Abstract
We recently reported that rhesus macaques inoculated with CD4-binding-competent and CD4-binding-defective soluble YU2-derived HIV-1 envelope glycoprotein (Env) trimers in adjuvant generate comparable levels of Env-specific binding antibodies (Abs) and T cell responses. We also showed that Abs directed against the Env coreceptor binding site (CoRbs) were elicited only in animals immunized with CD4-binding-competent trimers and not in animals immunized with CD4-binding-defective trimers, indicating that a direct interaction between Env and CD4 occurs in vivo. To investigate both the overall consequences of in vivo Env-CD4 interactions and the elicitation of CoRbs-directed Abs for protection against heterologous simian-human immunodeficiency virus (SHIV) challenge, we exposed rhesus macaques immunized with CD4-binding-competent and CD4-binding-defective trimers to the CCR5-tropic SHIV-SF162P4 challenge virus. Compared to unvaccinated controls, all vaccinated animals displayed improved control of plasma viremia, independent of the presence or absence of CoRbs-directed Abs prior to challenge. Immunization resulted in plasma responses that neutralized the heterologous SHIV challenge stock in vitro, with similar neutralizing Ab titers elicited by the CD4-binding-competent and CD4-binding-defective trimers. The neutralizing responses against both the SHIV-SF162P4 stock and a recombinant virus pseudotyped with a cloned SHIV-SF162P4-derived Env were significantly boosted by the SHIV challenge. Collectively, these results suggest that the capacity of soluble Env trimers to interact with primate CD4 in vivo and to stimulate the production of moderate titers of CoRbs-directed Abs did not influence the magnitude of the neutralizing Ab recall response after viral challenge or the subsequent control of viremia in this heterologous SHIV challenge model.
The external glycoprotein gp120 and the membrane-anchored glycoprotein gp41 of human immunodeficiency virus type 1 (HIV-1), collectively referred to as the envelope glycoproteins (Env), mediate viral entry and are the sole virally encoded targets for neutralizing antibodies (NAbs). Prior to binding the primary host cell receptor, CD4, the trimeric Env spike may sample multiple conformations on the surface of the virus. Which of these potential conformations display neutralizing Ab epitopes and are recognized by broadly reactive NAbs is currently unclear. A substantial conformational change occurs when the functional Env spike interacts with CD4, leading to the exposure and the formation of the bridging sheet, a highly conserved and immunogenic structure spanning the inner and outer domains of gp120 that contributes to coreceptor interaction (6, 14, 25, 30). CD4 binding is also thought to lead to the displacement of variable region 3 (V3) from a less exposed conformation in the packed functional spike to a more protruding conformation. Exposure of V3 is necessary for viral entry, as it also contributes to Env interaction with coreceptor (21). Additional or concurrent rearrangements of the functional spike structure may occur upon CD4 binding, as suggested by cryotomography (38), However, these rearrangements are less well understood due to the absence of a high-resolution structure of the static or CD4-liganded trimeric spike.
In attempts to elicit broadly reactive NAbs against HIV-1 through vaccination, a range of recombinant Env variants were designed and tested (reviewed in references 15, 26, 49, and 50). The capacity of such immunogens to elicit broadly reactive NAbs is often determined using standardized in vitro neutralization assays (34). However, the ability of HIV-1 Env vaccine-elicited B cell responses to mediate actual protective and functional responses against in vivo virus challenge is evaluated less frequently, since this requires the use of nonhuman primates (NHPs) and infection with chimeric simian-human immunodeficiency viruses (SHIVs). A series of SHIVs was developed, including those based on the HIV-1 Env glycoproteins from SF162 (40), 89.6 (54), ADA (45), BaL (48), DH12 (59), and 1157i (27). So far, few of these models, if any, fully mimic HIV-1 infection in humans. Currently, serially passaged CCR5-using SHIV-SF162 (SHIV-SF162P), which establishes transient or more prolonged viremia in macaques, represent a frequently used model to evaluate the protective effect of Env-based immunogens (2-5, 19, 20, 23, 24, 29, 53, 67). Depending on the number and nature of passages that this virus has been exposed to, the SHIV-SF162P stocks are more or less neutralization resistant (19, 62), allowing one to test the efficacy of a given vaccine candidate against a more or less rigorous form of viral challenge. Protection against mucosal SHIV-SF162P4 challenge after homologous SF162ΔV2 Env protein immunization of rhesus macaques was recently reported (2, 3). However, the nature and specificities of the vaccine-induced immune responses that mediate this effect remain incompletely defined.
