Abstract
Influenza virus polymerase initiates the biosynthesis of its own mRNAs with capped 10- to 13-nucleotide fragments cleaved from cellular (pre-)mRNAs. Two activities are required for this cap-snatching activity: specific binding of the cap structure and an endonuclease activity. Recent work has shown that the cap-binding site is situated in the central part of the PB2 subunit and that the endonuclease activity is situated in the N-terminal domain of the PA subunit (PA-Nter). The influenza endonuclease is a member of the PD-(D/E)XK family of nucleases that use divalent metal ions for nucleic acid cleavage. Here we analyze the metal binding and endonuclease activities of eight PA-Nter single-point mutants. We show by calorimetry that the wild-type active site binds two Mn2+ ions and has a 500-fold higher affinity for manganese than for magnesium ions. The endonuclease activity of the isolated mutant domains are compared with the cap-dependent transcription activities of identical mutations in trimeric recombinant polymerases previously described by other groups. Mutations that inactivate the endonuclease activity in the isolated PA-Nter knock out the transcription but not replication activity in the recombinant polymerase. We confirm the importance of a number of active-site residues and identify some residues that may be involved in the positioning of the RNA substrate in the active site. Our results validate the use of the isolated endonuclease domain in a drug-design process for new anti-influenza virus compounds.
Influenza virus is a segmented negative-strand RNA virus that replicates in the nucleus of infected cells. Its eight viral RNA (vRNA) segments are covered by the viral nucleoprotein (NP) with a stoichiometry of 24 nucleotides per nucleoprotein protomer (25). The 3′ and 5′ ends of the vRNA are bound to the RNA-dependent RNA polymerase, a heterotrimeric complex composed of PB1, PB2, and PA. The complex between vRNA, NP, and the polymerase is called a ribonucleoprotein particle (RNP). Both ends of the viral RNA are necessary for polymerase activity, and together they form the promoter for transcription (12, 18, 33). Influenza virus RNA polymerase differs from the polymerases of the nonsegmented negative-strand RNA viruses, in that it does not carry the enzymatic functions required for the 5′ capping of its own mRNAs (guanylyl- and methyltransferase activities). Instead, influenza virus mRNAs are capped by a unique cap-snatching mechanism (27). The polymerase binds to cellular pre-mRNAs via their cap structure and then cleaves them to generate capped 10- to 13-residue oligonucleotides, which are used as primers to initiate the transcription of viral mRNAs. The viral mRNAs are terminated by a 3′-end poly(A) sequence generated by the stuttering of the RNA polymerase at an oligo(U) motif located at the 5′ end of the template (28, 29). The endonuclease activity for cleavage of the host mRNAs is not active in recombinant trimeric polymerase in the absence of vRNA. For viral transcription, the binding of the 5′ end of the genomic vRNA is necessary to stimulate the cap binding (4). The binding of both ends of the vRNA is also required to stimulate the endonuclease activity (12), and direct binding of annealed 3′ and 5′ vRNA ends stimulates cap binding and endonuclease activity even more strongly (22). These results suggest that significant conformational changes may take place in the RNA polymerase complex upon binding of the vRNA. When the three-dimensional (3D) electron microscopy model of the polymerase on a recombinant RNP was compared with that of free recombinant polymerase, structural differences were indeed observed (1, 5, 35).
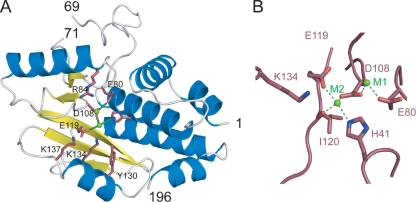
The cap-binding site was known to be located within the PB2 subunit (23) but was only recently shown by structural analysis to reside in an independently folding domain of the PB2 subunit, between amino acids 320 and 483 (10). This isolated domain binds to cap analogues in the absence of other parts of the RNA polymerase and of vRNA. The position of the endonuclease site was also controversial but has recently been proven to reside in an also independently folding amino-terminal domain of the PA subunit (6, 37). The crystal structure of the first 196 residues of PA (PA-Nter) (Fig. 1A) shows structural homology with nucleases of the PD-(D/E)XK superfamily, which contains bacterial restriction enzymes such as Escherichia coli EcoRV and Pyrococcus furiosus Holiday junction resolvase. This family of enzymes binds to one or two divalent metal ions, in particular, Mg2+ or Mn2+. One of the ions is clearly involved in catalysis, whereas the role of the second one is not clearly established and may have a modulatory role (inhibition or stimulation, depending on the concentration and the nature of the ion) (17, 26). PA-Nter shows endonuclease activity in the absence of the rest of the polymerase and has the same metal ion dependence as the polymerase in intact RNPs. The domain cleaves most efficiently with manganese ions, followed by cobalt ions (6, 7). In the crystal structure of PA-Nter determined by Dias et al., two divalent cation sites were identified, and both are occupied by manganese (6); metal site 1 (M1) is liganded by Glu80, Asp108, and Glu119 (through a water molecule); and metal site 2 (M2) is liganded by His41, Asp108, Glu119, and the carbonyl oxygen of Ile120 (Fig. 1A and B). On the other hand, Yuan and coworkers crystallized PA-Nter in the presence of only MgCl2 and observed a single Mg2+ ion in site M1 (37). The active site also contains Lys134, which could correspond to the catalytic lysine of the PD-(D/E)XK motif (6, 37, 38).
