Abstract
Elite controllers maintain undetectable levels of HIV-1 replication in the absence of antiretroviral therapy, but the correlates of immune protection in this patient population are ill defined. Here, we demonstrate that in comparison to patients with progressive HIV-1 infection or healthy persons not infected with HIV-1, elite controllers have circulating myeloid dendritic cells with significantly increased antigen-presenting properties, while their ability to secrete proinflammatory cytokines is substantially diminished. This unique functional profile is associated with a distinct surface expression pattern of immunomodulatory leukocyte-immunoglobulin-like receptors (LILR) and a strong and selective upregulation of LILRB1 and LILRB3. Blockade of these two receptors by monoclonal antibodies or short interfering RNA (siRNA) abrogated the specific antigen-presenting properties of dendritic cells, implying an important regulatory role of these molecules. These data reveal previously unrecognized innate components of immune protection against HIV-1 in elite controllers and offer novel perspectives for the manipulation of host immunity for the prevention and treatment of HIV-1 infection.
Elite human immunodeficiency virus type 1 (HIV-1) controllers represent a small group of HIV-1-infected individuals who are able to maintain undetectable HIV-1 viral loads in the absence of antiretroviral therapy (7, 33, 40). Understanding the mechanisms of immune protection in this specific patient population is of great interest for HIV-1 vaccine design, yet the identification of specific immune defense mechanisms that contribute to the spontaneous control of HIV-1 replication in these patients has been extremely challenging. Certain immunogenetic characteristics, such as specific HLA class I alleles (17) or chemokine receptor mutations (21, 46), have clearly been associated with control of HIV-1 viremia, yet it is now clear that a significant proportion of these patients can contain HIV-1 replication in the absence of such favorable genetic properties (36). Moreover, there is accumulating evidence from recent studies showing that elite controllers are infected with pathogenic, replication-competent viral species, indicating that defective viral replication is not the major reason underlying the elite controller phenotype (8, 34).
Myeloid dendritic cells (mDC) are the most effective naturally occurring antigen-presenting cells and have a central role in mediating pathogen-specific immune activity by connecting innate and adaptive mechanisms of host immune defense (47). They can prime naive T and B cells, expand antigen-specific memory cell responses, regulate immune activation by secretion of cytokines, and orchestrate immune defense mechanisms through cross talk with different effector immune cells (4, 39). These cells are therefore of outstanding interest for manipulating host immunity against HIV-1 with vaccines or immunogens. While numeric, phenotypic and functional deficiencies of myeloid dendritic cells in patients with progressive HIV-1 infection, both on and off highly active antiretroviral therapy (HAART), have been described in a series of prior studies (12, 16, 19), a potential beneficial role of these cells for HIV-1 immune protection in elite controllers has received limited attention. The functional profile of myeloid dendritic cells is regulated by a fine-tuned regulatory network of immunoreceptors that can affect their functional properties in either a stimulatory or inhibitory fashion. These regulatory molecules include the class of leukocyte immunoglobulin-like receptors (LILRs, also termed immunoglobulin-like transcripts [ILT] or CD85a to -m), a family of immunomodulatory receptors with genetic, structural, and functional similarities to killer-immunoglobulin receptors (KIR) (1, 10). These receptors can regulate dendritic cell function through immunoreceptor tyrosine-based inhibition motif (ITIM)- or immunoreceptor tyrosine-based activation motif (ITAM)-mediated signaling cascades that can specifically modify the antigen-presenting and cytokine-secretion properties of dendritic cells, and the results of a recent study suggested that interactions between such molecules and their respective ligands can significantly influence the clinical outcome of HIV-1 infection (20). How these receptors modulate the immune activity of dendritic cells in individuals with “elite” control of HIV-1 infection, however, is currently unknown.
In the present study, we describe specific antigen-presenting and cytokine-secretion properties of myeloid dendritic cells in elite controllers that clearly separate this specific patient population from HIV-1 progressors or HIV-1-negative persons and are in part regulated and maintained by a selective pattern of LILR receptor expression. These data suggest that LILR-mediated functional characteristics of dendritic cells represent a previously unrecognized innate component of HIV-1 immune protection in elite controllers and provide novel perspectives for immunological approaches to HIV-1 treatment and prevention through the manipulation of the functional properties of myeloid dendritic cells.
MATERIALS AND METHODS
Patients.
The HIV-1-infected individuals with chronic HIV-1 infection participating in this study were recruited at Massachusetts General Hospital. The clinical and demographic characteristics of the study patients are summarized in Table 1. All subjects gave written informed consent to participate, and the study was approved by the Massachusetts General Hospital Institutional Review Board.
TABLE 1.
Clinical and demographic characteristics of the study cohorts
| Cohort | Yrs of age [median (range)] | Sex ratio (male/female) | CD4 cell count/μl [median (range)] | Viral load/ml [median (range)] | Time (mos) since HIV-1 diagnosis [median (range)] |
|---|---|---|---|---|---|
| Progressors (n = 14) | 38.5 (30-51) | 13:1 | 412.5 (195-1,000) | 28,428 (8,104-449,000) | 50.5 (30-216) |
| Elite controllers (n = 13) | 50 (32-62) | 12:1 | 759 (188-1,156) | Undetectable (<49-110) | 183 (54-296) |
| HIV-1-negative persons (n = 21) | 24 (22-38) | 13:8 |
Dendritic cell isolation.
Myeloid dendritic cells were isolated from whole peripheral blood mononuclear cell (PBMC) samples by positive selection with blood dendritic cell antigen 1 (BDCA-1)- and BDCA-3-specific antibodies by using isolation kits from Miltenyi Biotech (Miltenyi, Auburn, CA). Purified BDCA-1+ and BDCA-3+ mDC were pooled together for further study. The isolated cells were >95% enriched for CD11c+ HLA-DR+ lin− cells, as determined by flow cytometry.
Preparation of monocyte-derived dendritic cells (MDDC).
Freshly isolated PBMC were washed several times in RPMI medium to remove platelets, plated into Corning T225 flasks in 5% pooled human serum medium, and incubated for 60 min at 37°C to adhere monocytes. Adherent monocytes were propagated in the presence of 50 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Amgen) for 5 days in RPMI 1640 medium supplemented with penicillin, streptomycin, l-glutamine, HEPES buffer, and 1% heparinized normal human plasma. On day 5, immature myelomonocytic cells were harvested using Hanks-based cell-dissociation buffer (Invitrogen), electroporated with ILT-specific short interfering RNAs (siRNAs), and then matured for 16 h using a previously described reagent cocktail containing interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), prostaglandin E2 (PGE2), and IL-6 (25) or Toll-like receptor 7/8 (TLR7/8) ligands.
