Abstract
Background: Supplementation in lactating HIV-1–infected women with preformed vitamin A and β-carotene (VA/BC) increases the risk of mother-to-child transmission of HIV through breastfeeding. Identifying a biological mechanism to explain this unexpected finding would lend support to a causal effect.
Objective: The aim of the study was to evaluate the effect of VA/BC or multivitamin (B complex, vitamin C, and vitamin E) supplementation of HIV-infected women on HIV shedding in breast milk during the first 2 y postpartum.
Design: We quantified viral (cell-free) and proviral (cell-associated) HIV loads in breast-milk samples collected ≤15 d after delivery and every 3 mo thereafter from 594 Tanzanian HIV-1–infected women who participated in a randomized trial. Women received 1 of the following 4 daily oral regimens in a 2 × 2 factorial fashion during pregnancy and throughout the first 2 y postpartum: multivitamin, VA/BC, multivitamin including VA/BC, or placebo.
Results: The proportion of breast-milk samples with detectable viral load was significantly higher in women who received VA/BC (51.3%) than in women who were not assigned to VA/BC (44.8%; P = 0.02). The effect was apparent ≥6 mo postpartum (relative risk: 1.34; 95% CI: 1.04, 1.73). No associations with proviral load were observed. The multivitamin had no effects. In observational analyses, β-carotene but not retinol breast-milk concentrations were significantly associated with an increased viral load in milk.
Conclusions: VA/BC supplementation in lactating women increases the HIV load in breast milk. This finding contributes to explaining the adverse effect of VA/BC on mother-to-child transmission. β-Carotene appears to have an effect on breast-milk viral load, independent of preformed vitamin A. This trial was registered at clinicaltrials.gov as NCT00197756.
INTRODUCTION
Pediatric HIV infection remains a major cause of morbidity and mortality in resource-limited countries. An estimated 430,000 new child infections occurred in 2008 (1), most of which were in sub-Saharan Africa. Mother-to-child transmission (MTCT), which may occur during pregnancy or delivery or via breastfeeding, accounts for most of these infections. Because early MTCT is largely preventable through perinatal interventions with antiretroviral agents, breastfeeding transmission of HIV has become responsible for a large proportion of all vertically acquired infections in breastfeeding populations (2, 3), and its prevention has remained a challenge (4).
We previously reported that daily supplementation of antiretroviral-naive HIV-infected women with vitamin A and β-carotene (VA/BC) during pregnancy and lactation increased the risk of breastfeeding MTCT (5). This effect seemed to be constant throughout the duration of breastfeeding. The mechanism to explain this unexpected effect is unknown; it could be related to a VA/BC-dependent increase in HIV excretion in breast milk, because breast-milk HIV concentrations are among the strongest risk factors for MTCT, and VA/BC supplementation was previously shown to increase viral shedding in other bodily fluids, such as cervicovaginal lavage (6). Finding an explanatory mechanism would lend support to an adverse causal effect of VA/BC supplementation on breastfeeding MTCT and would provide evidence to better understand potential adverse effects of supplementation with these nutrients in other scenarios.
We examined the effects of supplementation with VA/BC or multivitamins (B complex, C, and E) to HIV-infected women on viral (cell-free) and proviral (cell-associated) HIV shedding in breast milk during the first 2 y postpartum, in the context of a randomized trial conducted in Tanzania. In addition, we examined the associations between retinol or β-carotene concentrations and HIV shedding in breast milk, in an effort to identify the relations of each nutrient with viral excretion.
SUBJECTS AND METHODS
Study design and population
Between 1995 and 1997, 1078 HIV-1–infected women were enrolled in a randomized clinical trial in Dar es Salaam, Tanzania to examine the effect of vitamin supplements on maternal and child health outcomes. Details of the trial were described previously (7). In brief, women were enrolled at their first prenatal visit (gestation weeks 12–27) and randomly assigned in a 2-by-2 factorial manner to receive a daily oral dose of 1 of 4 regimens: 1) vitamin A with β-carotene (VA/BC: 5000 IU preformed vitamin A plus 30 mg β-carotene), 2) multivitamins (20 mg thiamine, 20 mg riboflavin, 25 mg vitamin B-6, 100 mg niacin, 50 μg vitamin B-12, 500 mg vitamin C, 30 mg vitamin E, and 0.8 mg folic acid), 3) multivitamins plus VA/BC, or 4) placebo. All women received 5 mg folic acid and 120 mg ferrous iron daily as part of standard prenatal care. At delivery, women in the 2 groups assigned to VA/BC received an additional dose of 200,000 IU vitamin A, whereas the other 2 groups received placebo.