FIG. 1.
Active site of PA-Nter. (A) Ribbon diagram of the structure of influenza A/Victoria/3/1975 PA-Nter (Protein Data Bank accession number 2W69) with α helices in blue and β strands in yellow. The key active-site residues mutated in this study are indicated in pink, and the manganese ions are in green. (B) Blowup of the active site of PA-Nter indicating the two metal binding sites and the metal binding ligands.
Both the isolated cap-binding domain of PB2 and the endonuclease domain of PA have significant advantages compared to intact viral RNPs for inhibitor screening and structure-based antiviral drug optimization. However, it needs to be ascertained that the isolated domains have the same function and activity as the domains inside the intact trimeric RNA polymerase. A mutational analysis of the cap-binding domain confirmed that mutations in the isolated domain have the same effects as those in the context of the intact polymerase (10). Here we present the results of a mutational analysis of the active site of the isolated endonuclease domain. The mutations include the active-site residues involved in metal binding (His41, Glu80, Asp108, Glu119), the putative catalytic Lys134 residue, and three strictly conserved residues located on the rim surrounding the active site: Arg84, Tyr130, and Lys137 (Fig. 1A). The effects of these mutations introduced in the isolated domain on endonuclease activity are compared to those already tested in the intact recombinant polymerase (13, 37). We also tested the affinities of Mn2+ and Mg2+ ions for the wild-type (wt) and mutant endonuclease domains and correlate metal binding with enzymatic activity.
MATERIALS AND METHODS
Mutants.
The clones containing the mutated DNA coding for PA-Nter (residues 1 to 209), cloned in pETM11, were obtained from Geneart (Germany). The clones code for a poly(His) sequence separated from the protein-coding sequence by a TEV cleavage site. The vectors were used to transform the E. coli BL21(DE3) RIL CodonPlus strain (Stratagene). The mutant proteins were expressed in LB medium overnight at 15°C after induction with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). All PA-Nter mutants were expressed in the soluble fraction of the bacterial cells and were purified by an immobilized metal affinity column (IMAC). A second IMAC step was performed after cleavage by the His-tagged TEV protease, followed by gel filtration on a Superdex 200 column (GE Healthcare). Finally, the protein was concentrated to 5 to 10 mg·ml−1. For H41A and H41E, the yields were only about 5% of those obtained for the other mutants. The elution profile during the final gel filtration step showed the presence of aggregated material for these mutants.
Biophysical characterization. (i) Far-UV CD spectra.
Far-UV circular dichroism (CD) spectra were recorded with a 1-mm path length at 20°C on a Jasco model J-810 CD spectropolarimeter equipped with a Peltier thermostat, as described previously (6). The PA-Nter concentration was 10 μM in 10 mM Tris-HCl, pH 7.0-10 mM NaCl.
(ii) Thermal shift assays.
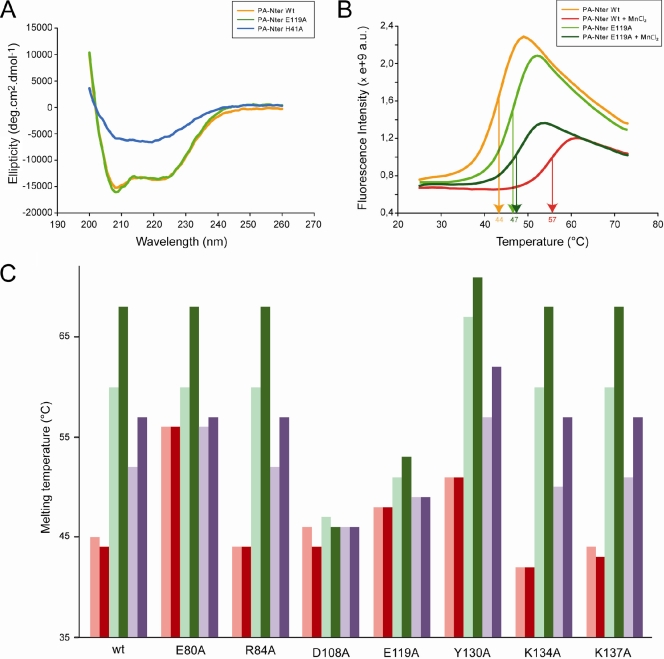
Thermal shift assays were performed with 10 μM PA-Nter in 20 mM Tris-HCl, pH 7.0-100 mM NaCl and a 5× dilution of SYPRO orange dye (Invitrogen), as described. The dye was excited at 490 nm, and the emission light was recorded at 575 nm while the temperature was increased by increments of 1°C per minute from 25 to 75°C. Control assays were carried out in the absence of protein or dye to check that no fluorescence signal was recorded. The experiments gave virtually identical results when they were performed under the same conditions. The variation between experiments came only from the estimation of the flexion point of the curve and was less than 0.5°C in triplicate experiments. For this reason, the results shown in Fig. 2C do not have error bars.
FIG. 2.
Biophysical characterization of PA-Nter mutants. (A) Circular dichroism on wt PA-Nter and two of the eight mutants. CD spectra of the wt, H41A, and E119A PA-Nters are in orange, blue, and green, respectively. (B) Thermal stabilization by MnCl2. Thermal shift assays on wt and E119A PA-Nters were done in the presence (red and dark green, respectively) or absence (orange and green, respectively) of 0.5 mM MnCl2. (C) Effects of metals and DPBA binding on the thermal stability. Thermal shift assays to test the metal ion stabilization were performed on wt PA-Nter and the corresponding mutants in the presence (dark colors) and absence (light colors) of DPBA. The proteins were incubated with 0.1 mM MnCl2 (green) or 5 mM MgCl2 (violet) or without any metal (red). All experiments were repeated at least three times on different occasions and with different protein preparations. The results were identical when the same experimental conditions were used.