Flow cytometric studies.
Dendritic cells were stained with lineage antibodies (CD3, CD14, CD16, CD19, CD20, and CD56) and CD11c and HLA-DR antibodies. Surface staining of mDC was performed using monoclonal antibodies directed against CD40, CD80, CD86, or CD83 (all antibodies from BD Biosciences) or a panel of antibodies directed against ILT1, -2, -4, and -5 that have no detectable cross-reactivity to alternative ILT (LILR) receptors (45). For the analysis of cytokine secretion patterns of mDC, PBMC were stimulated with TLR7/8 ligand CL097 (5 μg/ml; InvivoGen, San Diego, CA) for 20 h in the presence of brefeldin A. After fixation and permeabilization, intracellular cytokine staining was performed using monoclonal antibodies against TNF-α (BioLegend), IL-6 (eBioscience), and IL-12p70 (Miltenyi Biotec) according to standard protocols. For the phenotypic characterization of allogeneic T cells, cells were stained with monoclonal antibodies against CD127, CD62L, CD57, and CD45RA (BD Biosciences). Cells were analyzed on an LSRII cytometer using FACSDiva software. In some experiments, the cytokine secretion of mDC was measured in the presence of ILT2-blocking (clone M402; Amgen) and ILT5-blocking (clone 222821; R&D Systems) antibodies.
Mixed lymphocyte reactions.
T cell proliferation assays were performed in 96-well round-bottom microtiter plates in RPMI 1640 medium, containing 100 IU penicillin, 0.1 mg/ml streptomycin, 2 mM l-glutamine, and 10% human serum (Sigma). Purified mDC or MDDC were mixed with 2 × 105 allogeneic carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled T cells (isolated with a T cell enrichment kit; StemCell Technologies) at ratios of 1:25, 1:50, or 1:100 as appropriate. After 6 consecutive days of culture, cells were stained with monoclonal CD4+ and CD8+ antibodies and processed for flow cytometric analysis. When indicated, monoclonal antibodies directed against ILT2 (LILRB1) (clone M402; Amgen) or ILT5 (LILRB3) (clone 222821; R&D Systems) or control antibodies were added to mDC for 1 h (10 μg/ml); after washing, mDC were mixed with allogeneic T cells as described above.
siRNA-mediated gene knockout.
siRNA pools specific for ILT1 (LILRA2), ILT2 (LILRB1), ILT4 (LILRB2), or ILT5 (LILRB3) mRNA (ON-TARGETplus SMARTpool; Dharmacon) were used at concentrations of 1 nmol/million cells. Amounts of 1.0 × 106 MDDC were suspended in 300 μl Optimem in the presence of 1 nmol siRNA and transferred to a 4-mm electroporation cuvette (Bio-Rad Laboratories). After incubation on ice for 10 min, cells were electroporated (900 V, 0.75 msec square wave; Bio-Rad Genepulser Xcell).
Statistics.
Data are presented as box-and-whisker plots, reflecting the minimum, the maximum, and the 25th, 50th, and 75th percentiles. Differences were tested for statistical significance by use of analysis of variance (ANOVA), Mann-Whitney U test, or paired/unpaired t test as appropriate; a P value of <0.05 was considered significant.
RESULTS
Unique antigen-presenting properties of mDC in HIV-1 elite controllers.
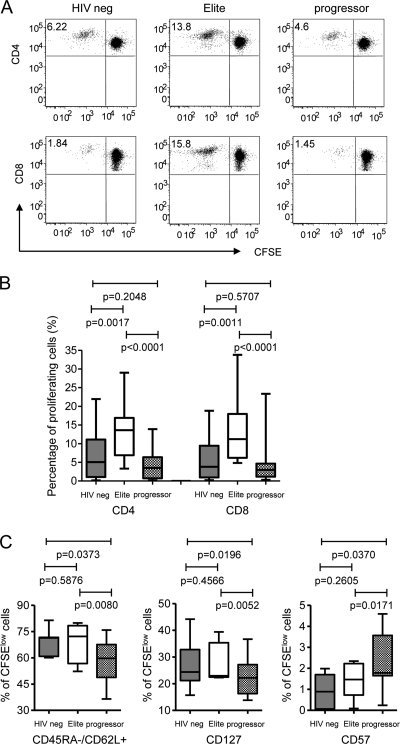
Using mixed lymphocyte reactions as a functional assay to evaluate the antigen-presenting properties of mDC, prior studies found weaker allostimulatory activities of peripheral blood mDC in HIV-1-infected patients than in healthy volunteers (16), while no differences were seen between treatment-naïve HIV-1 patients and persons with pharmacological suppression of HIV-1 viremia below detection thresholds (12). However, the functional antigen-presenting properties of mDC in elite controllers remain unclear. To address this, we purified myeloid cells positive for BDCA-1 (CD1c) or BDCA-3 (CD141) from whole PBMC preparations from elite controllers; mDC from HIV-1 patients with untreated chronic progressive disease and HIV-1-negative healthy volunteers were used as controls. The isolated cells strongly expressed CD11c and HLA-DR while being negative for lineage markers, as expected for BDCA-1+ or BDCA-3+ myeloid dendritic cells. Without any prior ex vivo culture or manipulation with exogenous cytokines, mDC from elite controllers, chronic progressors or HIV-1-negative donors were mixed with CFSE-labeled allogeneic T cells isolated from identical HIV-1-negative donors; subsequently, proliferating allogeneic T cell populations were flow cytometrically analyzed after 6 days in culture. Interestingly, mDC from controllers had a significantly enhanced ability to expand allogeneic T cell responses compared to mDC from both HIV-1-negative individuals and HIV-1-positive persons with progressive disease (Fig. 1 A and B). This was true for both allogeneic CD4+ and CD8+ T cells. The allostimulatory functions of mDC from HIV-1-negative persons were not significantly different from those of HIV-1 progressors in our study groups; however, there was a trend for a weaker allostimulatory capacity in HIV-1 progressors. There was no difference in the degree of HLA mismatches between allogeneic T cell donors and the persons from the three different study groups, and no HIV-1 antigens were detected in the culture supernatants from elite controllers or progressors that could have possibly influenced our findings (data not shown). Overall, these data show that mDC from elite controllers have unique abilities to prime and sensitize allogeneic T cell responses.