At baseline, information was collected on sociodemographic and anthropometric characteristics by trained study nurses. Physicians conducted a complete medical examination and obtained a blood sample that was used for analyses of hemoglobin, T cell subsets, micronutrient concentrations, and malaria smears. HIV disease stage was assessed according to the World Health Organization (WHO) criteria (8). Women were followed during pregnancy and after delivery at scheduled monthly clinic visits. Information on the risks and benefits of infant feeding options among HIV-infected women was provided according to WHO and Tanzanian Ministry of Health guidelines at the time, and the decision of whether to breastfeed was made by the mothers. Breastfeeding was adopted virtually by all mothers (>99%). At 12 mo postpartum, 94% of mothers were still lactating. By 18 mo, the proportion was 74% and by 24 mo it was 22%. During the first 2 y postpartum, ≈10 mL breast milk was collected by manual expression from either breast, ≈every 3 mo. There were no standardized requirements for the collection of samples in terms of time of the day, time since last breastfeeding, or other criteria. This was because offering formula to HIV-infected women was not viable, storage of expressed milk was impractical, or women did not always attend clinics at scheduled times. However, lack of standardization of breast-milk collection is not thought to distort measurements of HIV shedding (9); in addition, it is improbable that variability in HIV concentrations due to lack of sample standardization affects estimates of effect in the context of a randomized trial because it would likely be evenly distributed by treatment arm.
Compliance with the study regimen was assessed at the monthly visits as the proportion of tablets absent from bottles that were returned by each woman from the total number of tablets she should have taken. The median proportion was 83% by 2 y of follow-up. Antiretroviral treatment was not available in this setting at the time of the study. All women provided written informed consent for participation before enrollment. The protocol was approved by the Human Subjects Committee of the Harvard School of Public Health and the Research and Publications Committee of Muhimbili University of Health and Allied Sciences.
Laboratory methods
Hemoglobin was measured by using a CBC5 Coulter Counter (Coulter Corporation, Miami, FL). Plasma retinol and vitamin E concentrations were quantified with the use of HPLC. CD4+ T cell counts were determined with the use of FACSCount and FACSCan systems (Becton Dickinson, San Jose, CA).
Immediately after collection, breast-milk samples were placed on ice and transported to the Microbiology and Immunology Laboratory at Muhimbili National Hospital. On the same day, one aliquot of ≈5 mL whole breast milk was frozen at −70°C, and a second aliquot was centrifuged at 1500 × g for 12 min at 4°C. The cell-free aqueous milk fraction and the milk cell pellet were separately cryopreserved and shipped to the Harvard School of Public Health for testing. Viral RNA was isolated from the cell-free aqueous milk fraction by using the High Pure Viral RNA Kit (Roche Diagnostics, Indianapolis, IN). Quantification of viral load in the cell-free aqueous milk fraction was done by using the ultrasensitive protocol of the Amplicor HIV-1 Monitor Test (version 1.5; Roche Diagnostics). The lower limit of detection was 100 viral copies/mL. Cell pellets were washed with phosphate-buffered saline, and genomic DNA was extracted by using the QIAamp Blood Kit (Qiagen, Valencia, CA). The proportion of HIV-1 proviral copies per total cell numbers (cell-associated virus) was quantified by real-time polymerase chain reaction by using the FastStart DNA Master SYBR Green I mix and the LightCycler instrument (Roche Diagnostics), as previously described (10). The lower limit of detection was 5 proviral copies/10,000 cells.
Retinol and β-carotene concentrations were quantified in the whole-milk aliquot with the use of HPLC (Restek Corp, Bellefonte, PA), as previously described in detail (11). The minimum detection limits were 3.12 and 1.65 nmol/L for retinol and β-carotene, respectively.
