean gestational age: 9.5 wk) after women provided informed, written consent. The study was approved by the University of Pittsburgh Institutional Review Board.
At enrollment, women completed an interviewer-administered questionnaire to collect data on sociodemographic characteristics, medical, reproductive, and sexual history, and maternal behaviors. Also at enrollment, women provided a nonfasting blood sample that was banked for later analysis. A total of 546 eligible women enrolled in the study from June 2003 to December 2007 (75% response rate). Of these women, we excluded 163 women because they had a spontaneous or therapeutic abortion (n = 67), transferred care (n = 50), delivered a stillbirth (n = 5), were lost to follow-up (n = 31), or lacked data on covariates in the final model (n = 11). An additional 69 women were excluded because they did not have an adequate volume of banked serum to perform the folate assays. There were no meaningful differences in maternal characteristics or in the prevalence of preterm birth between women included and excluded from the analyses (data not shown). A total of 313 women were included in the final analyses.
Gestational age was based on the best obstetrical estimate from a reliable, self-reported estimate of last menstrual periods (LMPs) or ultrasounds early in pregnancy. Preterm birth was defined as a delivery of a live infant that occurred at <37 completed weeks of gestation. Spontaneous preterm birth (sPTB) was defined as a preterm delivery that occurred after preterm labor with intact membranes or a preterm prelabor rupture of the fetal membranes.
Quantification of folate species
Nonfasting serum samples of subjects were allowed to coagulate at room temperature for 30 min. Subsequently, tubes were kept on ice and covered with foil to protect them from light. Within 2 h of the blood draw, tubes were centrifuged, and serum was aliquotted into amber vials. Vials were stored at −80°F until they were transported on dry ice in 2009 to the local laboratory of Raman Venkataramanan. No samples were thawed before assay for folate species.
Folate species were quantified with high-pressure liquid chromatography–tandem mass spectrometry (LC-MS/MS) on the basis of a published method (17). The standard curve was prepared by using a mixture of standard folates added to blank human plasma from which endogenous folates were removed by treating the mixture with activated charcoal. Each analyte included was prepared at 0, 1.8, 4.5, 9.0, 13.5, 22.5, and 54 ng/mL. A total of 25 μl internal standard (1 μg/mL of a mixture of 13C5-5MeTHF, 13C5-5FoTHF and 13C5-FA) was added to 400 μl standard samples and/or 400 μl patient plasma samples, and samples were diluted with 0.9 mL solid-phase extraction sample buffer (10 g ammonium formate/L, 1 g ascorbic acid /L; pH 3.2). After a 20-min equilibration at 4°C, the diluted plasma sample was passed through Bond Elut PH 1-cc (100 mg) extraction cartridges (Varian, Palo Alto, CA) under vacuum. These columns were preconditioned with 2 mL methanol and 1 mL solid-phase extraction sample buffer (10 g ammonium formate/L and 1 g ascorbic acid/L; pH 3.2). After washing with 1 mL wash buffer (0.5 g ammonium formate/L and 0.05 g ascorbic acid/L; pH 3.4) and 150 μl elution buffer (400 mL methanol/L, 100 mL acetonitrile/L, 10 mL acetic acid/L, and 1 g ascorbic acid/L), folates were eluted with 150 μL elution buffer, and 20 μL of solution was injected into the high-pressure liquid chromatograph piloted by MassLynx 4.1 software (Waters Inc, Milford, MA). Chromatography was conducted with a C8 4 × 2.0-mm guard column and a Luna 5-μm C8 (2) 150 × 3.0-mm analytic column (Phenomenex, Torrance, CA) that was maintained at 30°C by using isocratic elusion with a mobile phase that consisted of 400 mL methanol/L, 100 mL acetonitrile/L , and 10 mL acetic acid/L at flow rate of 0.25 mL/min.
Analyses were conducted with a Waters Quattro micro atmospheric pressure ionization mass spectrometer (Waters Inc) with positive electrospray ionization made using multiple reaction monitoring. The ratios of the peak areas of 5MeTHF, 5FoTHF, and folic acid to the corresponding stable isotope-labeled internal standard were linearly related to the concentrations of 5MeTHF, 5FoTHF, and folic acid (R2 = 0.9985, 0.9906, and 0.9909, respectively) in the concentration range of 1.8–54 ng/mL for all 3 analytes. The mean percentage deviations were <15% for each concentration of all calibration curves including the lower limit of quantification of 1.8 ng/mL. The intraday and interday CV was<10%. The method was also accurate with a bias of ≤15% acceptable limits at all concentrations tested.