We recently showed that Abs against the HIV-1 gp120 coreceptor binding site (CoRbs) are elicited as a consequence of in vivo interactions between Env and primate CD4 during immunization with soluble CD4 (sCD4)-binding-competent Env trimers (14). We subsequently showed that rhesus macaques inoculated with CD4-binding competent and CD4-binding defective soluble YU2-derived gp140-F trimers in adjuvant generate comparable levels of Env-specific binding Abs and T cell responses but that CoRbs-directed Abs are elicited only in animals immunized with wild-type (wt) CD4-binding competent Env trimers (13). So far, the impact of Env-CD4 in vivo interactions during Env immunization and the role of CoRbs-directed Abs in protection against SHIV infection remain incompletely understood. A majority of the well-characterized CoRbs-directed monoclonal Abs (MAbs) lack the capacity to neutralize primary viruses in vitro (7, 31). However, it has been suggested that Abs directed against this region may contribute to the neutralizing Ab response seen in some HIV-1-infected individuals (18, 35, 58) and to the protection observed in some SHIV challenge experiments (12).
The distinct difference in the capacity of the CD4-binding competent and CD4-binding defective Env trimers to elicit CoRbs-directed Abs described in our previous study presented an opportunity to evaluate the protective effect of CoRbs-directed Abs in the SHIV model. The availability of animals immunized with these Env immunogens also allowed us to ask the more general question about whether in vivo interactions between soluble Env trimers and CD4-expressing host cells would influence the outcome of heterologous SHIV-SF162P4 infection. We show here that Env trimer-immunized animals displayed improved control of SHIV-SF162P4 viremia compared to unimmunized control animals, independent of whether they were inoculated with CD4-binding-competent or CD4-binding-defective trimers. These results suggest that the capacity of soluble Env trimers to interact with CD4 in vivo and to stimulate the production of CoRbs-directed Abs did not measurably influence the protective effect of the vaccine-elicited immune responses in this SHIV challenge model.
MATERIALS AND METHODS
Construction of Env immunogens.
The Env immunogens used in the present study were described elsewhere (13). Briefly, cleavage-defective soluble YU2gp140 trimers (here referred to as wt) (66) were modified using site-directed mutagenesis (Stratagene). The 368 trimer was generated by modifying the codon for amino acid 368 from aspartic acid to arginine (368D/R), while the 423/425/431 variant trimer was generated by altering codons 423, 425, and 431 from isoleucine to methionine (423I/M), asparagine to lysine (425N/K), and glycine to glutamic acid (431G/E), respectively.
Expression and purification of Env immunogens.