Data analysis
We randomly selected 720 women for inclusion in the breast-milk study. Of them, 594 had one or more samples that were suitable for viral load determinations, and this constituted the study sample. We examined the effect of vitamin supplements on HIV shedding in breast milk during the first 2 y postpartum following the intent-to-treat principle. For both viral and proviral loads, we considered detectable concentrations as a dichotomous endpoint. Outcomes were not considered as continuous variables because the distributions were left-censored due to the detection limits of the assays. We first assessed whether there were significant interactions between the VA/BC and multivitamin arms. Because these interactions were not present, we assessed the effect of VA/BC by comparing women in the 2 treatment groups who received VA/BC (VA/BC with placebo and VA/BC with multivitamins) with women in the 2 treatment groups who did not (multivitamins with placebo and placebo only). An analogous approach was followed to determine the effect of multivitamins. We first estimated treatment effects overall and according to the time postpartum when the samples were collected [0 mo (early postpartum, ≤15 d), 3 mo, 6 mo, 9 mo, 12 mo, and >12 mo [second-year postpartum)]. Relative risks (RRs) with 95% CIs for detectable viral load were calculated from generalized estimating equation models with the binomial distribution, the log-link function, and an exchangeable correlation matrix to account for within-person correlations of repeated measurements. Predictors were treatment assignment and time of sample collection. Interactions with time were assessed by testing cross-product terms between treatment and time with the use of the Wald test. Given that multivitamin supplements were previously shown to decrease the risk of MTCT among women with poor immunologic or nutritional status at baseline (5), we also assessed interactions between the multivitamin regimen and low maternal baseline CD4 cell counts, hemoglobin concentrations, and midupper arm circumference.
We conducted supplemental analyses to examine the cross-sectional associations between the concentrations of retinol or β-carotene and viral shedding in the same breast-milk sample, irrespective of treatment assignment, as a means to differentiate the effects of the 2 VA/BC arm components. Nutrient concentrations were divided in quartiles according to the within-postpartum period distributions, and prevalence ratios for detectable HIV load were estimated by using binomial generalized estimating equations with the lowest quartile as the reference category. These models included both retinol and β-carotene as predictors, considering that they shared a common source (the VA/BC treatment arm) in half of the subjects. Models were also adjusted for time postpartum of sample collection. Tests for linear trend were obtained by introducing into the models an ordinal variable representing the nutrient quartiles as a continuous predictor.
Analyses were carried out with the use of the Statistical Analysis Software version 9.1 (SAS Institute, Cary, NC).
RESULTS
There were 1319 breast-milk samples available from the 594 women included in the analyses of HIV shedding; the mean number of samples per woman was 2.2 ± 1.3, and the proportions of women with 1, 2, 3, and ≥4 samples were 39.7%, 21.9%, 23.2%, and 15.2%, respectively. Lack of availability of breast-milk samples was mainly due to missed clinic visits, low breast-milk output at the time of collection, or weaning. Women with and without breast-milk samples did not differ regarding treatment assignment, age, educational level, HIV-disease stage, or other baseline characteristics (data not shown). The women's characteristics at enrollment were not different by treatment arm (Table 1).
TABLE 1.
Baseline characteristics of women in the breast-milk study by treatment group assignment1
| Placebo (n = 142) | Vitamin A/β-carotene (n = 140) | Multivitamins (n = 154) | Multivitamins and vitamin A/β-carotene (n = 158) | |
| Gestation at randomization (y) | 20.3 ± 3.82 | 20.1 ± 3.6 | 20.6 ± 3.2 | 20.2 ± 3.2 |
| Age (y) | 25.2 ± 4.8 | 24.7 ± 5.1 | 24.8 ± 4.7 | 24.6 ± 4.3 |
| Completed primary education [% (n)] | 87.3 (124) | 88.6 (124) | 85.7 (132) | 91.1 (144) |
| Primigravida [% (n)] | 26.4 (37) | 35.5 (49) | 35.8 (54) | 29.9 (47) |
| Cohabits with a partner [% (n)] | 90.9 (129) | 86.4 (121) | 89.0 (137) | 88.6 (140) |
| Height (cm) | 155.9 ± 5.8 | 156.5 ± 5.5 | 156.5 ± 5.3 | 156.3 ± 5.8 |
| BMI (kg/m2) | 23.4 ± 3.3 | 23.1 ± 3.5 | 23.7 ± 3.6 | 23.4 ± 3.1 |
| Plasma vitamin A (μmol/L) | 0.88 ± 0.44 | 0.83 ± 0.35 | 0.83 ± 0.33 | 0.87 ± 0.29 |
| Plasma vitamin E (μmol/L) | 10.0 ± 2.8 | 10.0 ± 3.0 | 9.7 ± 2.9 | 10.1 ± 2.8 |
| Hemoglobin (g/L) | 95 ± 17 | 92 ± 17 | 94 ± 15 | 92 ± 16 |
| Baseline CD4+ T count (cells/μL) | 418 ± 178 | 409 ± 194 | 426 ± 197 | 418 ± 204 |
| HIV symptomatic [% (n)]3 | 18.3 (26) | 23.7 (33) | 18.3 (28) | 18.6 (29) |
| Malaria parasitemia [% (n)] | 16.3 (23) | 20.1 (28) | 15.3 (23) | 19.9 (31) |
There were no significant differences between the groups.