Covariates
Women self-reported their race-ethnicity as non-Hispanic white or non-Hispanic black. Data on maternal age, smoking status in the 3 mo before pregnancy, smoking since becoming pregnant, maternal education, marital status, parity, household yearly income, employment status, use of marijuana and alcohol since becoming pregnant, and date of last live birth were ascertained from the baseline interviewer-administered questionnaire. The prepregnancy body mass index (BMI; in kg/m2) was based on maternal self-report of pregravid weight and measured height at enrollment. BMI categories were underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obese (≥30) (18). Because only 2 women were underweight, we grouped them with normal-weight women.
Statistical analyses
Maternal serum folate species were categorized into thirds on the basis of tertiles of their distributions. Pearson chi-square test was used to determine differences in maternal characteristics by total folate tertile and preterm-birth status. We tested for trends across folate tertiles by preterm-birth status. Spearman's rank correlation coefficient and linear regression were used to assess correlations between 5MeTHF and 5FoTHF concentrations. We tested for equality in the medians of concentrations of each folate species by preterm-birth status by using the Kruskal-Wallis one-factor analysis of variance. Multivariable log-binomial regression models were used to assess the independent association between folate species and the risk of preterm birth and sPTB. Log-binomial models were chosen because we wanted to estimate risk ratios (RRs), and preterm birth was not a rare disease (>10% prevalence). Potential confounders were race-ethnicity, smoking before and during pregnancy, education, marital status, parity, income, employment, pregravid BMI, use of alcohol and marijuana since becoming pregnant, and interpregnancy interval. Confounding was defined as a >10% change in the adjusted RR after exclusion of the covariate from the full model. Only race-ethnicity, education, smoking, and obesity in models of preterm birth and education and smoking in models of sPTB met our definition of confounding. We tested for a statistical interaction between serum 5MeTHF and 5FoTHF concentrations on the risk of preterm birth by using a likelihood ratio test (α = 0.10). To illustrate the results of this model, adjusted RRs (95% CIs) were calculated by using linear combinations of model β coefficients for pairs of 5MeTHF and 5FoTHF concentrations. Concentrations of 5MeTHF were chosen at the 10th, 50th, and 90th percentiles of the distribution, and concentrations of 5FoTHF were chosen at the 10th, 25th, 50th, 75th, and 90th percentiles of the distribution.
Regular multivitamin use in the periconceptional period was not measured in our study, but it is a potential confounder of the folate–preterm birth association. The regular use of periconceptional multivitamins is associated with a reduced risk of preterm birth (19, 20) and higher concentrations of serum folate (21, 22). In the absence of multivitamin use data, we conducted a sensitivity analysis for unmeasured confounding by regular periconceptional multivitamin use that was adapted from the work of Lash and Fink (23) as previously described (24). These methods allow investigators to use a known or expected range of effect estimates of the exposure-confounder and confounder-outcome relations to adjust their final model results for the unmeasured covariate. To quantify the degree of unmeasured confounding by multivitamin use, we parameterized the relative risk that was due to confounding by using a trapezoidal distribution. The limit of the relative risk that was due to confounding was calculated according to the methods of Flanders and Khoury (25).We compared the RRs (95% CIs) from the conventional logistic regression model of total folate and preterm birth risk with estimates obtained from the sensitivity-analysis iterations, which reflected systematic error and random error associated with missing data on regular multivitamin use (23). Stata Software, version 10 (StataCorp, College Station, TX) and SAS software, version 9.2 (SAS, Cary, NC) were used for analyses.
RESULTS
The majority of women in the cohort were 20–29 y old, multiparous, high-school educated, unmarried, unemployed, and had low-incomes (Table 1). Approximately one-half of the women were non-Hispanic blacks, and one-half of the women were smokers. Over 60% of women were overweight or obese. The median (interquartile range) gestational age at enrollment was 9.4 wk (7.5–12.1 wk). Mothers in the highest third of the folate distribution (tertile 3) were significantly more likely to be non-Hispanic white, nulliparous, nonsmokers, and married than were mothers in the other groups.
TABLE 1.