FIG. 1.
Unique antigen-presenting properties of circulating myeloid dendritic cells from elite controllers. (A) Dot plots reflect the proportion of proliferating allogeneic T cells after stimulation with isolated circulating mDC from the indicated patient groups. (B) Box-and-whisker plots summarize the proportions of proliferating allogeneic T cells after exposure to mDC from the three study cohorts (n = 12 each) (see “Statistics” in Materials and Methods). (C) Phenotypic properties of allogeneic T cell responses following stimulation with mDC from three different study cohorts. Data reflect the proportions of proliferating, CFSEdim allogeneic CD4+ T cells with the indicated phenotypic characteristics. Experiments were performed with mDC from 7 different donors from each group.
We subsequently tested whether these quantitative differences in allostimulatory activities of mDC translated into qualitative changes in the allogeneic T cells responding to stimulation. To analyze this, we assessed the phenotypic properties of allogeneic CD4 T cells proliferating in response to stimulation with mDC from the three study cohorts. As shown by the results in Fig. 1C, mDC from controllers and HIV-1-negative persons generated memory cell-like CD4 T cells with strong expression of the lymph node homing marker CD62L and the IL-7 receptor CD127, while the expression of CD57, a surface marker associated with replicative senescence (9), was reduced. In contrast, mDC from progressors induced an activated effector T cell-like profile with low CD62L/CD127 and increased CD57 expression. No differences in the phenotypic properties of nonproliferating allogeneic T cells were observed between the three study cohorts (the P value was >0.05 for all comparisons). Overall, these data suggest that mDC from controllers or HIV-1-negative persons preferentially induce memory-like T cells, while mDC from progressors generate more differentiated effector-like T cell responses.
Distinct cytokine secretion pattern of mDC in elite controllers.
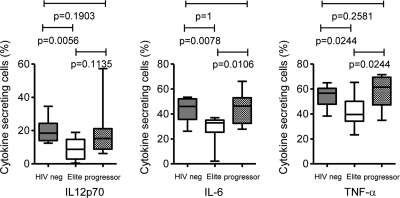
In addition to their functions as professional antigen-presenting cells, myeloid dendritic cells can secrete cytokines and other soluble factors after sensing pathogens through pattern recognition receptors, such as Toll-like receptors (47). These cytokines have important roles for regulating immune activation and for coordinating the functional activities of effector immune cells. To analyze the cytokine secretion properties of mDC in our three study cohorts, we stimulated whole PBMC with TLR7/8 ligands and assessed the secretion of cytokines in lin− HLA-DR+ CD11c+ mDC by intracellular staining using multiparameter flow cytometry. Interestingly, we observed that mDC from elite controllers differed from mDC of HIV-1 progressors or HIV-1-negative persons in secreting significantly smaller amounts of the proinflammatory cytokines TNF-α, IL-6, and IL-12p70 (Fig. 2). Thus, these data show that mDC from elite controllers are characterized by a unique combination of increased antigen-presenting and decreased cytokine secretion properties.
FIG. 2.
Distinct cytokine secretion properties of circulating mDC from elite controllers. Box-and-whisker plots reflect the proportion of lin− HLA-DR+ CD11c+ mDC secreting the indicated cytokine, as determined by intracellular cytokine staining following stimulation with TLR7/8 ligands in three different study cohorts (n = 9 each).
Specific signatures of LILR receptor surface expression on mDC from HIV-1 controllers.
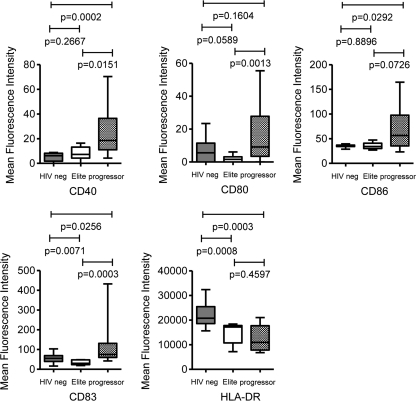
To investigate the mechanisms accounting for the unique functional properties of mDC from elite controllers, we initially focused on the analysis of costimulatory molecules and dendritic cell maturation markers, which have important effects on the antigen-presenting properties of dendritic cells. For this purpose, we flow cytometrically analyzed the surface phenotypes of lin− HLA-DR+ CD11c+ mDC from the peripheral blood of the three study cohorts. Overall, the total proportions of mDC in the three study cohorts were not statistically different (P = 0.7856, one-way ANOVA). In progressors, we found a significant upregulation of CD83, CD40, CD86, and CD80 on mDC, while no systematic differences were found between HIV-1 controllers and HIV-1-negative persons (Fig. 3). These expression patterns are in line with a partial activation and maturation of mDC during progressive HIV-1 infection (2, 15), but they do not correspond to the unique functional profile of mDC in controllers described above.
FIG. 3.
Surface expression of costimulatory molecules and dendritic cell maturation markers on circulating lin− HLA-DR+ CD11c+ mDC from HIV-1-negative persons (n = 15), HIV-1 elite controllers (n = 11), or HIV-1 progressors (n = 12). Box-and-whisker plots reflect the mean fluorescence intensities of the antibodies corresponding to the indicated phenotypic markers.