Mean ± SD (all such values).
Symptomatic women were at stages 2 or 3 according to the World Health Organization staging system of HIV disease.
Effect of vitamins on breast-milk viral load
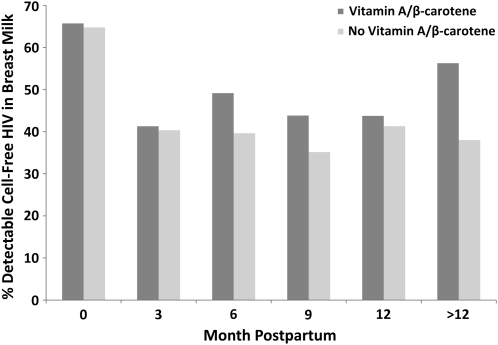
The proportions of samples with detectable HIV-1 load at 0, 3, 6, 9, 12, and >12 mo postpartum were 65.5%, 43.6%, 44.0%, 40.6%, 44.7%, and 48.5%, respectively. Overall, compared with women who did not receive VA/BC, women assigned to VA/BC had a significantly higher proportion of samples with detectable viral load (51.3% compared with 44.8%; P = 0.02). No significant differences between women who received multivitamins and those who did not (47.7% compared with 48.8%; P = 0.69) were observed. We next examined the effect of supplements at each postpartum period. When more than one sample per woman was available at each time point, we chose the one closest to the scheduled date of collection. We noted that the effect of VA/BC was apparent at or after 6 mo postpartum compared with earlier periods; there was no apparent effect at 0 or 3 mo (Figure 1). At or after 6 mo postpartum, VA/BC resulted in a 34% increase in the risk of HIV shedding in milk (95% CI: 4%, 73%), whereas no effect was observed during the first 6 mo (P for interaction with time = 0.10) (Table 2). These results did not vary when the analysis was restricted to women who provided a sample at month 0 plus at least one additional sample thereafter or among women who had a first sample available at 3 mo plus at least another thereafter. No time-related effects were observed for the multivitamins (data not shown).
FIGURE 1.
Effect of vitamin A/β-carotene (VA/BC) supplementation in lactating women on cell-free HIV shedding in breast milk. Sample sizes in the VA/BC and no VA/BC groups at 0, 3, 6, 9, 12, and >12 mo postpartum were 140/122, 172/176, 118/106, 89/74, 48/46, and 64/50, respectively. Corresponding proportions (P from chi-square test) with detectable viral loads were 65.7/64.8 (P = 0.87), 41.3/40.3 (P = 0.86), 49.2/39.6 (P = 0.15), 43.8/35.1 (P = 0.26), 43.8/41.3 (P = 0.81), and 56.3/38.0 (P = 0.05).
TABLE 2.
Effect of vitamin supplements on cell-free HIV concentration in breast milk
| Vitamin A/β-carotene |
Multivitamins |
|||||||
| Yes | No | RR (95% CI)1 | P | Yes | No | RR (95% CI)1 | P | |
| <6 mo postpartum [n (no. of samples)] | 265 (468) | 258 (434) | 273 (473) | 250 (429) | ||||
| Breast-milk viral load (/mL) | ||||||||
| 50th Percentile | 129 | <100 | 107 | <100 | ||||
| 75th Percentile | 887 | 668 | 617 | 932 | ||||
| 90th Percentile | 4248 | 4522 | 4428 | 4228 | ||||
| Detectable viral load (%) | 51.9 | 48.2 | 1.04 (0.90, 1.21) | 0.57 | 50.5 | 49.7 | 1.04 (0.90, 1.20) | 0.61 |
| ≥6 mo postpartum [n (no. of samples)] | 33 (224) | 38 (193) | 39 (219) | 32 (198) | ||||
| Breast-milk viral load (/mL) | ||||||||
| 50th Percentile | <100 | <100 | <100 | <100 | ||||
| 75th Percentile | 496 | 241 | 313 | 440 | ||||
| 90th Percentile | 2597 | 1327 | 2073 | 2116 | ||||
| Detectable viral load (%) | 50.0 | 37.3 | 1.34 (1.04, 1.73) | 0.03 | 41.6 | 47.0 | 0.90 (0.70, 1.16) | 0.41 |
Relative risks (RRs) and 95% CIs are from binomial generalized estimating equation models; an exchangeable covariance matrix was specified to account for within-person correlations of repeated measurements. P values are from the Wald test. P for interaction with time >0.05 for vitamin A/β-carotene and multivitamins.