(iii) Isothermal titration calorimetry.
Isothermal titration calorimetry (ITC) experiments were performed using a high-precision VP-ITC system (Microcal Inc., Northampton, MA). Proteins were first extensively dialyzed against the titration buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl). All solutions were filtered, degassed to avoid bubble formation, and equilibrated to the corresponding temperature before each experiment. Protein solutions at about 60 μM in the calorimetric cell were titrated with the appropriate metal (0.8 to 9 mM) dissolved in dialysis buffer. Depending on the binding affinities, titrations were carried out either by constant-volume injections (30 injections of 7.5 μl) or by increasing-volume injections (27 injections from 4 to 20 μl) in order to better define the titration curves. The heat evolved after each metal injection was obtained from the integral of the calorimetric signal. The resulting binding isotherms were analyzed by nonlinear least-squares fitting of the experimental data to models corresponding to a single set of identical sites or corresponding to two sets of independent sites. Analysis of the data was performed using the Microcal Origin program (OriginLab Corporation, Northampton, MA).
Endonuclease assays.
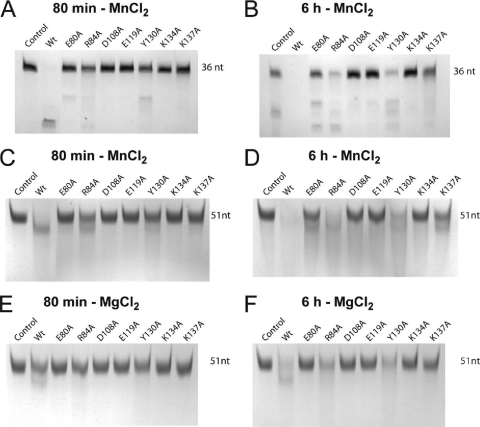
Endonuclease assays were carried out using an unstructured U-rich RNA probe of 51 nucleotides (6, 31) or a short panhandle RNA of 36 nucleotides comprising just the conserved 3′ and 5′ ends of the viral RNA with a short linker (6). RNA cleavage was performed by incubating 13 μM PA-Nter with various RNA substrates (all at 10 μM) at 37°C in a final volume of 50 μl. The reaction buffer was 20 mM Tris-HCl, pH 8, 100 mM NaCl, and 10 mM β-mercaptoethanol plus 1 mM MnCl2 or 1 mM MgCl2. Incubations were stopped by addition of EGTA at a final concentration of 20 mM. The reaction products were loaded on 8 M urea-15% polyacrylamide gels and stained with methylene blue. The results of these assays were not quantified. The activities were estimated from the disappearance of the band of the intact panhandle RNA after incubation for 80 min and 6 h as ++ for wt enzyme, + for mutants like R84A and Y130A that digested less than 50% of the substrate RNA after 80 min but all of it after 6 h, ± for mutants like K137A that still had a significant amount of intact RNA after 6 h, and − for mutants like D108A, E119A, and K134A that were inactive.
RESULTS
All PA-Nter mutants were expressed and purified, and all mutants except the H41A mutant had yields comparable to the yield of the wt. This mutant yielded only about 5% of that for the wild type, and the elution profile of the final gel filtration step showed the presence of aggregates. The folding of the mutants was checked by circular dichroism. All mutants showed a spectrum identical to that of the wt PA-Nter, as shown in Fig. 2A only for the E119A mutant, except that the H41A mutant showed little secondary structure (Fig. 2A).
Thermal stability.
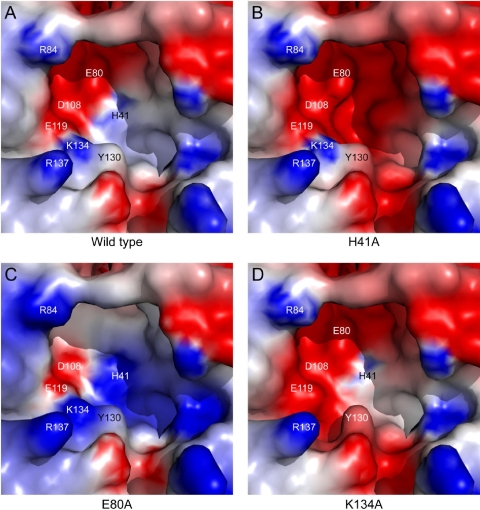
The thermal stability of the mutants was tested by Thermofluor assays in which a hydrophobic fluorophore has little affinity for native proteins but binds to denatured proteins, leading to an increase of the fluorescence (9). The apparent melting temperature (Tm) of denaturation can be obtained from the temperature dependence of the fluorescence (Fig. 2B). The thermal stability of the H41A mutant could not be derived since the fluorescent probe bound to the protein at room temperature, another indication that it is not properly folded. The R84A, D108A, and K137A PA-Nters have the same Tm as wt PA-Nter (Fig. 2C, pink bars) and the K134A PA-Nter is slightly less stable, whereas the E80A, E119A, and Y130A PA-Nters are more stable (Fig. 2C). The active site of wt PA-Nter is strongly negatively charged (Fig. 3A). In general, mutations that increase the negative charge in the active-site pocket destabilize the domain (e.g., see Fig. 3B and D for the H41A and K134A PA-Nters, respectively), whereas mutations that reduce the negative charge increase the stability of the domain (e.g., Fig. 3C for the E80A PA-Nter). The fact that the H41A PA-Nter does not fold properly suggests that a basic residue at position 41 is important to provide the necessary electrostatic compensation for correct folding.