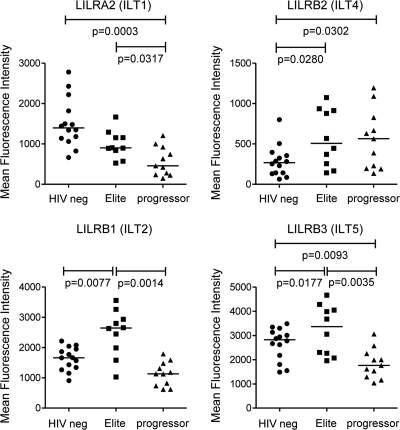
LILRs represent a group of immunomodulatory molecules that can have a dominant effect on dendritic cell function and, thus, may be involved in the regulation of the unique functional profile observed in mDC from elite controllers. To analyze this, we investigated the surface expression patterns of LILR receptors on mDC in whole PBMC samples from our three study cohorts using a panel of monoclonal antibodies (45) that were highly specific for their target receptors, as confirmed by selective staining of CHO cell lines transfected with plasmids encoding ILT1 (LILRA2), ILT2 (LILRB1), ILT3 (LILRB4), ILT4 (LILRB2), ILT5 (LILRB3), or LILRA1 (data not shown). Strikingly, we observed a significant upregulation of the receptors ILT2 (LILRB1) and ILT5 (LILRB3) (13) in elite controllers compared to the expression of these receptors in both HIV-1-negative individuals and HIV-1 progressors (Fig. 4). Moreover, both ILT2 (LILRB1) and ILT5 (LILRB3) were significantly more strongly expressed in HIV-1-negative persons than in HIV-1 progressors. The inhibitory receptor ILT4 (LILRB2) (11, 14) was more strongly expressed on mDC from HIV-1-infected persons than on those from HIV-1-negative individuals, with no significant difference being detectable between controllers and progressors. In contrast, the expression of the stimulatory receptor ILT1 (LILRA2) (35) was significantly stronger in HIV-1-negative persons than in HIV-1 progressors and controllers (Fig. 4). This expression profile of ILT (LILR) receptors was highly selective for circulating mDC, and no similar patterns of ILT surface expression were observed on T cells, B cells, or monocytes (data not shown). Overall, these data show that the unique combination of increased allostimulatory and decreased cytokine-secretion properties of mDC from elite controllers is associated with a distinct ILT (LILR) surface expression phenotype.
FIG. 4.
Distinct surface expression phenotype of LILRs in elite controllers. Surface expression of ILT1 (LILRA2), ILT2 (LILRB1), ILT4 (LILRB2), and ILT5 (LILRB3) on circulating lin− HLA-DR+ CD11c+ mDC in three study cohorts was determined by flow cytometry. Data reflect the mean fluorescence intensities of the antibodies corresponding to the respective ILT receptors.
Allostimulatory activities of mDC from elite controllers are maintained by ILT2 (LILRB1) and ILT5 (LILRB3).
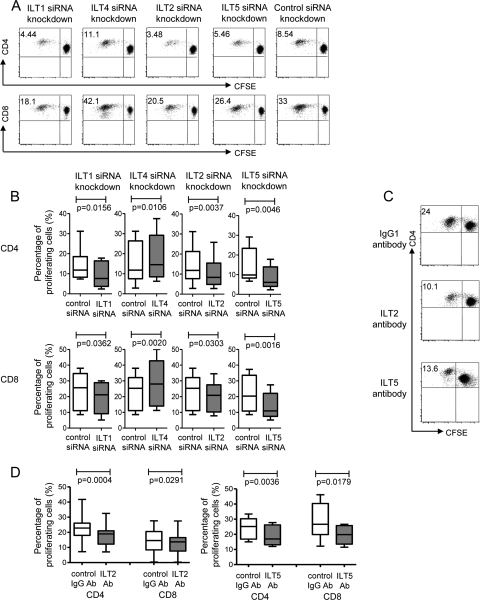
To directly test whether the specific antigen-presenting properties of mDC in elite controllers are regulated by ILTs (LILRs), we generated monocyte-derived dendritic cells (MDDC) from HIV-1-negative donors and assessed their allostimulatory properties after targeted siRNA-mediated knockout of selected ILT (LILR) receptors using mixed-lymphocyte reaction mixtures with allogeneic T cells from HIV-1-seronegative donors. LILR-specific siRNA reliably and selectively reduced the surface expression of the corresponding receptor by >60% (data not shown). Following electroporation with ILT5 (LILRB3)-specific siRNA, we observed a significantly weaker ability of dendritic cells than of cells electroporated with nontargeting control siRNA to sensitize and expand allogeneic T cells. A similar reduction of allostimulatory dendritic cell activity was observed after targeted downregulation of ILT2 (LILRB1), although the measurable effect was weaker, specifically for allogeneic CD8+ T cells (Fig. 5 A and B). In line with prior reports of the stimulatory activity of ILT1 (LILRA2) (35), the allostimulatory properties of MDDC were also significantly weaker after inhibition of ILT1. In contrast, we observed that siRNA-mediated silencing of ILT4 (LILRB2) led to an opposite effect and increased the allostimulatory properties of dendritic cells, consistent with reports of an inhibitory effect of this molecule on dendritic cells or monocytes (11, 28). Overall, these data suggest that the selective upregulation of ILT2 (LILRB1) and ILT5 (LILRB3) is critically involved in mediating the unique antigen-presenting properties of mDC in elite controllers. To further confirm this, we tested the allostimulatory properties of mDC isolated directly from whole PBMC populations of our study cohorts following selective blockade of ILT2 (LILRB1) or ILT5 (LILRB3) using monoclonal antibodies. For these experiments, mDC purified by selection of BDCA-1/3-positive cells were incubated with ILT2- and ILT5-specific antibodies or control antibodies for 1 h; after washing, mDC were incubated with allogeneic T cells from identical HIV-1-negative donors. In agreement with our prior data, these experiments showed that antibody-mediated inhibition of ILT2 and ILT5 led to significantly weaker abilities of freshly isolated mDC to sensitize allogeneic T cells (Fig. 5C and D).
FIG. 5.
Unique antigen-presenting properties of elite controllers are mediated by ILT2 (LILRB1) and ILT5 (LILRB3). (A and B) Proliferation of CFSE-labeled allogeneic T cells following stimulation with MDDC electroporated with siRNA targeting the indicated ILT transcript or nontargeting control siRNA. (A) Dot plots show results from one representative experiment. (B) Summarization of allostimulatory activities of MDDC collected from 8 HIV-1-negative donors after incubation with allogeneic T cells from an HIV-1-negative donor. (C and D) Proliferative activities of allogeneic T cells after exposure to isolated circulating mDC in the presence of ILT2- or ILT5-specific antibodies. (C) Dot plots show results from one representative experiment. (D) Summarization of allostimulatory properties of mDC from 15 HIV-1-negative donors for ILT2 and 6 HIV-1-negative donors for ILT5 after incubation with allogeneic T cells from an HIV-1-negative donor. Ab, antibody.
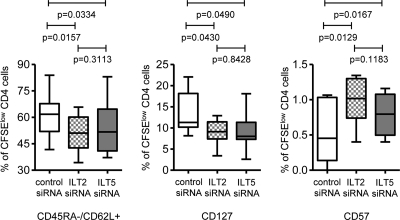
To analyze whether ILT2 (LILRB1) and ILT5 (LILRB3) also affect the ability of mDC to induce a memory cell-like phenotype in T cells, we assessed the phenotype of allogeneic T cells that proliferated after exposure to MDDC manipulated with siRNA targeting ILT2 or ILT5. As shown by the results in Fig. 6, downregulation of ILT2 (LILRB1) and ILT5 (LILRB3) in MDDC resulted in lower expression intensities of the memory markers CD62L and CD127 on proliferating allogeneic T cells while significantly enhancing the expression of CD57. These data indicate that ILT2 (LILRB1) and ILT5 (LILRB3) can contribute to the ability of mDC to generate allostimulatory T cell responses with memory cell-like properties.