Effect of vitamins on breast-milk proviral load
Because of logistic constraints, the proviral load was analyzed in a subsample of 680 breast-milk samples from 460 women (mean: 1.5 ± 0.7 samples per woman). Women with proviral load determinations did not differ from women without determinations in terms of treatment assignment or according to most sociodemographic or disease-stage indicators; however, they tended to be older and were less likely to be primigravida. Baseline characteristics did not differ by treatment arm in this subsample. The proportions of samples with detectable proviral load at 0, 3, 6, 9, 12, and >12 mo postpartum were 63.7%, 59.2%, 39.2%, 63.3%, 88.2%, and 72.1%, respectively. No significant effects on the proviral load of VA/BC (RR: 0.93; 95% CI: 0.80, 1.07; P = 0.29) or multivitamins (RR: 1.01; 95% CI: 0.88, 1.17; P = 0.86) were observed.
No significant interactions were observed between the multivitamin arms and indicators of the woman's immunologic and nutritional status at baseline, including CD4 cell counts, hemoglobin concentrations, or midupper arm circumference, on either viral or proviral load in breast milk (data not shown).
HIV shedding in relation to breast-milk retinol and beta-carotene concentrations
Breast-milk retinol was not significantly associated with viral load after adjustment for β-carotene concentrations; however, breast-milk β-carotene concentrations were related to increased detectable viral load in milk (Table 3). The estimates of association were comparable for quartiles 2, 3, and 4, possibly suggesting a “threshold” effect. The prevalence ratio for β-carotene concentrations at or above quartile 2 compared with quartile 1 was 1.21 (95% CI: 1.03, 1.41; P = 0.02). Breast-milk retinol or β-carotene concentrations were not significantly associated with proviral load. Prevalence ratios (95% CI) in quartiles 2, 3, and 4 compared with quartile 1 were 1.00 (0.79, 1.25), 1.15 (0.92, 1.43), and 1.19 (0.94, 1.51) for retinol and 0.95 (0.77, 1.19), 0.95 (0.76, 1.20), and 0.78 (0.59, 1.02) for β-carotene. Adjustment for fat content of the breast-milk samples did not change any of these results (data not shown).
TABLE 3.
Cell-free HIV load in breast milk according to quartile (Q) of breast-milk retinol and β-carotene concentrations
| Detectable viral load |
|||
| No. of samples | Percentage | Adjusted PR (95% CI)1 | |
| Retinol | |||
| Q1 | 288 | 43.8 | 1.00 |
| Q2 | 288 | 47.6 | 1.03 (0.87, 1.22) |
| Q3 | 290 | 49.0 | 0.99 (0.83, 1.19) |
| Q4 | 288 | 53.5 | 1.11 (0.91, 1.36) |
| P for trend2 | 0.43 | ||
| β-Carotene | |||
| Q1 | 286 | 43.4 | 1.00 |
| Q2 | 290 | 46.2 | 1.18 (1.00, 1.39) |
| Q3 | 288 | 52.1 | 1.28 (1.06, 1.55) |
| Q4 | 290 | 52.1 | 1.17 (0.93, 1.49) |
| P for trend2 | 0.19 | ||
Prevalence ratios (PRs) and 95% CIs are from binomial generalized estimating equation models with detectable (yes or no) viral load as the outcome and predictors that included indicator variables for quartiles of retinol and β-carotene concentrations and month postpartum when the sample was collected; an exchangeable covariance matrix was specified to account for within-person correlations of repeated measurements.
Wald test of a variable representing the quartiles of retinol or β-carotene concentrations that was introduced into the model as a continuous predictor.
DISCUSSION
In this randomized clinical trial, the administration of vitamin A and β-carotene supplements to HIV-1–infected women during pregnancy and lactation resulted in increased viral load in breast milk at or after 6 mo postpartum. Considering that excretion of HIV in breast milk is one of the strongest risk factors for MTCT (10, 12), these results provide a mechanistic explanation to the previously reported adverse effect of VA/BC on MTCT of HIV through breastfeeding in this population (5).