FIG. 3.
Electrostatic surface potentials of the active sites of wt and three mutant PA-Nters. The surface of wt PA-Nter (A) was calculated from the crystal structure (Protein Data Bank accession number 2W69), whereas those of mutant PA-ters H41A (B), E80A (C), and K134A (D) correspond to models generated in silico. The electrostatic surfaces were calculated using the DelPhi program (30) with H41 fully protonated. The potential scales range from −5.0 kT/e (red) to 5.0 kT/e (blue).
Nuclease activity.
The RNase activity of the mutants was tested in the presence of 1 mM MnCl2 or 1 mM MgCl2 using short panhandle RNA (36 nucleotides) and unstructured U-rich RNA (51 nucleotides) as substrates (6). The substrate RNA was analyzed on gels after digestion for 80 min or 6 h, as shown in Fig. 4 and summarized in Table 1. Two of three mutants with mutations of acidic residues directly involved in metal binding (E80A, D108A, and E119A) are inactive both with MnCl2 and with MgCl2. The exception was the E80A mutant, which retained activity only in the presence of manganese ions. The K134A mutant is also completely inactive, which is consistent with it being the catalytic lysine. The R84A, Y130A, and K137A mutants situated on the active-site rim are all less active than the wild type to various degrees but are not inactive. Also note that the activities of the wild type and all active mutants were higher in the presence of 1 mM MnCl2 than in the presence of 1 mM MgCl2 (Fig. 4C to F).
FIG. 4.
Endonuclease activities of PA-Nter mutants.(A and B) RNase activities of wild-type and mutant PA-Nters in the presence of 1 mM MnCl2 using a short panhandle RNA of 36 nucleotides (6) for 80 min (A) and 6 h (B). (C to F) The same experiment described for panels A and B but using U-rich RNA (51 nucleotides) plus 1 mM MnCl2 (C and D) and using U-rich RNA plus 1 mM MgCl2 (E and F). RNA cleavage was performed by incubating 13 μM PA-Nter for 80 min (A, C, and E) or 6 h (B, D, and F) with 10 μM RNA at 37°C in a final volume of 50 μl.
TABLE 1.
Effects of mutations on RNase activity of PA-Nter and comparison with transcription and replication activities of the recombinant trimeric polymerase
| Mutation | Role | RNase activity |
Transcriptiona | Replicationa | |
|---|---|---|---|---|---|
| MnCl2 | MgCl2 | ||||
| wt | ++ | ++ | + | + | |
| H41A | Ligand Mn2 | NDb | ND | − | − |
| E80A | Ligand Mn1 | ± | − | − | + |
| R84A | RNA positioning | + | + | + | + |
| D108A | Ligands Mn1 and Mn2 | − | − | − | + |
| E119A | Ligand Mn2 | − | − | − | + |
| Y130A | RNA positioning | + | + | + | + |
| K134A | Catalytic | − | − | − | + |
| K137A | RNA positioning | ± | ± | + | + |
Metal binding monitored by thermal stabilization.
We previously showed that the addition of 0.5 mM Mn2+ ions significantly enhances the thermal stability of wt PA-Nter and that 0.5 mM dioxo-4-phenylbutanoic acid (DPBA), a known inhibitor of the influenza virus endonuclease (34), supershifts the Tm but only in the presence of metal ions (6). It is likely that the three oxygens on this inhibitor ligate the two resident metal ions in a similar manner, as has recently been observed in the integrase-inhibitor complex of retroviruses (14). To monitor metal binding to the mutant proteins, we therefore used the Thermofluor assay to measure the Tm in the presence of 0.1 mM MnCl2 (Fig. 2C, light green), 5 mM MgCl2 (Fig. 2C, light violet), or no metal (Fig. 2C, pink). We also measured the Tm in the presence of metal ions plus DPBA (Fig. 2C, red, dark green, and dark violet). Two of the three mutations that knock out the metal-ligating acidic residues (E80A, D108A, and E119A) also abolish metal ion binding. The exception was the E80A mutant, which was stabilized by Mn2+ but not by Mg2+ ions, consistent with the results of the endonuclease assay. All other mutations were stabilized by both types of cations and were further stabilized when DPBA was added in the presence of metal ions, similar to the result for the wt. In the absence of ions, the inhibitor did not stabilize the proteins.
Metal binding measured by ITC.
In the PD-(D/E)XK nuclease superfamily, the positions equivalent to Asp108, Glu119, and Lys134 are always present, giving the family its name. Glu80 and His41 are not always conserved, and indeed, this combination seems to be specific for the influenza virus endonuclease (6, 37). Glu80 is particularly interesting, as E80A shows binding and residual nuclease activity with Mn2+ ions but not with Mg2+ ions. In order to understand these observations, we directly measured Mn2+ and Mg2+ ion binding to wt PA-Nter and E80A by ITC (Fig. 5 and Table 2), which is the most appropriate technique for directly measuring the thermodynamics of protein-ligand binding (21). We find that binding of Mn2+ ions to wt PA-Nter is exothermic (Fig. 5A), whereas binding of Mg2+ ions is endothermic (Fig. 5B). The dissociation constants for the Mn2+ and Mg2+ ions are obtained by model fitting (Table 2), in which the number of binding sites and the values for the disassociation complex (Kd−1) and enthalpy change (ΔH) are variables.