FIG. 6.
Effect of ILT2/ILT5 expression on dendritic cells on memory phenotype of allogeneic CD4 T cell responses. Box-and-whisker plots reflect the proportions of proliferating allogeneic T cells expressing the indicated surface markers following stimulation with MDDC manipulated with siRNA targeting ILT2/ILT5 or nontargeting control siRNA. Results using MDDC from 8 HIV-1-negative donors are shown.
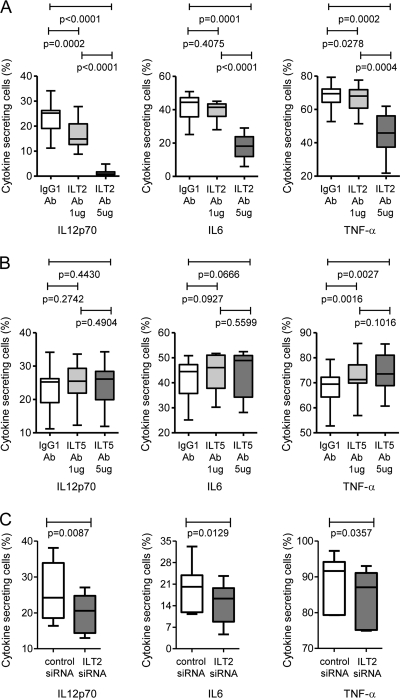
ILT2 (LILRB1) and ILT5 (LILRB3) differentially affect the cytokine secretion properties of mDC.
In our last series of experiments, we analyzed the effect of ILT2 (LILRB1) and ILT5 (LILRB3) on the cytokine secretion properties of mDC. For this purpose, PBMC were stimulated with TLR7/8 ligands in the presence or absence of ILT2/5-blocking monoclonal antibodies, and the secretion of TNF-α, IL-6, and IL-12p70 by mDC was assessed after 20 h using flow cytometry. As shown by the results in Fig. 7 A and B, blocking of ILT2 resulted in reduced secretion of all of these cytokines, as opposed to ILT5-inhibiting antibodies, which moderately increased the secretion of IL-6 and TNF-α but did not affect the secretion of IL-12p70. While an ILT5-mediated inhibition of cytokine secretion is in line with prior data (13), a stimulation of cytokine secretion by ILT2 is unexpected based on previous studies (50). We therefore further studied the effect of ILT2 on the cytokine secretion profile of mDC using MDDC manipulated with ILT2-specific siRNA. These experiments showed that ILT2 silencing reduced the secretion of all three cytokines, thus confirming our initial observations made after blocking mDC with ILT2-specific antibodies (Fig. 7C). Overall, these data show that ILT2 (LILRB1) and ILT5 (LILRB3) have opposing effects on the cytokine secretion properties of mDC.
FIG. 7.
Influence of ILT2/ILT5 on the cytokine secretion properties of dendritic cells. (A and B) Secretion of IL-12p70, IL-6, or TNF-α by mDC after stimulation with TLR7/8 ligands in the presence of ILT2 (A) or ILT5 (B) antibodies (n = 10 study persons). (C) Proportions of MDDC secreting the indicated cytokines after manipulation with ILT2-specific siRNA (n = 6 study persons).
DISCUSSION
HIV-1 elite controllers can spontaneously control HIV-1 viremia to undetectable levels and may serve as a model for successful and highly efficient immune activity against HIV-1. The identification of immunological characteristics specific for this patient population is therefore one of the most promising approaches for detecting components of immune protection against HIV-1. Here, we describe unique antigen-presenting and cytokine-secretion properties of circulating mDC in elite controllers that clearly distinguish this specific patient population from individuals with progressive HIV-1 infection, as well as from HIV-1-negative persons. These specific functional characteristics of mDC were associated with a unique phenotypic upregulation of ILT2 (LILRB1) and ILT5 (LILRB3), and functional assays indicated that these receptors are indeed critically involved in mediating and maintaining the specific antigen-presenting properties of mDC in elite controllers. Since the observed functional properties of dendritic cells in controllers differed from those in both HIV-1 progressors and HIV-1-negative individuals, they are unlikely to merely reflect an epiphenomenon of controlled HIV-1 viremia but may indeed represent a key element of immune protection against HIV-1 infection.
Prior data have linked the elite controller phenotype mainly to HIV-1-specific T cell immune responses, while a specific contribution of innate and humoral components of the immune system to HIV-1-directed host defense mechanisms in elite controllers has been less well documented (7). Our data are the first to show that myeloid dendritic cells, a key component of the innate immune system, have specific and distinct functional properties in elite controllers that clearly distinguish them from populations with an alternative HIV status. In terms of cellular immunity, we found that dendritic cells from elite controllers had significantly enhanced antigen-presenting characteristics and were able to sensitize allogeneic T cells more effectively than dendritic cells from HIV-1-negative persons or HIV-1 progressors. Moreover, in addition to these quantitative differences in allostimulatory functions, dendritic cells from elite controllers preferentially induced a memory cell-like T cell response, as opposed to dendritic cells from progressors, which generated more differentiated effector cell-like T cell responses. Strong HIV-1-specific T cell responses with polyfunctional and memory cell-like properties have indeed been described in elite controllers by numerous investigators (6, 29, 32), but the mechanisms involved in the generation and maintenance of such polyfunctional memory-like T cell responses in elite controllers have remained unclear so far. Since we were unable to identify HIV-1-infected T cell donors whose HLA alleles completely matched those of our study patients, we did not directly assess the effect of dendritic cells on proliferative HIV-1-specific T cell responses; however, our data suggest that the unique allostimulatory properties of dendritic cells from elite controllers may play a critical role in the development of T cell immunity against HIV-1 and contribute to a specific network between innate dendritic cells and adaptive T cell immune responses in elite controllers.