The specific mechanisms through which vitamin A or β-carotene could increase HIV shedding in milk are not well understood. MacDonald et al (13) postulated that vitamin A enhances the differentiation of myeloid and lymphoid cells, which leads to increased expression of the chemokine (C-C motif) receptor 5 (CCR5)—one of the membrane co-receptors that HIV uses to enter its target cells. Retinoic acid indeed appears to facilitate the differentiation of monocytes to macrophages, and this differentiation is accompanied by up-regulation of CCR5 (14). Nonetheless, it is unknown whether retinoic acid has the same effect in nonreplicating macrophages, which are the most abundant in breast milk (15) and the principal cellular carriers of productive HIV-1 infection in milk (16). We found no effects of VA/BC supplementation on proviral load, but only on viral load; thus, mechanisms other than macrophageal CCR5 up-regulation could be at play. One of those mechanisms might include mastitis—an inflammatory process of the breast during which tight junctions in the mammary epithelium are disrupted, allowing both cell-free virus in extracellular fluid and HIV-infected cells to leak from plasma into milk, hence elevating the risk of MTCT (12, 17). Randomized supplementation trials however, have not reported significant effects of vitamin A or β-carotene alone (18) or in combination with other micronutrients (19, 20) on mastitis. We have no information on the prevalence of clinical mastitis in this population, but ≥17% of women had evidence of subclinical mastitis in a small case-control study (17), as documented by an elevated ratio of sodium to potassium in milk—an indicator of inflammation. The effect of vitamin supplements on mastitis in this study population remains to be ascertained. The lack of an effect of VA/BC on breast-milk viral shedding during early postpartum, a period when HIV load in milk was highest, might suggest that the effect of VA/BC on MTCT <6 mo is not necessarily mediated through increases in viral shedding, but could be related to other mechanisms. For example, vitamin A might increase CCR5 expression of infant mucosal cells, thus enhancing their susceptibility to infection.
Determining whether β-carotene or retinol had independent effects on MTCT-related endpoints is an important research question because the finding that supplementation with both nutrients increased MTCT (5) may have introduced uncertainty about the safety of vitamin A supplementation programs. β-Carotene could be converted into vitamin A through central cleavage; but, when administered at high doses (>20 mg/d), it might have effects that are different from those expected of preformed vitamin A (21). Because vitamin A and β-carotene were administered together in this trial, it is not possible to ascertain the individual effect of each nutrient following an intent-to-treat analytic approach. Nevertheless, in an effort to identify the separate effects of each nutrient, we evaluated the associations between viral or proviral HIV concentrations and retinol or β-carotene concentrations in breast milk using an observational, cross-sectional design in which we took advantage of the treatment's large effect on the variability of breast-milk nutrient concentrations (11). A key aspect of these analyses was the simultaneous adjustment for retinol and β-carotene concentrations in the models to overcome confounding of the effect of one nutrient by the other's given that they shared the same major source (treatment assignment) in half of the population. Our results provide some indication that β-carotene might have increased the viral load in breast milk, independent of preformed vitamin A. Biological mechanisms underlying this association are speculatory, although previous clinical trials have shown adverse effects of β-carotene supplementation at ≥20 mg/d on the risk of other outcomes, including lung cancer (21, 22), and cardiovascular disease in population subgroups (23). Several pathways have been posited to explain these effects. Whereas β-carotene is a potent antioxidant, high-dose intake could result in prooxidant activity (24), which might interfere with antioxidant defense systems (25). In addition, breakdown products from excentric cleavage of β-carotene could disrupt mitochondrial function (26). Results from previous β-carotene supplementation trials conducted among HIV-infected persons have not consistently indicated that it increases the risk of adverse events. High doses of β-carotene (180 mg/d) had no effects on CD4+ cell counts (27, 28) or plasma viral load (28) in 2 different studies. Also, in a trial of natural mixed carotenoids equivalent to 72 mg β-carotene/d, no significant effect on mortality was observed in HIV-infected adults (29). The doses of β-carotene used in these trials were much higher than the amounts obtained from typical diets in populations in whom HIV infection is widely prevalent. Even in areas where interventions to improve vitamin A status through intake of carotenoid-rich food sources have been evaluated, daily intake of β-carotene in pregnant or lactating women would range from 1.1 to 2.4 mg/d (30, 31).