FIG. 5.
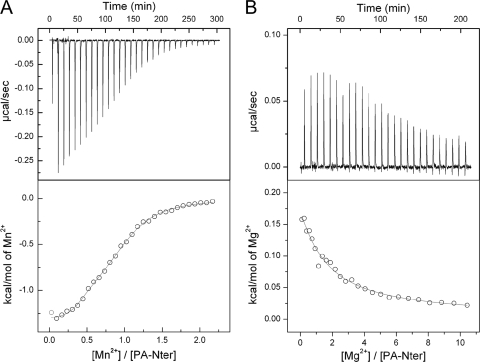
Isothermal titration calorimetry of wild-type PA-Nter with metal ions. (A) MnCl2 at 0.3 mM was added to 60 μM PA-Nter at 25°C in 20 mM Tris-HCl (pH 8.0) plus 100 mM NaCl; (B) MgCl2 at 9 mM was added to 60 μM PA-Nter at 25°C in 20 mM Tris-HCl (pH 8.0) plus 100 mM NaCl. In the lower panels, the circles represent experimental data and the continuous lines correspond to the best fit to a model with two binding sites.
TABLE 2.
Metal specificity of PA-Ntera
| Ion | Site |
Kd (μM) |
|
|---|---|---|---|
| wt | E80A | ||
| Mn2+ | High affinity | 0.3 | 5.2 |
| Low affinity | 6.5 | 46 | |
| Mg2+ | High affinity | 148 | |
| Low affinity | 4,000 | ||
Isothermal titration calorimetry experiments were performed on a VP-ITC calorimeter using protein solutions at a concentration of 60 μM and metal solution concentrations between 0.8 and 9 mM.
For the wt protein, the titration data for both Mn2+ and Mg2+ are best fitted with a two-site model since the quality of the fit (by the chi-square test) is better by a factor of 2 than that for a single-site model. Note that at this stage that we make no assumption about whether the two Mn2+ sites revealed by ITC are the same as the two Mg2+ sites or whether they correspond to sites M1 and M2 defined by the crystal structure (6). The affinity of wt PA-Nter for Mn2+ ions (Kds for the two sites, 0.3 and 6.5 μM) is 500 to 600 times higher than that for Mg2+ ions (Kds for the two sites, 148 and 4,000 μM). This is consistent with the higher endonuclease activity shown by wt PA-Nter in the presence of 1 mM Mn2+ compared to that in the presence of 1 mM Mg2+ ions (compare Fig. 4C and E). The measured affinities lie in the range of those obtained for other divalent cation binding proteins, such as RNAses and proteases (3, 20, 36). The fact that the binding enthalpy is exothermic for Mn2+ ions and endothermic for Mg2+ ions suggests a more optimal coordination for bound Mn2+ ions, which is reflected in a more favorable enthalpy.
Addition of MgCl2 to the E80A mutant produced no heat effect, in agreement with the absence of thermal stabilization and nuclease activity upon addition of Mg2+ ions. The binding data for Mn2+ could be modeled using a single site with a Kd of 77 μM and an occupancy of 2.1 or with two sites with Kd values of 5.2 and 46 μM. Because the chi-square test of the single-site model provides a value twice that of the two-site-model, we favor the two-site model. Although the Mn2+ binding affinities for the two-site model are only 10 times lower than those for wt PA-Nter, the endonuclease activity of this mutant is impaired in the presence of 1 mM MnCl2, which may be due to a difference in the geometry of metal binding due to the absence of Glu80.
DISCUSSION
This work was undertaken with dual aims: first, to determine whether mutations in the isolated endonuclease domain had the same effects on activity as the equivalent mutations made in the trimeric polymerase and, second, to clarify whether the nuclease activity of the domain depends on binding to one or two Mg2+ or Mn2+ ions.
Concerning the first aim, all the alanine mutations that we made in PA-Nter have already been studied in the context of the trimeric recombinant polymerase (13, 37). As Table 1 shows, mutations that inactivate the endonuclease activity in the isolated PA-Nter domain knock out transcription but not replication in the intact polymerase. The experiments with the intact recombinant polymerase were performed in the presence of MgCl2 without added MnCl2. Bearing in mind our results for this mutant, this could explain why the intact polymerase containing the E80A mutation was inactive in transcription in the assay of Hara and coworkers (13). The results for this mutation presented by Yuan and coworkers (37) are more ambiguous; although the mutant was inactive in the endonuclease assay, some globin mRNA-primed transcription activity was retained. Concerning the conserved Arg84, Tyr130, and Lys137 residues on the active-site rim, we hypothesized that these could be necessary for the binding and correct positioning of the substrate RNA in the active site. In particular, Arg84 is bound to a well-ordered sulfate ion in the crystal structure of PA-Nter (6). This sulfate is in the same position as one of the phosphates of the EcoRV restriction enzyme product complex (16). Tyr130 and Lys137 coordinate water molecules that bind to the monophosphate group in a complex of PA-Nter with nucleoside monophosphates (38). In the isolated endonuclease domain, the enzymatic activities of R84A, Y130A, and K137A were reduced, whereas in the context of the intact trimeric polymerase, the mutations retain full transcription activity (13). We may explain this difference by the fact that in the intact polymerase the prebinding of the cellular mRNA to the PB2 cap-binding domain probably considerably enhances substrate affinity by increasing the local concentration. Furthermore, the presence of other positively charged polymerase domains such as the highly basic surfaces of the PB2 627 domain (19, 32) and the C-terminal two-thirds of PA (15, 24) may also assist with the correct positioning of the substrate RNA over the endonuclease active site.