The Env proteins were produced by transient transfection of Freestyle 293F suspension cells (Invitrogen) as previously described (14). Briefly, cells were transfected at a density of 1.1 × 106/ml in GIBCOFreestyle293 expression medium using 293Fectin, according to the manufacturer's instructions (Invitrogen). Supernatants were collected 4 days after transfection. Following collection, all supernatants were centrifuged at 3,500 × g to remove cells or cell debris, filtered through a 0.22-μm filter, supplemented with Complete EDTA-free protease inhibitor cocktail (Roche) and penicillin-streptomycin (Invitrogen), and stored at 4°C until further purification. The wt, 368, and 423/425/431 trimers used for immunizations were purified by a three-step process. First, the proteins were captured via glycans by lentil-lectin affinity chromatography (GE Healthcare, Uppsala, Sweden). After extensive washing with phosphate-buffered saline (PBS) supplemented with 0.5 M NaCl, the proteins were eluted with 1 M methyl-α-d-mannopyranoside and captured in the second step via the His tag by nickel chelation chromatography (GE Healthcare). After washing with 40 mM imidazole (IM) and 0.5 M NaCl in PBS, proteins were eluted with 300 mM IM in PBS. Trimers were separated from lower-molecular-weight forms by gel filtration chromatography using a Superdex200 26/60 preparative-grade column with the ÄKTA fast protein liquid chromatography system (GE Healthcare).
Animals.
Twenty-three female rhesus macaques of Chinese origin, 5 to 6 years old, were housed in the Astrid Fagraeus laboratory at the Swedish Institute for Infectious Disease Control. Housing and care procedures were in compliance with the provisions and general guidelines of the Swedish Animal Welfare Agency. All procedures were approved by the Local Ethical Committee on Animal Experiments. Before entering the study, all animals were confirmed negative for simian immunodeficiency virus (SIV), simian T cell lymphotropic virus, and simian retrovirus type D.
Immunizations and sampling.
Fifteen of the rhesus macaques (three groups of five animals) were previously inoculated with the immunogens described above (13). Immunizations were performed monthly by the intramuscular (i.m.) route of injection. All immunizations were administered with protein in combination with 500 μg CpG ODN2395 (Coley Pharmaceutical Group) and 75 μg ABISCO-100 (Isconova AB) as adjuvant. Protein doses were 200 μg per animal for the first inoculation and 100 μg for the following injections. Blood samples were taken before and 2 weeks after each immunization.
SHIV challenge and viral load determination.
The 15 immunized animals and 8 Env-naïve control animals were used for the challenge experiment. A virus challenge stock, based on the CCR5-tropic SHIV-SF162P4 (20, 62), was prepared. Briefly, plasma from an acutely SHIV-SF162P4 (29)-infected rhesus macaque, kindly provided by L. Stamatatos, was inoculated intravenously (i.v.) into a naïve rhesus macaque. At peak viremia, blood and bone marrow were injected into a second macaque (32 M). Virus was reisolated at peak viremia by coculture with naïve rhesus peripheral blood mononuclear cells (PBMC) and subsequently injected i.v. into a naïve rhesus macaque of Indian origin (M661), and reisolation was done as described above. The resulting virus was passaged in vitro in the human T cell line C8166-CCR5 to produce the challenge stock used in the present study, designated SHIV-SF162P4. On the day of challenge the SHIV-SF162P4 stock was diluted 1/10,000 in PBS, and 1 ml, corresponding to 22 50% tissue culture infectious doses (TCID50) (measured in C8166-CCR5 cells) or approximately 18 50% monkey infectious doses (MID50) (based on a limited in vivo titration in nine Chinese rhesus macaques), was inoculated i.v. 4 weeks after the last immunization. Viral loads were monitored weekly for 4 weeks and thereafter at week 6 and 10 postchallenge using the Exavir Load kit (Cavidi, Sweden) according to the manufacturer's instructions. Anamnestic Ab responses were evaluated at 1 to 10 weeks postchallenge by enzyme-linked immunosorbent assay (ELISA).
Analysis of Env-binding Abs in the sera.