Despite the suggestion that increases in breast-milk β-carotene concentrations during lactation might underlie the effect of VA/BC supplementation on breast-milk viral load, an adverse effect of preformed vitamin A on MTCT cannot be completely ruled out. A trial testing the efficacy of a single large dose of preformed vitamin A alone given to women during the early postpartum period (200,000 IU) and/or to neonates (50,000 IU) in Zimbabwe found an increased risk of infant HIV infection or death when vitamin A was provided to either, but not both, the mother or infant, compared with placebo (32). This suggests that even if preformed vitamin A does not increase HIV shedding in breast milk, it might enhance the infant's susceptibility to HIV infection. Future studies evaluating the associations between breast-milk retinol and β-carotene concentrations and breastfeeding MTCT might provide additional insights into the separate effects of these nutrients.
In conclusion, vitamin A and β-carotene supplementation to HIV-infected lactating women increases viral load in breast milk at or after 6 mo postpartum. There is some indication that this association might be related to increases in the breast-milk concentrations of β-carotene. These findings contribute to explain the adverse effect of vitamin A and β-carotene supplementation on MTCT of HIV, reported previously, and support the notion that daily supplementation of HIV-infected lactating women with high doses of vitamin A and β-carotene cannot be safely recommended.
Acknowledgments
The authors’ responsibilities were as follows—EV: designed the study, obtained funding, conducted the data analysis, interpreted the results, and wrote the first draft of the manuscript; INK: contributed to the study design, established protocols for virologic analyses, conducted the viral load determinations, and participated in the interpretation of results; SA and CM: contributed to the identification of samples for analysis and the viral load determinations; RJB: participated in the study design and provided statistical guidance; KPM: contributed to field activities and interpretation of the results; and WWF: participated in the study design and data interpretation. All authors helped write the manuscript. None of the authors had a conflict of interest in relation to this manuscript.
REFERENCES
- 1.UNAIDS AIDS epidemic update. Geneva, Switzerland: United Nations Program on HIV/AIDS, 2009 [Google Scholar]
- 2.Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis 2004;189:2154–66 [DOI] [PubMed] [Google Scholar]
- 3.Becquet R, Bland R, Leroy V, et al. Duration, pattern of breastfeeding and postnatal transmission of HIV: pooled analysis of individual data from West and South African cohorts. PLoS ONE 2009;4:e7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becquet R, Ekouevi DK, Arrive E, et al. Universal antiretroviral therapy for pregnant and breast-feeding HIV-1-infected women: towards the elimination of mother-to-child transmission of HIV-1 in resource-limited settings. Clin Infect Dis 2009;49:1936–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawzi WW, Msamanga GI, Hunter D, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS 2002;16:1935–44 [DOI] [PubMed] [Google Scholar]
- 6.Fawzi W, Msamanga G, Antelman G, et al. Effect of prenatal vitamin supplementation on lower-genital levels of HIV type 1 and interleukin type 1 beta at 36 weeks of gestation. Clin Infect Dis 2004;38:716–22 [DOI] [PubMed] [Google Scholar]
- 7.Fawzi WW, Msamanga GI, Spiegelman D, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med 2004;351:23–32 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization Interim proposal for a WHO staging system for HIV infection and disease. Wkly Epidemiol Rec 1990;65:221–4 [PubMed] [Google Scholar]
- 9.Ghosh MK, Kuhn L, West J, et al. Quantitation of human immunodeficiency virus type 1 in breast milk. J Clin Microbiol 2003;41:2465–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koulinska IN, Villamor E, Chaplin B, et al. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. J Acquir Immune Defic Syndr 2006;41:93–9 [DOI] [PubMed] [Google Scholar]
- 11.Webb AL, Aboud S, Furtado J, et al. Effect of vitamin supplementation on breast milk concentrations of retinol, carotenoids and tocopherols in HIV-infected Tanzanian women. Eur J Clin Nutr 2009;63:332–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semba RD, Kumwenda N, Hoover DR, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis 1999;180:93–8 [DOI] [PubMed] [Google Scholar]