Maternal characteristics for the total population and by category of total folate concentration at <16 wk gestation
| Maternal serum total folate |
Outcome |
|||||
| Total population | Bottom tertile | Middle tertile | Upper tertile | Term birth | Preterm birth | |
| Maternal race [n (%)] | ||||||
| Non-Hispanic white | 138 (44) | 28 (27) | 43 (41) | 67 (64)1 | 125 (46) | 13 (33) |
| Non-Hispanic black | 175 (56) | 76 (73) | 62 (59) | 37 (36) | 148 (54) | 27 (67) |
| Maternal age [n (%)] | ||||||
| <20 y | 39 (12) | 10 (10) | 13 (12) | 16 (15) | 32 (12) | 7 (18) |
| 20–29 y | 227 (73) | 80 (77) | 74 (70) | 73 (70) | 203 (74) | 24 (60) |
| ≥30 y | 47 (15) | 14 (13) | 18 (17) | 15 (15) | 38 (14) | 9 (22) |
| Parity [n (%)]2 | ||||||
| 0 | 45 (14) | 10 (10) | 11 (10) | 24 (23)1 | 40 (15) | 5 (13) |
| 1 | 139 (45) | 38 (38) | 53 (51) | 48 (46) | 123 (45) | 16 (40) |
| ≥2 | 126 (41) | 53 (52) | 41 (39) | 32 (31) | 107 (40) | 19 (48) |
| Smoking status 3 mo before pregnancy [n (%)] | ||||||
| Nonsmoker | 138 (44) | 37 (36) | 44 (42) | 57 (55)3 | 117 (43) | 21 (53) |
| Smoker | 175 (56) | 67 (64) | 61 (58) | 47 (45) | 156 (57) | 19 (47) |
| Maternal education [n (%)] | ||||||
| Less than high school | 71 (23) | 28 (27) | 28 (27) | 15 (14)1 | 65 (24) | 6 (15) |
| High school or equivalent | 211 (67) | 71 (68) | 70 (67) | 70 (67) | 181 (66) | 30 (75) |
| Greater than high school | 31 (10) | 5 (5) | 7 (6) | 19 (18) | 27 (10) | 4 (10) |
| Marital status [n (%)] | ||||||
| Unmarried | 265 (85) | 92 (88) | 93 (88) | 80 (77)3 | 231 (85) | 34 (85) |
| Married | 48 (15) | 12 (12) | 12 (12) | 24 (23) | 42 (15) | 6 (15) |
| Employment status [n (%)] | ||||||
| Unemployed | 156 (50) | 54 (52) | 55 (52) | 47 (45) | 135 (49) | 135 (49) |
| Employed part time | 71 (23) | 24 (23) | 22 (21) | 25 (24) | 65 (24) | 65 (24) |
| Employed full time | 86 (27) | 26 (25) | 28 (27) | 32 (31) | 73 (27) | 73 (27) |
| Family's yearly income [n (%)]4 | ||||||
| <$10,000 | 129 (42) | 49 (48) | 45 (44) | 35 (34) | 114 (42) | 15 (38) |
| $10,000 to <$25,000 | 112 (36) | 38 (37) | 38 (37) | 36 (35) | 96 (36) | 16 (41) |
| ≥$25,000 | 66 (22) | 15 (14) | 20 (19) | 31 (31) | 58 (22) | 8 (21) |
| Prepregnancy BMI [n (%)] | ||||||
| <25.0 kg/m2 | 121 (38) | 42 (40) | 36 (34) | 43 (41) | 104 (38) | 17 (42) |
| 25.0–29.9 kg/m2 | 83 (27) | 20 (20) | 31 (30) | 32 (31) | 72 (26) | 11 (28) |
| ≥30.0 kg/m2 | 109 (35) | 42 (40) | 38 (36) | 29 (28) | 97 (36) | 12 (30) |
P < 0.01 (Pearson's chi-square test).
n = 3 with missing data on parity.
P < 0.05 (Pearson's chi-square test).
n = 6 with missing data on income.