An additional observation is that the active site of the endonuclease is very acidic, like that of other endonucleases (Fig. 3A); and mutations that increase the negative charge destabilize the protein, whereas those that decrease the acidity stabilize PA-Nter. The single exception is the D108A mutant PA-Nter, which has the same stability as wt PA-Nter, for which we do not have an explanation. The H41A mutation in the context of the intact polymerase results in disruption not only of transcription but also of replication (13, 37). Therefore, it seems that the correct folding or the stability of PA-Nter is a prerequisite to the correct formation of the active site of the polymerase on the PB1 subunit. These observations also suggest a rationale for why PA-Nter has a histidine at position 41 rather than a glutamate, as in EcoRV (5).
In conclusion, the accordance of the activities of PA-Nter with those of the intact recombinant trimer suggests that isolated PA-Nter has the same structure in the context of the intact trimer and validates the use of the isolated domain for drug screening and structure-based design. A similar conclusion was drawn for the PB2 cap-binding domain, i.e., that the isolated domain has the same structure as that in recombinant RNP (10). This would imply that activation of the cap-binding and endonuclease functions in the intact polymerase, by the binding of the 5′ end of the vRNA or a 3′-5′ duplex (12, 22), are likely not due to the induced formation of the two active sites but rather to the removal of inhibition (perhaps steric) of these functions. The structural differences that are observed between free polymerase and RNP-bound polymerase (1, 5, 35) suggest important domain rearrangements that may change the disposition of the cap-binding and endonuclease sites and the overall binding of host mRNA by the intact polymerase.
Concerning the second aim of our study, in a careful enzymatic analysis of the endonuclease activity of purified RNPs, Doan et al. showed that there are two interacting metal binding sites that need to be occupied with divalent metal ions for full nuclease activity (7). They found that manganese ions are two times stronger than magnesium ions at activating the endonuclease and showed that the affinity for Mn2+ ions is stronger than for Mg2+ ions. The metal dependence of the endonuclease activity shown here is in agreement with these results. The nuclease activity of PA-Nter and the mutants is higher in the presence of 1 mM MnCl2 than in the presence of 1 mM MgCl2, and the shift in Tm is higher in the presence of 0.1 mM Mn2+ ions than in the presence of 5 mM Mg2+ ions.
Yuan and coworkers grew crystals of PA-Nter in the presence of 100 mM MgCl2 and observed a single metal ion only in position M1, even in the presence of mononucleotide phosphates (38). Therefore, in the absence of substrate, Mg2+ ions seem to bind only to the M1 site even at concentrations that lay several orders of magnitude above the low affinity Kd (4 mM). Because a second magnesium ion was never seen in the active site, it is possible that the low-affinity binding site for Mg2+ lies outside the active site. Histidine is one of the ligands of the M2 site in the crystal structure (6). Whereas manganese ions can be favorably coordinated by both acidic residues and histidine (2, 11), ligation of Mg2+ ions by histidine is uncommon (8). This agrees with the biochemical and enzymatic data presented here for the E80A mutant (Glu80 is an M1 ligand), which showed neither RNase activity nor binding of Mg2+ ions, although the mutant could still bind two manganese ions. All crystallographic data (37, 38) and our enzymatic and ITC data presented here suggest that only a single magnesium ion can bind to the enzymatic cavity of PA-Nter in the absence of substrate. However, Doan et al. (7) found a Hill coefficient of 2 for the endonuclease activity of intact viral RNPs in the presence of Mg2+, suggesting that two ions can bind in the presence of substrate. The finding that the stability of wt PA-Nter supershifts in the presence of Mg2+ plus the inhibitor also suggests that two ions can bind when PA-Nter is stabilized by the inhibitor.
Dias et al. grew crystals in a mixture of 2.5 mM MnCl2 and 5 mM MgCl2 and located two Mn2+ ions in positions M1 and M2 (6). Two manganese ions were also found by Zhao and coworkers upon adding MnCl2 (38). Our ITC results explain these structural observations, since they show that Mn2+ can bind with a high affinity to two sites (Kds, 0.3 and 6.5 μM) and that Mn2+ binding to its second site is 20-fold higher than that of Mg2+ ions to their high-affinity site (Kd, 148 μM). Although with these data we cannot prove that the two Mn2+ binding sites obtained through ITC correlate with the two binding sites observed in the crystal structure, it is likely that it is the case. This is supported by the results on activity and Mn2+ binding of the E80A mutant, which has an active site that resembles that of EcoRV, which also binds to two manganese ions.
In conclusion, our results are consistent with previous results on the metal dependence of the endonuclease in intact RNPs. We show quantitatively that the endonuclease active site binds to two Mn2+ ions and has a significantly higher affinity for Mn2+ ions than for Mg2+ ions. As was mentioned by Zhao et al., the cellular concentration of free magnesium ions is in the millimolar range, whereas that of manganese ions is in the micromolar range (38), making roles for both ions in the endonuclease activity during infection by influenza virus entirely possible. This suggestion is strengthened by the findings of Doan et al. that indicated a synergistic activation of cleavage activity with combinations of different metal ions (7).