In contrast to the significantly enhanced allostimulatory properties of dendritic cells from elite controllers, their humoral cytokine secretion activities were significantly reduced in comparison to those of our reference cohorts. In particular, we found that dendritic cells from elite controllers secreted less TNF-α, IL-6, and IL-12p70. These molecules have key regulatory roles for recruiting and activating inflammatory cells and are likely involved in the elevated levels of immune activation that have been shown to play prominent parts in the pathogenesis of progressive HIV-1 infection (3, 26). Thus, the reduced secretion of proinflammatory cytokines in elite controllers may represent a specific protective mechanism against immune overactivation and, in this way, contribute to resistance against HIV-1 disease manifestations. Indeed, a significant proportion of elite controllers, including the patients in this study, maintain normal CD4 T cell counts and have no evidence of increased immune activation (5, 23, 44), despite the fact that low-level HIV-1 viremia remains detectable in the majority of these patients with the use of ultrasensitive assays (37). However, a smaller proportion of elite controllers do have signs of progressive HIV-1 infection with declining CD4 T cell counts and higher levels of immune activation (22, 23), and it will be important to determine the functional properties of mDC in this subset of elite controllers in future studies.
Using experiments with blocking monoclonal antibodies and siRNA-mediated gene knockout, we found that the antigen-presenting properties of mDC in elite controllers are mediated at least in part by a selective upregulation of the immunomodulatory receptors ILT2 (LILRB1) and ILT5 (LILRB3). Whether this upregulation occurs constitutively as a genetically determined process or is part of a specific immune response to HIV-1 infection in elite controllers is unclear at present; experiments involving biological relatives of elite controllers or the analysis of epigenetic factors influencing ILT (LILR) receptor expression will be helpful in that context. Moreover, the genes for ILT2 (LILRB1) (27) and ILT5 (LILRB3) (13, 38) are highly polymorphic, and specific genetic mutations within these receptors might be critically involved in the unique expression and function of these molecules in elite controllers. Notably, both ILT2 (LILRB1) and ILT5 (LILRB3) cause intracellular signal transduction via immunoreceptor tyrosine-based motifs (13) and have been implicated in inhibitory immune regulation in T cells (24, 41) and NK cells (49), although ILT2 (LILRB1)-mediated activation in lymphocytes and NK cells has also been reported (42, 43). In dendritic cells, ILT2 (LILRB1) receptor engagement with antibodies or cytomegalovirus-encoded human HLA homologues resulted in a number of different effects, including alterations of cytokine production, protection against apoptosis, and increased survival in tissue culture systems (50). Our experiments extend these observations by showing that both ILT2 (LILRB1) and ILT5 (LILRB3) can increase the allostimulatory properties of mDC and enhance their ability to generate T cell responses with phenotypic properties of memory cells. Moreover, based on the results of our experiments with blocking antibodies, these receptors had an opposing effect on the cytokine secretion properties of dendritic cells, with stimulatory effects mediated by ILT2 (LILRB1) and inhibitory impulses mediated by ILT5 (LILRB3). Overall, these data suggest that the regulation of the specific cytokine secretion profile of mDC in elite controllers cannot simply be attributed to the effects of ILT2 and ILT5 but is likely to be more complex. Future studies will therefore be necessary to determine how alternative immunomodulatory receptors, such as C-type lectins (18), or possible differences in TLR signaling may contribute to the diminished cytokine secretion properties of mDC in elite controllers.
The data shown here emphasize the important role that mDC may play in the future design of immunological strategies for HIV-1 treatment and prevention and suggest that the clinical manipulation of LILR-mediated functional properties of dendritic cells might offer novel perspectives for the design of prophylactic or therapeutic HIV-1 vaccines. Notably, dendritic cell-based immunotherapeutic interventions are among the few that have ever led to beneficial clinical effects in both simian immunodeficiency virus (SIV)-infected monkeys (31) and HIV-1-infected humans (30), although the molecular mechanisms responsible for their clinical efficacy were less clear. Our data suggest that the manipulation of ILT2 (LILRB1)- and ILT5 (LILRB3)-mediated functional activities of mDC might be a promising strategy for increasing host immune defense mechanisms against HIV-1 and, thus, may be considered in the future development of dendritic cell-based approaches to HIV-1 treatment and prevention.
In conclusion, our data reveal a unique combination of increased antigen-presenting properties with reduced secretion of proinflammatory cytokines in mDC from HIV-1 elite controllers that is in part mediated and maintained by a distinct and selective expression pattern of immunoregulatory LILRs. This specific functional profile of mDC in elite controllers may allow for the highly effective generation of memory-like T cell responses while simultaneously protecting against HIV-1-associated immune overactivation. These observations contribute importantly to the understanding of specific immunological properties of elite controllers and will be helpful for the rational design of immunological approaches for HIV-1 treatment and prevention.
Acknowledgments
This study was supported by the U.S. National Institutes of Health (grants AI078799 and AI074415 to X.G.Y.). X.G.Y. and M.L. are both recipients of a Doris Duke Clinical Scientist Development Award. The recruitment of study patients was supported by the William and Melinda Gates Foundation and the Mark and Lisa Schwartz Foundation.