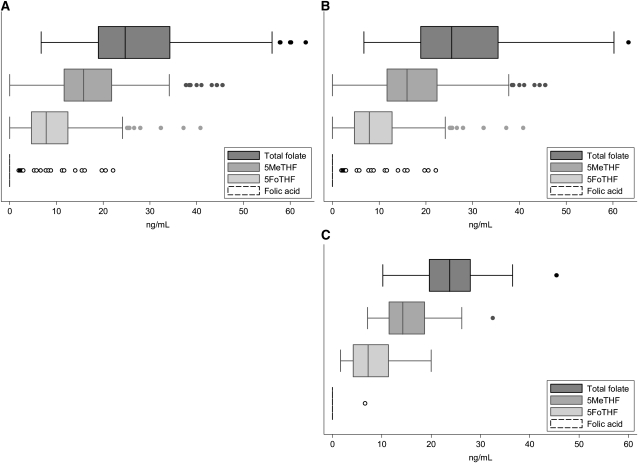
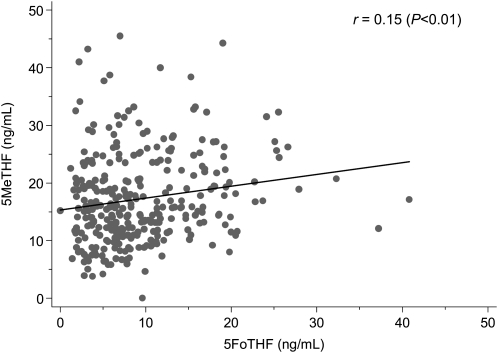
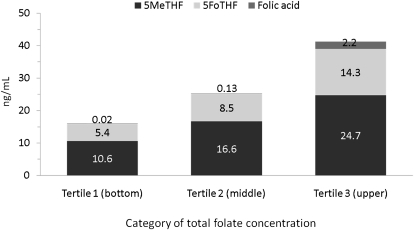
None of the women were folate deficient (serum total folate concentration <3 ng/mL) (26). The medians (interquartile ranges) for serum total folate, 5MeTHF, and 5FoTHF concentrations were 24.7 (19.0–34.3), 15.8 (11.6–21.9), and 7.8 (4.7–12.5) ng/mL, respectively (Figure 2A). Serum 5MeTHF and 5FoTHF concentrations made up 65% (interquartile range: 53–77%) and 33% (interquartile range: 21–45%) of total folate concentrations, respectively. There was a weak, but significant linear positive association between serum 5MeTHF and 5FoTHF concentrations (r = 0.15, P < 0.01; Figure 3).Only 8% of women (n = 21) had detectable serum folic acid concentrations, and among these women, serum folic acid contributed from 3.5 to 53% of their total folate concentrations. The distributions of folate species by total folate tertile are shown in Figure 4.
FIGURE 2.
Distributions of folate species in maternal serum at <16 wk gestation in the total cohort (A; n = 313) and in term (B; n = 273) and preterm (C; n = 40) births. 5MeTHF, 5-methyltetrahydrofolate; 5FoTHF, 5-formyltetrahydrofolate. There were no significant differences between median concentrations of folate species by Kruskal-Wallis one-factor ANOVA.
FIGURE 3.
Univariate association between maternal serum 5-methyltetrahydrofolate (5MeTHF) and 5-formyltetrahydrofolate (5FoTHF) concentrations at <16 wk gestation on the basis of Spearman's rank correlation coefficient and linear regression (n = 313).
FIGURE 4.
Mean concentrations of folate species by category of total folate concentration (n = 313). 5MeTHF, 5-methyltetrahydrofolate; 5FoTHF, 5-formyltetrahydrofolate.
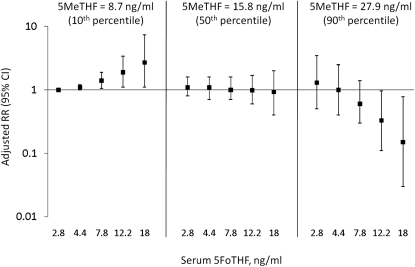
There were 40 cases of preterm birth (12.8%), including 27 cases of sPTB (9.0%), in the cohort. There were no significant differences in unadjusted median folate concentrations by preterm birth status (Figure 2, B and C). However, after adjustment for race-ethnicity, education, smoking, and obesity, women in the highest tertile of total folate concentrations had a 60% reduction in the risk of preterm birth [RR (95% CI): 0.4 (0.1, 0.9); Table 2]. The results were less precise and included the null value after accounting for unmeasured confounding by regular periconceptional multivitamin use [point estimate (95% sensitivity analysis interval): 0.4 (0.1, 1.0)]. The association between the highest 5MeTHF concentration tertile and preterm birth was of borderline statistical significance (P = 0.08). Importantly, we observed a significant interaction between serum 5MeTHF and 5FoTHF concentrations on the risk of preterm birth (P = 0.01; Figure 5). When serum 5MeTHF concentrations were low (8.7 ng/mL; 10th percentile), there was a positive linear relation between 5FoTHF and risk of preterm birth. Indeed, the RRs (95% CIs) for 5FoTHF concentrations of 7.8, 12.2, and 18 ng/mL were 1.4 (1.1, 1.9), 1.9 (1.1, 3.4), and 2.7 (1.1, 7.4), respectively, compared with the referent of 2.8 ng/mL, in women with low 5MeTHF concentrations. When serum 5MeTHF concentration was at the median of the distribution, no relation was observed with preterm birth regardless of the serum 5FoTHF concentration. In contrast, when 5MeTHF concentrations were high (27.9 ng/mL; 90th percentile), there was a strong negative relation between 5FoTHF and preterm birth. Concentrations of 5FoTHF of 12.2 and 18 ng/mL combined with concentrations of 5MeTHF of 27.9 ng/mL were protective against preterm birth [RR (95% CI): 0.33 (0.11, 0.97) and 0.15 (0.03, 0.78), respectively].
TABLE 2.