Acknowledgments
We acknowledge the Partnership for Structural Biology for an integrated structural biology environment.
The work was partially funded by the EU FLUPOL contract (SP5B-CT-2007-044263), the ANR FLU INTERPOL contract (ANR-06-MIME-014-02), and Lyon Biopôle. Alexandre Dias was the recipient of a Ph.D. fellowship from the French MENRT.
Footnotes
Published ahead of print on 30 June 2010.
REFERENCES
- 1.Area, E., J. Martin-Benito, P. Gastaminza, E. Torreira, J. M. Valpuesta, J. L. Carrascosa, and J. Ortin. 2004. 3D structure of the influenza virus polymerase complex: localization of subunit domains. Proc. Natl. Acad. Sci. U. S. A. 101:308-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bewley, M. C., and J. M. Flanagan. 2001. Arginase, p. 952-962. In A. Messerschmidt, R. Huber, T. Poulos, and K. Wieghart (ed.), Handbook of metalproteins. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 3.Brazier, M. W., P. Davies, E. Player, F. Marken, J. H. Viles, and D. R. Brown. 2008. Manganese binding to the prion protein. J. Biol. Chem. 283:12831-12839. [DOI] [PubMed] [Google Scholar]
- 4.Cianci, C., L. Tiley, and M. Krystal. 1995. Differential activation of the influenza virus polymerase via template RNA binding. J. Virol. 69:3995-3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coloma, R., J. M. Valpuesta, R. Arranz, J. L. Carrascosa, J. Ortin, and J. Martin-Benito. 2009. The structure of a biologically active influenza virus ribonucleoprotein complex. PLoS Pathog. 5:e1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dias, A., D. Bouvier, T. Crepin, A. A. McCarthy, D. J. Hart, F. Baudin, S. Cusack, and R. W. Ruigrok. 2009. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 458:914-918. [DOI] [PubMed] [Google Scholar]
- 7.Doan, L., B. Handa, N. A. Roberts, and K. Klumpp. 1999. Metal ion catalysis of RNA cleavage by the influenza virus endonuclease. Biochemistry 38:5612-5619. [DOI] [PubMed] [Google Scholar]
- 8.Dupureur, C. M. 2008. Roles of metal ions in nucleases. Curr. Opin. Chem. Biol. 12:250-255. [DOI] [PubMed] [Google Scholar]
- 9.Ericsson, U. B., B. M. Hallberg, G. T. Detitta, N. Dekker, and P. Nordlund. 2006. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Anal. Biochem. 357:289-298. [DOI] [PubMed] [Google Scholar]
- 10.Guilligay, D., F. Tarendeau, P. Resa-Infante, R. Coloma, T. Crepin, P. Sehr, J. Lewis, R. W. Ruigrok, J. Ortin, D. J. Hart, and S. Cusack. 2008. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat. Struct. Mol. Biol. 15:500-506. [DOI] [PubMed] [Google Scholar]
- 11.Guss, J. M., and H. C. Freeman. 2001. Aminopeptidase, p. 973-980. In A. Messerschmidt, R. Huber, T. Poulos, and K. Wieghart (ed.), Handbook of metalproteins. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 12.Hagen, M., T. D. Chung, J. A. Butcher, and M. Krystal. 1994. Recombinant influenza virus polymerase: requirement of both 5′ and 3′ viral ends for endonuclease activity. J. Virol. 68:1509-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara, K., F. I. Schmidt, M. Crow, and G. G. Brownlee. 2006. Amino acid residues in the N-terminal region of the PA subunit of influenza A virus RNA polymerase play a critical role in protein stability, endonuclease activity, cap binding, and virion RNA promoter binding. J. Virol. 80:7789-7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hare, S., S. S. Gupta, E. Valkov, A. Engelman, and P. Cherepanov. 2010. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature 464:232-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, X., J. Zhou, M. Bartlam, R. Zhang, J. Ma, Z. Lou, X. Li, J. Li, A. Joachimiak, Z. Zeng, R. Ge, Z. Rao, and Y. Liu. 2008. Crystal structure of the polymerase PA(C)-PB1(N) complex from an avian influenza H5N1 virus. Nature 454:1123-1126. [DOI] [PubMed] [Google Scholar]
- 16.Horton, N. C., and J. J. Perona. 2004. DNA cleavage by EcoRV endonuclease: two metal ions in three metal ion binding sites. Biochemistry 43:6841-6857. [DOI] [PubMed] [Google Scholar]
- 17.Imhof, P., S. Fischer, and J. C. Smith. 2009. Catalytic mechanism of DNA backbone cleavage by the restriction enzyme EcoRV: a quantum mechanical/molecular mechanical analysis. Biochemistry 48:9061-9075. [DOI] [PubMed] [Google Scholar]
- 18.Klumpp, K., R. W. Ruigrok, and F. Baudin. 1997. Roles of the influenza virus polymerase and nucleoprotein in forming a functional RNP structure. EMBO J. 16:1248-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuzuhara, T., D. Kise, H. Yoshida, T. Horita, Y. Murazaki, A. Nishimura, N. Echigo, H. Utsunomiya, and H. Tsuge. 2009. Structural basis of the influenza A virus RNA polymerase PB2 RNA-binding domain containing the pathogenicity-determinant lysine 627 residue. J. Biol. Chem. 284:6855-6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai, B., Y. Li, A. Cao, and L. Lai. 2003. Metal ion binding and enzymatic mechanism of Methanococcus jannaschii RNase HII. Biochemistry 42:785-791. [DOI] [PubMed] [Google Scholar]