- 13.MacDonald KS, Malonza I, Chen DK, et al. Vitamin A and risk of HIV-1 seroconversion among Kenyan men with genital ulcers. AIDS 2001;15:635–9 [DOI] [PubMed] [Google Scholar]
- 14.Moriuchi H, Moriuchi M, Fauci AS. Differentiation of promonocytic U937 subclones into macrophagelike phenotypes regulates a cellular factor(s) which modulates fusion/entry of macrophagetropic human immunodeficiency virus type 1. J Virol 1998;72:3394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guay LA, Hom DL, Mmiro F, et al. Detection of human immunodeficiency virus type 1 (HIV-1) DNA and p24 antigen in breast milk of HIV-1-infected Ugandan women and vertical transmission. Pediatrics 1996;98:438–44 [PubMed] [Google Scholar]
- 16.Southern SO. Milk-borne transmission of HIV. Characterization of productively infected cells in breast milk and interactions between milk and saliva. J Hum Virol 1998;1:328–37 [PubMed] [Google Scholar]
- 17.Kantarci S, Koulinska IN, Aboud S, Fawzi WW, Villamor E. Subclinical mastitis, cell-associated HIV-1 shedding in breast milk, and breast-feeding transmission of HIV-1. J Acquir Immune Defic Syndr 2007;46:651–4 [DOI] [PubMed] [Google Scholar]
- 18.Filteau SM, Rice AL, Ball JJ, et al. Breast milk immune factors in Bangladeshi women supplemented postpartum with retinol or beta-carotene. Am J Clin Nutr 1999;69:953–8 [DOI] [PubMed] [Google Scholar]
- 19.Hindle LJ, Gitau R, Filteau SM, et al. Effect of multiple micronutrient supplementation during pregnancy on inflammatory markers in Nepalese women. Am J Clin Nutr 2006;84:1086–92 [DOI] [PubMed] [Google Scholar]
- 20.Gomo E, Filteau SM, Tomkins AM, Ndhlovu P, Michaelsen KF, Friis H. Subclinical mastitis among HIV-infected and uninfected Zimbabwean women participating in a multimicronutrient supplementation trial. Trans R Soc Trop Med Hyg 2003;97:212–6 [DOI] [PubMed] [Google Scholar]
- 21.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150–5 [DOI] [PubMed] [Google Scholar]
- 22.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 1994;330:1029–35 [DOI] [PubMed] [Google Scholar]
- 23.Tornwall ME, Virtamo J, Korhonen PA, et al. Effect of alpha-tocopherol and beta-carotene supplementation on coronary heart disease during the 6-year post-trial follow-up in the ATBC study. Eur Heart J 2004;25:1171–8 [DOI] [PubMed] [Google Scholar]
- 24.Palozza P. Prooxidant actions of carotenoids in biologic systems. Nutr Rev 1998;56:257–65 [DOI] [PubMed] [Google Scholar]
- 25.McGill CR, Green NR, Meadows MC, Gropper SS. Beta-carotene supplementation decreases leukocyte superoxide dismutase activity and serum glutathione peroxidase concentration in humans. J Nutr Biochem 2003;14:656–62 [DOI] [PubMed] [Google Scholar]
- 26.Siems W, Wiswedel I, Salerno C, et al. Beta-carotene breakdown products may impair mitochondrial functions–potential side effects of high-dose beta-carotene supplementation. J Nutr Biochem 2005;16:385–97 [DOI] [PubMed] [Google Scholar]
- 27.Coodley GO, Coodley MK, Lusk R, et al. Beta-carotene in HIV infection: an extended evaluation. AIDS 1996;10:967–73 [DOI] [PubMed] [Google Scholar]
- 28.Nimmagadda AP, Burri BJ, Neidlinger T, O'Brien WA, Goetz MB. Effect of oral beta-carotene supplementation on plasma human immunodeficiency virus (HIV) RNA levels and CD4+ cell counts in HIV-infected patients. Clin Infect Dis 1998;27:1311–3 [DOI] [PubMed] [Google Scholar]
- 29.Austin J, Singhal N, Voigt R, et al. A community randomized controlled clinical trial of mixed carotenoids and micronutrient supplementation of patients with acquired immunodeficiency syndrome. Eur J Clin Nutr 2006;60:1266–76 [DOI] [PubMed] [Google Scholar]
- 30.Lietz G, Henry CJ, Mulokozi G, et al. Comparison of the effects of supplemental red palm oil and sunflower oil on maternal vitamin A status. Am J Clin Nutr 2001;74:501–9 [DOI] [PubMed] [Google Scholar]
- 31.Radhika MS, Bhaskaram P, Balakrishna N, Ramalakshmi BA. Red palm oil supplementation: a feasible diet-based approach to improve the vitamin A status of pregnant women and their infants. Food Nutr Bull 2003;24:208–17 [DOI] [PubMed] [Google Scholar]
- 32.Humphrey JH, Iliff PJ, Marinda ET, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis 2006;193:860–71 [DOI] [PubMed] [Google Scholar]