Association between tertiles of folate species at <16 wk and risk of preterm birth (PTB) and spontaneous preterm birth (sPTB)1
| Term | PTB | Adjusted RR (95% CI)2 | Term | sPTB | Adjusted RR (95% CI)3 | |
| n (%) | n (%) | n (%) | n (%) | |||
| Total folate | ||||||
| T1 (16.8 ng/mL)4 | 89 (86) | 15 (14) | 1.0 (ref) | 89 (88) | 12 (12) | 1.0 (ref) |
| T2 (24.7 ng/mL)4 | 86 (82) | 19 (18) | 1.3 (0.7, 2.4) | 86 (88) | 12 (12) | 1.0 (0.5, 2.2) |
| T3 (38.9 ng/mL)4 | 98 (94) | 6 (6) | 0.4 (0.1, 0.9) | 98 (97) | 3 (3) | 0.2 (0.1, 0.8) |
| 1-SD (12-ng/mL) increase | 0.7 (0.5, 1.0) | 0.6 (0.4, 0.9) | ||||
| 5MeTHF | ||||||
| T1 (10.2 ng/mL)4 | 89 (84) | 17 (16) | 1.0 (ref) | 89 (86) | 14 (14)5 | 1.0 (ref) |
| T2 (15.8 ng/mL)4 | 90 (86) | 15 (14) | 0.9 (0.5, 1.7) | 90 (91) | 9 (9) | 0.7 (0.3, 1.5) |
| T3 (25.1 ng/mL)4 | 94 (92) | 8 (8) | 0.5 (0.2, 1.1) | 94 (96) | 4 (4) | 0.3 (0.1, 0.8) |
| 1-SD (7.9 ng/mL) increase | 0.8 (0.5, 1.1) | 0.6 (0.4, 0.9) | ||||
| 5FoTHF | ||||||
| T1 (3.7 ng/mL)4 | 90 (87) | 13 (13) | 1.0 (ref) | 90 (94) | 6 (6) | 1.0 (ref) |
| T2 (7.8 ng/mL)4 | 91 (88) | 13 (12) | 1.1 (0.5, 2.2) | 91 (88) | 13 (12) | 2.0 (0.8, 5.2) |
| T3 (15.5 ng/mL)4 | 92 (87) | 14 (13) | 1.2 (0.6, 2.4) | 92 (92) | 8 (8) | 1.3 (0.5, 3.6) |
| 1-SD (12 ng/mL) increase | 0.9 (0.7, 1.2) | 0.9 (0.7, 1.14) |
T1, bottom tertile; T2, middle tertile; T3, upper tertile; RR, risk ratio; ref, reference; 5MeTHF, 5-methyltetrahydrofolate; 5FoTHF, 5-formyltetrahydrofolate.
Adjusted for race-ethnicity, education, smoking, and obesity.
Adjusted for education and smoking.
Values in parentheses are median values for the tertile.
P < 0.05 (test of trend).
FIGURE 5.
Interaction between maternal serum 5-methyltetrahydrofolate (5MeTHF) and 5-formyltetrahydrofolate (5FoTHF) on the risk of preterm birth (n = 313; P = 0.01). Serum 5MeTHF concentrations were chosen at the 10th, 50th, and 90th percentiles of the distribution, and 5FoTHF concentrations were chosen at the 10th, 25th, 50th, 75th, and 90th percentiles of the distribution. Risk ratios (RRs) were generated on the basis of linear combinations of the log-binomial regression-model coefficients by using the concentrations of 5FoTHF and 5MeTHF provided. All RRs were adjusted for race-ethnicity, smoking before pregnancy, education, and obesity.
When we studied sPTB as the outcome, serum total folate concentrations in the highest tertile were associated with an 80% reduction in risk of sPTB [RR (95% CI): 0.2 (0.1, 0.8)] compared with the bottom tertile after confounder adjustment (Table 2). In fact, when total folate was entered into the model as a continuous variable, there was a linear relation between total folate concentrations and the risk of sPTB. A 1-SD increase in serum total folate concentrations was associated with a 40% reduction in sPTB. The relations between serum total folate concentrations and sPTB were driven by serum 5MeTHF concentrations, as evidenced by the similar sPTB adjusted RR. We did not observe an interaction between 5MeTHF and 5FoTHF concentrations and the risk of sPTB.
DISCUSSION
Despite the absence of clinical folate deficiency in our cohort, we observed negative linear associations between total folate concentrations and the risk of sPTB as well as between 5MeTHF concentrations and the risk of sPTB. We also observed an interaction between 5MeTHF and 5FoTHF concentration and the risk of preterm birth, whereby low concentrations of 5MeTHF and high concentrations of 5FoTHF increased the risk, whereas high concentrations of 5MeTHF and 5FoTHF reduced the risk. These relations were shown in a cohort recruited after mandated folic acid fortification of grain products in the United States.