HIV-1 Env-specific serum IgG was measured by ELISA as previously described (43), with some modifications. For postchallenge Ab detection, the plasma was first inactivated by incubation at 56°C for 1 h. Briefly, insect-cell-produced YU2 gp120 protein or SF162 gp120 was applied to Nunc Maxisorp microtiter plates at 1 μg/ml in 50 mM carbonate buffer (pH 9.6) overnight at +4°C. After blocking in PBS containing 2% milk, serum samples were added and incubated for 1.5 h at 37°C. The gp120-specific IgG was detected by adding secondary horseradish peroxidase (HRP)-conjugated anti-human IgG Ab (Jackson ImmunoResearch, Suffolk, United Kingdom) followed by o-phenylenediamine (OPD) (Sigma, Schnelldorf, Germany). Between each incubation step, the plates were washed six times with PBS 0.05%-Tween 20. Reactions were terminated by adding 2.5 M H2SO4, and the optical density (OD) was read at 492 nm. The OD50 titer for each sample was calculated by interpolation from mean 50% OD values calculated from controls using the formula [(ODmax − ODmin)/2] + ODmin.
Virus neutralization assays.
Plasma or MAbs were tested for neutralization capacity against chosen HIV-1 Envs or a cloned SHIV Env (clone 41.1). Neutralization assays were performed using single-round infectious HIV-1 Env pseudovirus or replication-competent SHIV-SF162P4 stock with protease inhibitors (42) and TZM-bl target cells as previously described (34, 60). For evaluation of CoRbs Abs, HIV-2 was preincubated with or without 9 nM soluble CD4 before plasma was added (10). In peptide absorption experiments, plasma was preincubated with either a control peptide (V3 scrambled; NKGTHNIPTARNIYGFPTSRRGT) or the YU2 V3 peptide (V3 peptide; TRPNNNTRKSINIGPGRALYTTG) as done previously (36). Results are reported as serum neutralization ID50, which is the reciprocal of the serum dilution producing 50% virus neutralization.
Cloning of Env sequences contained within the SHIV-SF162P4 stock.
Viral RNA was extracted from the concentrated SHIV-SF162P4 challenge stock using the QIAamp viral RNA mini kit (Qiagen). The RNA was reverse transcribed into cDNA using Superscript III (Invitrogen) and oligo(dT)18 according to the manufacturer's instructions. The full-length gp160 Env gene was amplified with the high-fidelity PCR master mix (Roche), forward primer EnvSHIV5in (5′-GCAGAAGACAGTGGCAATGA-3′), and reverse primer EnvSHIV3in (5′-GCGAGTATCCATCTTCCACCT-3′). The product was gel extracted, purified (Qiagen), cloned into the pJET1.2-blunt (Fermentas) or pCR-blunt (Invitrogen) vector, transformed into DH5α bacteria, and grown under ampicillin selection. Positive colonies were screened for insert length and direction before being excised and cloned into the pcDNA3.1 expression vector for sequencing and pseudovirus production. The full-length gp160 gene was sequenced with four overlapping reads, translated into amino acids, and aligned with the parental SF162 sequence (NCBI accession number EU123924). Fourteen Env sequences were cloned and analyzed. Consistent amino acid changes between the parental SF162 sequence and the SHIV-SF162P4 clones were observed on only three positions in Env gp120. One mutation was present in 6 out of 14 clones and was positioned in variable region 1 (R164K). The other two changes were located just outside the V3 region at positions 332 and 336, the latter in a predicted NNT glycosylation site. In 12 of 14 clones the 332G (glycine) and 336N (asparagine) were substituted for arginine and aspartic acid, respectively, abolishing the glycosylation site. The SHIV Env clone 41.1 exhibited the G332R and N336D substitutions but not the R164K substitution.
Statistical analyses.
In all comparisons with two groups, statistical significance was determined with the nonparametric Mann-Whitney U test. When comparing ≥3 groups, values were log10 transformed and analysis of variance (ANOVA) was used. To evaluate significance concerning two factors, time and groups, a repeated-measures two-way ANOVA was applied on log10-transformed values using Graph Pad Prism software version 5. Significance was indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01; and ***, P ≤ 0.001. Correlations were determined with the Spearman correlation test. The two-tailed P value and Spearman r are presented.
RESULTS
Antigenicity and immunogenicity of wt and CD4-binding-defective soluble Env trimers.