- 21.Leavitt, S., and E. Freire. 2001. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 11:560-566. [DOI] [PubMed] [Google Scholar]
- 22.Lee, M. T., K. Klumpp, P. Digard, and L. Tiley. 2003. Activation of influenza virus RNA polymerase by the 5′ and 3′ terminal duplex of genomic RNA. Nucleic Acids Res. 31:1624-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, M. L., P. Rao, and R. M. Krug. 2001. The active sites of the influenza cap-dependent endonuclease are on different polymerase subunits. EMBO J. 20:2078-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obayashi, E., H. Yoshida, F. Kawai, N. Shibayama, A. Kawaguchi, K. Nagata, J. R. Tame, and S. Y. Park. 2008. The structural basis for an essential subunit interaction in influenza virus RNA polymerase. Nature 454:1127-1131. [DOI] [PubMed] [Google Scholar]
- 25.Ortega, J., J. Martin-Benito, T. Zurcher, J. M. Valpuesta, J. L. Carrascosa, and J. Ortin. 2000. Ultrastructural and functional analyses of recombinant influenza virus ribonucleoproteins suggest dimerization of nucleoprotein during virus amplification. J. Virol. 74:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pingoud, V., W. Wende, P. Friedhoff, M. Reuter, J. Alves, A. Jeltsch, L. Mones, M. Fuxreiter, and A. Pingoud. 2009. On the divalent metal ion dependence of DNA cleavage by restriction endonucleases of the EcoRI family. J. Mol. Biol. 393:140-160. [DOI] [PubMed] [Google Scholar]
- 27.Plotch, S. J., M. Bouloy, I. Ulmanen, and R. M. Krug. 1981. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell 23:847-858. [DOI] [PubMed] [Google Scholar]
- 28.Poon, L. L., D. C. Pritlove, E. Fodor, and G. G. Brownlee. 1999. Direct evidence that the poly(A) tail of influenza A virus mRNA is synthesized by reiterative copying of a U track in the virion RNA template. J. Virol. 73:3473-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson, J. S., M. Schubert, and R. A. Lazzarini. 1981. Polyadenylation sites for influenza virus mRNA. J. Virol. 38:157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocchia, W., S. Sridharan, A. Nicholls, E. Alexov, A. Chiabrera, and B. Honig. 2002. Rapid grid-based construction of the molecular surface and the use of induced surface charge to calculate reaction field energies: applications to the molecular systems and geometric objects. J. Comput. Chem. 23:128-137. [DOI] [PubMed] [Google Scholar]
- 31.Saito, T., D. M. Owen, F. Jiang, J. Marcotrigiano, and M. Gale, Jr. 2008. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarendeau, F., T. Crepin, D. Guilligay, R. W. Ruigrok, S. Cusack, and D. J. Hart. 2008. Host determinant residue lysine 627 lies on the surface of a discrete, folded domain of influenza virus polymerase PB2 subunit. PLoS Pathog. 4:e1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiley, L. S., M. Hagen, J. T. Matthews, and M. Krystal. 1994. Sequence-specific binding of the influenza virus RNA polymerase to sequences located at the 5′ ends of the viral RNAs. J. Virol. 68:5108-5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomassini, J., H. Selnick, M. E. Davies, M. E. Armstrong, J. Baldwin, M. Bourgeois, J. Hastings, D. Hazuda, J. Lewis, W. McClements, et al. 1994. Inhibition of cap (m7GpppXm)-dependent endonuclease of influenza virus by 4-substituted 2,4-dioxobutanoic acid compounds. Antimicrob. Agents Chemother. 38:2827-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torreira, E., G. Schoehn, Y. Fernandez, N. Jorba, R. W. Ruigrok, S. Cusack, J. Ortin, and O. Llorca. 2007. Three-dimensional model for the isolated recombinant influenza virus polymerase heterotrimer. Nucleic Acids Res. 35:3774-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehenkel, A., M. Bellinzoni, F. Schaeffer, A. Villarino, and P. M. Alzari. 2007. Structural and binding studies of the three-metal center in two mycobacterial PPM Ser/Thr protein phosphatases. J. Mol. Biol. 374:890-898. [DOI] [PubMed] [Google Scholar]
- 37.Yuan, P., M. Bartlam, Z. Lou, S. Chen, J. Zhou, X. He, Z. Lv, R. Ge, X. Li, T. Deng, E. Fodor, Z. Rao, and Y. Liu. 2009. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature 458:909-913. [DOI] [PubMed] [Google Scholar]
- 38.Zhao, C., Z. Lou, Y. Guo, M. Ma, Y. Chen, S. Liang, L. Zhang, S. Chen, X. Li, Y. Liu, M. Bartlam, and Z. Rao. 2009. Nucleoside monophosphate complex structures of the endonuclease domain from the influenza virus polymerase PA subunit reveal the substrate binding site inside the catalytic center. J. Virol. 83:9024-9030. [DOI] [PMC free article] [PubMed] [Google Scholar]