To our knowledge, this is the first study of individual folate species in relation to preterm birth occurrence. Our results on total serum folate concentrations are consistent with those shown in other pregnancy cohorts. For instance, in 2468 North Carolina women, Siega-Riz et al (3) observed that serum total folate concentrations <16.3 ng/mL (which corresponds to our lower tertile of total folate), red cell total folate concentrations ≤627 ng/mL, and intake of ≤500 μg dietary folate/d at 24–28 wk was associated with 50–90% increases in preterm birth and sPTB. In a low-income group of 832 New Jersey mothers, Scholl et al (4) showed a negative linear relation between serum total folate concentrations at 28 wk and preterm birth and an elevated risk of preterm birth with midpregnancy folate intake of ≤400 μg folate/d. These results are supported by 2 randomized trials of folic acid plus iron compared with iron alone that were started in late pregnancy, which showed an increased mean gestational age with folic acid and iron treatment (5, 7).
In one of the few studies published that used a cohort post–folic acid fortification, 70% and 50% reductions in the risk of sPTB at 20 to <28 and 28 to <32 wk, respectively, were shown in women who reported using preconceptional multivitamins containing folic acid for ≥1 y (27). Results were similar in 2 other studies of preconceptional (28) and periconceptional (29) folic acid–containing multivitamin use. However, because these researchers combined individuals who used folic acid alone or folic acid in a multivitamin, it is impossible to determine whether the risk reduction was a result of folate or other micronutrients taken with folate.
In contrast, no relation between folate intake and preterm birth was shown in several observational investigations of folate biomarkers (8–10) and randomized trials of folic acid supplementation (11–14). However, it is notable that these studies relied solely on the mother's report of the first day of her LMP to determine gestational age at delivery. LMP-derived gestational age is error prone because mothers may not perfectly recall their LMP and may misidentify a LMP that was due to postconception bleeding, delayed ovulation, or intervening early miscarriage. A Hungarian randomized trial of folic acid–containing multivitamins compared with trace elements only taken before conception to 12 wk gestation reported no effect on preterm birth (30), but the supplementation may have ended too early to have seen effects on the length of gestation, and folic acid was not studied alone. Despite these negative studies, the preponderance of evidence suggests that improved folate status is associated with a reduced risk of preterm birth.
Although a growing body of literature implicates an effect of folate in preterm birth, little is known about the underlying mechanisms. Folate is central to a variety of complex biological processes including purine and thymidine synthesis, DNA methylation, and metabolism of homocysteine. The different folate species are involved preferentially in these distinct biological pathways. For example, 5MeTHF, by using vitamin B-12 as a cofactor, donates a methyl group to convert homocysteine to methionine. Methionine is necessary for protein biosynthesis and the generation of S-adenosyl methionine (SAM). SAM is critical in DNA methylation. In contrast, 5FoTHF is central to many biological pathways including purine and pyrimidine synthesis. Our data of the interaction between 5MeTHF and 5FoTHF suggest that certain biological pathways may be more critical for determining preterm birth risk.
Our finding of the interaction between 5MeTHF and 5FoTHF concentrations on preterm birth also suggests that the balance of these species, rather than the total folate concentration, has important biological consequences. This observation highlights the need to understand the determinants of the relative concentrations of folate species. At least 2 lines of future investigation are warranted. First, genetic polymorphisms in enzymes involved in the folate pathway may play a key role in determining the relative concentrations of the different folate species and, thus, the risk of preterm birth. Engel et al (31) showed that a gene-sequence variation in the SHMT1 gene, which is associated with less serine hydroxymethyltransferase 1 transcriptional activity, interacted with a low dietary intake of folate to increase odds of a sPTB. Our findings provide some biologic corroboration to these results. A second important area of investigation is the effect of different sources of folate on the proportion of folate species. It is possible that folate derived from supplements compared with from diets could contribute differentially to the relative proportion of folate species. Unfortunately, our collection of dietary and supplement use data in this ongoing cohort study began after 80% of women in this study were enrolled, so we do not have adequate data to understand the determinants of folate species in our population.