We thank Rachel Allen (University of London, United Kingdom) and Daniel Kavanagh (Ragon Institute, Boston, MA) for helpful discussions and a critical review of the manuscript.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Allan, D. S., A. J. McMichael, and V. M. Braud. 2000. The ILT family of leukocyte receptors. Immunobiology 202:34-41. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, M., M. Cordero, J. Almeida, and A. Orfao. 2005. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS 19:261-271. [PubMed] [Google Scholar]
- 3.Appay, V., and D. Sauce. 2008. Immune activation and inflammation in HIV-1 infection: causes and consequences. J. Pathol. 214:231-241. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 5.Bello, G., C. A. Velasco-de-Castro, V. Bongertz, C. A. Rodrigues, C. B. Giacoia-Gripp, J. H. Pilotto, B. Grinsztejn, V. G. Veloso, and M. G. Morgado. 2009. Immune activation and antibody responses in non-progressing elite controller individuals infected with HIV-1. J. Med. Virol. 81:1681-1690. [DOI] [PubMed] [Google Scholar]
- 6.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blankson, J. N. 2010. Effector mechanisms in HIV-1 infected elite controllers: highly active immune responses? Antiviral Res. 85:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blankson, J. N., J. R. Bailey, S. Thayil, H. C. Yang, K. Lassen, J. Lai, S. K. Gandhi, J. D. Siliciano, T. M. Williams, and R. F. Siliciano. 2007. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 81:2508-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley, J. M., N. J. Karandikar, M. R. Betts, D. R. Ambrozak, B. J. Hill, L. E. Crotty, J. P. Casazza, J. Kuruppu, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711-2720. [DOI] [PubMed] [Google Scholar]
- 10.Brown, D., J. Trowsdale, and R. Allen. 2004. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 64:215-225. [DOI] [PubMed] [Google Scholar]
- 11.Chang, C. C., R. Ciubotariu, J. S. Manavalan, J. Yuan, A. I. Colovai, F. Piazza, S. Lederman, M. Colonna, R. Cortesini, R. Dalla-Favera, and N. Suciu-Foca. 2002. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat. Immunol. 3:237-243. [DOI] [PubMed] [Google Scholar]
- 12.Chehimi, J., L. Azzoni, M. Farabaugh, S. A. Creer, C. Tomescu, A. Hancock, A. Mackiewicz, L. D'Alessandro, S. Ghanekar, A. S. Foulkes, K. Mounzer, J. Kostman, and L. J. Montaner. 2007. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J. Immunol. 179:2642-2650. [DOI] [PubMed] [Google Scholar]
- 13.Colonna, M., F. Navarro, T. Bellon, M. Llano, P. Garcia, J. Samaridis, L. Angman, M. Cella, and M. Lopez-Botet. 1997. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 186:1809-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colonna, M., J. Samaridis, M. Cella, L. Angman, R. L. Allen, C. A. O'Callaghan, R. Dunbar, G. S. Ogg, V. Cerundolo, and A. Rolink. 1998. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J. Immunol. 160:3096-3100. [PubMed] [Google Scholar]
- 15.Dillon, S. M., K. B. Robertson, S. C. Pan, S. Mawhinney, A. L. Meditz, J. M. Folkvord, E. Connick, M. D. McCarter, and C. C. Wilson. 2008. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J. Acquir. Immune Defic. Syndr. 48:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101:4505-4511. [DOI] [PubMed] [Google Scholar]
- 17.Fellay, J., K. V. Shianna, D. Ge, S. Colombo, B. Ledergerber, M. Weale, K. Zhang, C. Gumbs, A. Castagna, A. Cossarizza, A. Cozzi-Lepri, A. De Luca, P. Easterbrook, P. Francioli, S. Mallal, J. Martinez-Picado, J. M. Miro, N. Obel, J. P. Smith, J. Wyniger, P. Descombes, S. E. Antonarakis, N. L. Letvin, A. J. McMichael, B. F. Haynes, A. Telenti, and D. B. Goldstein. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317:944-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T. B., and S. I. Gringhuis. 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9:465-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassi, F., A. Hosmalin, D. McIlroy, V. Calvez, P. Debre, and B. Autran. 1999. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS 13:759-766. [DOI] [PubMed] [Google Scholar]
- 20.Huang, J., J. J. Goedert, E. J. Sundberg, T. D. Cung, P. S. Burke, M. P. Martin, L. Preiss, J. Lifson, M. Lichterfeld, M. Carrington, and X. G. Yu. 2009. HLA-B*35-Px-mediated acceleration of HIV-1 infection by increased inhibitory immunoregulatory impulses. J. Exp. Med. 206:2959-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, Y., W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang, T. He, S. Kang, D. Ceradini, Z. Jin, K. Yazdanbakhsh, K. Kunstman, D. Erickson, E. Dragon, N. R. Landau, J. Phair, D. D. Ho, and R. A. Koup. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 2:1240-1243. [DOI] [PubMed] [Google Scholar]
- 22.Hunt, P. W. 2009. Natural control of HIV-1 replication and long-term nonprogression: overlapping but distinct phenotypes. J. Infect. Dis. 200:1636-1638. [DOI] [PubMed] [Google Scholar]
- 23.Hunt, P. W., J. Brenchley, E. Sinclair, J. M. McCune, M. Roland, K. Page-Shafer, P. Hsue, B. Emu, M. Krone, H. Lampiris, D. Douek, J. N. Martin, and S. G. Deeks. 2008. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 197:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ince, M. N., B. Harnisch, Z. Xu, S. K. Lee, C. Lange, L. Moretta, M. Lederman, and J. Lieberman. 2004. Increased expression of the natural killer cell inhibitory receptor CD85j/ILT2 on antigen-specific effector CD8 T cells and its impact on CD8 T-cell function. Immunology 112:531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavanagh, D. G., D. E. Kaufmann, S. Sunderji, N. Frahm, S. Le Gall, D. Boczkowski, E. S. Rosenberg, D. R. Stone, M. N. Johnston, B. S. Wagner, M. T. Zaman, C. Brander, E. Gilboa, B. D. Walker, and N. Bhardwaj. 2006. Expansion of HIV-specific CD4+ and CD8+ T cells by dendritic cells transfected with mRNA encoding cytoplasm- or lysosome-targeted Nef. Blood 107:1963-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelleher, P. 2008. Immune activation and viraemia: reciprocal interactions in disease pathogenesis. J. HIV Ther. 13:76-78. [PubMed] [Google Scholar]