The folate status of our population was similar to that of the National Health and Nutrition Examination Survey 1999–2004 that was based of women of all ages (16) after application of the equations to convert the Bio-Rad radioassay results to LC-MS/MS (32). Also, compared with others (17), we observed smaller contributions of 5MeTHF and folic acid and larger contributions of 5FoTHF to total folate concentrations. Folic acid was observed in serum after recent consumption of >300 μg folic acid from supplements or fortified food (33). Data on oral intake of folic acid from our population is needed to understand these low concentrations. Although some investigators have suggested that 5FoTHF in serum actually may be 5MeTHF that has degraded as a result of improper handling of samples (34), our careful sample management makes this explanation unlikely. There may be an alteration in folate metabolism during pregnancy to cause this increase in 5FoTHF concentrations, but we are not aware of studies that have addressed this issue (33).
Our study was limited by a lack of data on folate intake and measurements of other key metabolites in the folate pathway, including homocysteine, vitamin B-12, methionine, SAM, and S-adenosylhomocysteine. Serum folate concentrations do not represent long-term folate status and may be affected by recent folate intake, given that our samples were nonfasting. As with all observational studies, our study can only suggest associations, not causation. We attempted to account for factors that are associated with folate status and preterm birth, including regular periconceptional multivitamin use (an unmeasured confounder). Nevertheless, we cannot exclude the possibility that confounding by additional unmeasured factors, such as physical activity, family support, psychosocial stress, household conditions, and genetic factors, may have biased our results, and confounders we did collect may have been measured with error. Our sample size limited our ability to study subtypes of preterm birth (eg, early compared with late preterm birth), which may provide more evidence into mechanisms by which folate may protect against early delivery. Nonetheless, our study's prospective design, rigorously defined preterm-birth phenotype, and quantification of 3 folate metabolites with the gold-standard method of LC-MS/MS are major strengths.
In conclusion, the prevalence of preterm birth has risen steadily over the past 20 y (35), with no decline after the nationwide folic acid fortification of grain products. If others confirm our results, the relative concentrations of folate species may prove more critical than total folate concentrations in preventing preterm birth. Furthermore, these data may be important in the design of clinical trials aimed at determining the optimal composition of folate supplements and food fortification. An improved understanding of folate metabolism during pregnancy may lead to targeted intervention strategies that decrease the rate of preterm birth.
Acknowledgments
We thank Christine Pfeiffer for helpful discussions about the results of this study and Timothy Lash and Matthew Fox for providing the program used to generate the sensitivity-analysis results.
The authors’ responsibilities were as follows—LMB and HNS: designed the research; RV and HNS: provided the data; LMB and J-YC: analyzed data; JLM: conducted the literature review; LMB, KPH, and HNS: wrote the manuscript; and J-YC, VR, RWE, and JLM: provided significant advice and critically edited the manuscript. All authors read and approved the final manuscript. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1. US Department of Health and Human Services Healthy people 2010, 2nd ed. With understanding and improving health and objectives for improving health. 2 vols. Washington, DC: US Government Printing Office, 2000 [Google Scholar]
- 2.Institute of Medicine Preterm birth: causes, consequences, and prevention. Washington, DC: National Academy of Sciences, 2006 [Google Scholar]
- 3.Siega-Riz AM, Savitz DA, Zeisel SH, Thorp JM, Herring A. Second trimester folate status and preterm birth. Am J Obstet Gynecol 2004;191:1851–7 [DOI] [PubMed] [Google Scholar]
- 4.Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Dietary and serum folate: their influence on the outcome of pregnancy. Am J Clin Nutr 1996;63:520–5 [DOI] [PubMed] [Google Scholar]
- 5.Tchernia G, Blot I, Rey A, Kaltwasser JP, Zittoun J, Papiernik E. Maternal folate status, birthweight and gestational age. Dev Pharmacol Ther 1982;4(suppl):58–65 [PubMed] [Google Scholar]
- 6.Whiteside MG, Ungar B, Cowling DC. Iron, folic acid and vitamin B12 levels in normal pregnancy, and their influence on birth-weight and the duration of pregnancy. Med J Aust 1968;1:338–42 [DOI] [PubMed] [Google Scholar]
- 7.Blot I, Papiernik E, Kaltwasser JP, Werner E, Tchernia G. Influence of routine administration of folic acid and iron during pregnancy. Gynecol Obstet Invest 1981;12:294–304 [DOI] [PubMed] [Google Scholar]
- 8.Daniel WA, Jr, Mounger JR, Perkins JC. Obstetric and fetal complications in folate-deficient adolescent girls. Am J Obstet Gynecol 1971;111:233–8 [DOI] [PubMed] [Google Scholar]
- 9.Martin JD, Davis RE, Stenhouse N. Serum folate and vitamin B12 levels in pregnancy with particular reference to uterine bleeding and bacteriuria. J Obstet Gynaecol Br Commonw 1967;74:697–701 [DOI] [PubMed] [Google Scholar]
- 10.Hibbard BM. Folates and the fetus. S Afr Med J 1975;49:1223–6 [PubMed] [Google Scholar]
- 11.Giles PF, Harcourt AG, Whiteside MG. The effect of prescribing folic acid during pregnancy on birth-weight and duration of pregnancy. A double-blind trial. Med J Aust 1971;2:17–21 [PubMed] [Google Scholar]
- 12.Christian P, Khatry SK, Katz J, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher J, Gurr A, Fellingham FR, Prankerd TA, Brant HA, Menzies DN. The value of folic acid supplements in pregnancy. J Obstet Gynaecol Br Commonw 1971;78:781–5 [DOI] [PubMed] [Google Scholar]
- 14.Fleming AF, Martin JD, Hahnel R, Westlake AJ. Effects of iron and folic acid antenatal supplements on maternal haematology and fetal wellbeing. Med J Aust 1974;2:429–36 [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid, final rule. Fed Regist 1996;61:8781–97 [Google Scholar]
- 16.Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988-2004. Am J Clin Nutr 2007;86:718–27 [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer CM, Fazili Z, McCoy L, Zhang M, Gunter EW. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin Chem 2004;50:423–32 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995;854:1–452 [PubMed] [Google Scholar]
- 19.Vahratian A, Siega-Riz AM, Savitz DA, Thorp JM., Jr Multivitamin use and the risk of preterm birth. Am J Epidemiol 2004;160:886–92 [DOI] [PubMed] [Google Scholar]
- 20.Catov JM, Bodnar LM, Ness RB, Markovic N, Roberts JM. Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. Am J Epidemiol 2007;166:296–303 [DOI] [PubMed] [Google Scholar]
- 21.Baker PN, Wheeler SJ, Sanders TA, et al. A prospective study of micronutrient status in adolescent pregnancy. Am J Clin Nutr 2009;89:1114–24 [DOI] [PubMed] [Google Scholar]
- 22.Scholl TO, Hediger ML, Bendich A, Schall JI, Smith WK, Krueger PM. Use of multivitamin/mineral prenatal supplements: influence on the outcome of pregnancy. Am J Epidemiol 1997;146:134–41 [DOI] [PubMed] [Google Scholar]
- 23.Lash TL, Fink AK. Semi-automated sensitivity analysis to assess systematic errors in observational data. Epidemiology 2003;14:451–8 [DOI] [PubMed] [Google Scholar]
- 24.Bodnar LM, Tang G, Ness RB, Harger G, Roberts JM. Periconceptional multivitamin use reduces the risk of preeclampsia. Am J Epidemiol 2006;164:470–7 [DOI] [PubMed] [Google Scholar]
- 25.Flanders WD, Khoury MJ. Indirect assessment of confounding: graphic description and limits on effect of adjusting for covariates. Epidemiology 1990;1:239–46 [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B-6, folate, vitamin B-12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998 [PubMed] [Google Scholar]
- 27.Bukowski R, Malone FD, Porter FT, et al. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med 2009;6:e1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolschau J, Kristoffersen K, Ulrich M, Grinsted P, Schaumburg E, Foged N. The influence of folic acid supplement on the outcome of pregnancies in the county of Funen in Denmark. Part I. Eur J Obstet Gynecol Reprod Biol 1999;87:105–10, discussion 103–4 [PubMed] [Google Scholar]
- 29.Shaw GM, Liberman RF, Todoroff K, Wasserman CR. Low birth weight, preterm delivery, and periconceptional vitamin use. J Pediatr 1997;130:1013–4 [DOI] [PubMed] [Google Scholar]
- 30.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5 [DOI] [PubMed] [Google Scholar]
- 31.Engel SM, Olshan AF, Siega-Riz AM, Savitz DA, Chanock SJ. Polymorphisms in folate metabolizing genes and risk for spontaneous preterm and small-for-gestational age birth. Am J Obstet Gynecol 2006;195:1231:e1–11 [DOI] [PubMed] [Google Scholar]
- 32.Fazili Z, Pfeiffer CM, Zhang M. Comparison of serum folate species analyzed by LC-MS/MS with total folate measured by microbiologic assay and Bio-Rad radioassay. Clin Chem 2007;53:781–4 [DOI] [PubMed] [Google Scholar]
- 33.Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr 1997;65:1790–5 [DOI] [PubMed] [Google Scholar]
- 34.Hannisdal R, Ueland PM, Eussen SJ, Svardal A, Hustad S. Analytical recovery of folate degradation products formed in human serum and plasma at room temperature. J Nutr 2009;139:1415–8 [DOI] [PubMed] [Google Scholar]
- 35.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2006. Natl Vital Stat Rep 2009;57:1–104 [PubMed] [Google Scholar]