- 27.Kuroki, K., N. Tsuchiya, M. Shiroishi, L. Rasubala, Y. Yamashita, K. Matsuta, T. Fukazawa, M. Kusaoi, Y. Murakami, M. Takiguchi, T. Juji, H. Hashimoto, D. Kohda, K. Maenaka, and K. Tokunaga. 2005. Extensive polymorphisms of LILRB1 (ILT2, LIR1) and their association with HLA-DRB1 shared epitope negative rheumatoid arthritis. Hum. Mol. Genet. 14:2469-2480. [DOI] [PubMed] [Google Scholar]
- 28.Lichterfeld, M., D. G. Kavanagh, K. L. Williams, B. Moza, S. K. Mui, T. Miura, R. Sivamurthy, R. Allgaier, F. Pereyra, A. Trocha, M. Feeney, R. T. Gandhi, E. S. Rosenberg, M. Altfeld, T. M. Allen, R. Allen, B. D. Walker, E. J. Sundberg, and X. G. Yu. 2007. A viral CTL escape mutation leading to immunoglobulin-like transcript 4-mediated functional inhibition of myelomonocytic cells. J. Exp. Med. 204:2813-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichterfeld, M., D. Mou, T. D. Cung, K. L. Williams, M. T. Waring, J. Huang, F. Pereyra, A. Trocha, G. J. Freeman, E. S. Rosenberg, B. D. Walker, and X. G. Yu. 2008. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood 112:3679-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu, W., L. C. Arraes, W. T. Ferreira, and J. M. Andrieu. 2004. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 10:1359-1365. [DOI] [PubMed] [Google Scholar]
- 31.Lu, W., X. Wu, Y. Lu, W. Guo, and J. M. Andrieu. 2003. Therapeutic dendritic-cell vaccine for simian AIDS. Nat. Med. 9:27-32. [DOI] [PubMed] [Google Scholar]
- 32.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 33.Migueles, S. A., C. M. Osborne, C. Royce, A. A. Compton, R. P. Joshi, K. A. Weeks, J. E. Rood, A. M. Berkley, J. B. Sacha, N. A. Cogliano-Shutta, M. Lloyd, G. Roby, R. Kwan, M. McLaughlin, S. Stallings, C. Rehm, M. A. O'Shea, J. Mican, B. Z. Packard, A. Komoriya, S. Palmer, A. P. Wiegand, F. Maldarelli, J. M. Coffin, J. W. Mellors, C. W. Hallahan, D. A. Follman, and M. Connors. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura, T., M. A. Brockman, C. J. Brumme, Z. L. Brumme, J. M. Carlson, F. Pereyra, A. Trocha, M. M. Addo, B. L. Block, A. C. Rothchild, B. M. Baker, T. Flynn, A. Schneidewind, B. Li, Y. E. Wang, D. Heckerman, T. M. Allen, and B. D. Walker. 2008. Genetic characterization of human immunodeficiency virus type 1 in elite controllers: lack of gross genetic defects or common amino acid changes. J. Virol. 82:8422-8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima, H., J. Samaridis, L. Angman, and M. Colonna. 1999. Human myeloid cells express an activating ILT receptor (ILT1) that associates with Fc receptor gamma-chain. J. Immunol. 162:5-8. [PubMed] [Google Scholar]
- 36.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563-571. [DOI] [PubMed] [Google Scholar]
- 37.Pereyra, F., S. Palmer, T. Miura, B. L. Block, A. Wiegand, A. C. Rothchild, B. Baker, R. Rosenberg, E. Cutrell, M. S. Seaman, J. M. Coffin, and B. D. Walker. 2009. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 200:984-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfistershammer, K., A. Lawitschka, C. Klauser, J. Leitner, R. Weigl, M. H. Heemskerk, W. F. Pickl, O. Majdic, G. A. Bohmig, G. F. Fischer, H. T. Greinix, and P. Steinberger. 2009. Allogeneic disparities in immunoglobulin-like transcript 5 induce potent antibody responses in hematopoietic stem cell transplant recipients. Blood 114:2323-2332. [DOI] [PubMed] [Google Scholar]
- 39.Reis e Sousa, C. 2004. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 16:21-25. [DOI] [PubMed] [Google Scholar]
- 40.Saez-Cirion, A., M. Sinet, S. Y. Shin, A. Urrutia, P. Versmisse, C. Lacabaratz, F. Boufassa, V. Avettand-Fenoel, C. Rouzioux, J. F. Delfraissy, F. Barre-Sinoussi, O. Lambotte, A. Venet, and G. Pancino. 2009. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J. Immunol. 182:7828-7837. [DOI] [PubMed] [Google Scholar]
- 41.Saverino, D., M. Fabbi, F. Ghiotto, A. Merlo, S. Bruno, D. Zarcone, C. Tenca, M. Tiso, G. Santoro, G. Anastasi, D. Cosman, C. E. Grossi, and E. Ciccone. 2000. The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J. Immunol. 165:3742-3755. [DOI] [PubMed] [Google Scholar]
- 42.Saverino, D., F. Ghiotto, A. Merlo, S. Bruno, L. Battini, M. Occhino, M. Maffei, C. Tenca, S. Pileri, L. Baldi, M. Fabbi, A. Bachi, A. De Santanna, C. E. Grossi, and E. Ciccone. 2004. Specific recognition of the viral protein UL18 by CD85j/LIR-1/ILT2 on CD8+ T cells mediates the non-MHC-restricted lysis of human cytomegalovirus-infected cells. J. Immunol. 172:5629-5637. [DOI] [PubMed] [Google Scholar]
- 43.Scott-Algara, D., V. Arnold, C. Didier, T. Kattan, G. Pirozzi, F. Barre-Sinoussi, and G. Pancino. 2008. The CD85j+ NK cell subset potently controls HIV-1 replication in autologous dendritic cells. PLoS One 3:e1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sedaghat, A. R., D. A. Rastegar, K. A. O'Connell, J. B. Dinoso, C. O. Wilke, and J. N. Blankson. 2009. T cell dynamics and the response to HAART in a cohort of HIV-1-infected elite suppressors. Clin. Infect. Dis. 49:1763-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sloane, D. E., N. Tedla, M. Awoniyi, D. W. Macglashan, Jr., L. Borges, K. F. Austen, and J. P. Arm. 2004. Leukocyte immunoglobulin-like receptors: novel innate receptors for human basophil activation and inhibition. Blood 104:2832-2839. [DOI] [PubMed] [Google Scholar]
- 46.Smith, M. W., M. Dean, M. Carrington, C. Winkler, G. A. Huttley, D. A. Lomb, J. J. Goedert, T. R. O'Brien, L. P. Jacobson, R. Kaslow, S. Buchbinder, E. Vittinghoff, D. Vlahov, K. Hoots, M. W. Hilgartner, and S. J. O'Brien. 1997. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science 277:959-965. [DOI] [PubMed] [Google Scholar]
- 47.Steinman, R. M., and H. Hemmi. 2006. Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 311:17-58. [DOI] [PubMed] [Google Scholar]
- 48.Reference deleted.
- 49.Vitale, M., R. Castriconi, S. Parolini, D. Pende, M. L. Hsu, L. Moretta, D. Cosman, and A. Moretta. 1999. The leukocyte Ig-like receptor (LIR)-1 for the cytomegalovirus UL18 protein displays a broad specificity for different HLA class I alleles: analysis of LIR-1+ NK cell clones. Int. Immunol. 11:29-35. [DOI] [PubMed] [Google Scholar]
- 50.Young, N. T., E. C. Waller, R. Patel, A. Roghanian, J. M. Austyn, and J. Trowsdale. 2008. The inhibitory receptor LILRB1 modulates the differentiation and regulatory potential of human dendritic cells. Blood 111:3090-3096. [DOI] [PubMed] [Google Scholar]