We recently investigated the potential impact of in vivo interactions between Env and primate CD4 on the immunogenicity of soluble trimeric Env immunogens in transgenic rabbits expressing human CD4 (14) and in rhesus macaques (13). For the latter study, we generated two Env trimer variants rendered defective in their capacity to bind CD4 by two distinct mechanisms. The first variant harbored a substitution of amino acid 368 (46) (368D/R; here referred to as 368 trimers) in the CD4 binding site (CD4bs) of gp120 and was therefore expected to be severely impaired in its capacity to bind CD4 but to retain the capacity to bind CoRbs-directed Abs, as this binding surface is distal to position 368. The second variant contained three substitutions in the beta 20 strand of the outer domain of the unformed bridging sheet of gp120 (423I/M, 425N/K, and 431G/E; here referred to as 423/425/431 trimers). These mutations were predicted to favor a helical structure in this region of unliganded gp120 and thereby dually to disrupt the formation of the CoRbs and to eliminate stable binding to CD4 by interfering with the gp120 conformational changes needed for this high-affinity interaction (65).
The antigenic and immunogenic properties of the wt, 368, and 423/425/431 trimers were described in our previous work (13). Here, we extended these studies to investigate the protective effect of the immune responses elicited by these Env variants in response to heterologous SHIV-SF162P4 challenge. A summary of the antigenic properties of the three Env variants is shown in Table 1. In brief, both the 368 and 423/425/431 trimers were severely defective for binding to soluble CD4 (sCD4) compared to the wt trimers. The 368 trimers were also defective for recognition by all CD4bs-directed MAbs tested, while the 423/425/431 trimers retained binding by this vaccine-relevant class of Abs. In contrast, the 368 trimers but not the 423/425/431 trimers retained the ability to interact with CoRbs-directed MAbs, while all three trimer variants were equally well recognized by the V3-directed MAb, 447. A comprehensive analysis of the Env-specific B and T cell responses elicited by the three trimer variants was described previously (13). Additional data of particular relevance for the SHIV-SF162P4 challenge study are presented here.
TABLE 1.
Antigenic profile of Env immunogens
| Antibody | Titera of YU2 trimer variant |
||
|---|---|---|---|
| wt | 368 | 423/425/431 | |
| sCD4 | 1 | —b | — |
| CD4bs MAbs | |||
| b12 | 1 | — | 0.99 |
| b6 | 1 | 0.28 | 1.07 |
| F91 | 1 | 0.15 | 1.31 |
| F105 | 1 | — | 0.90 |
| CoRbs MAbs | |||
| 17b | 1 | 1.57 | — |
| 48d | 1 | 2.72 | — |
| E51 | 1 | 1.29 | — |
| 2.1C | 1 | 1.51 | — |
| V3 MAb 447 | 1 | 0.90 | 1.06 |
ELISA binding titer relative to that of wt trimers.
—, no detectable binding.
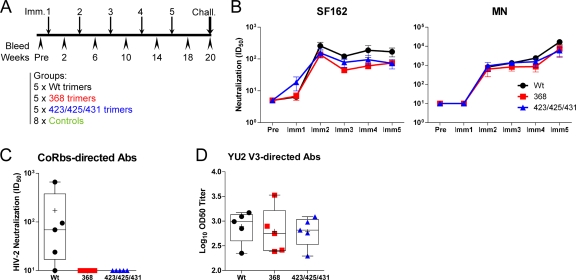
The study outline is shown in Fig. 1 A. In brief, 15 animals (5 per group) were inoculated with wt, 368, or 423/425/431 trimers in adjuvant at monthly intervals. After five protein inoculations, these animals and eight Env-naïve control animals were challenged intravenously with SHIV-SF162P4. Env-specific Abs capable of neutralizing the parental SF162 virus were elicited in all animals. The Ab titers reached a plateau at 2 weeks following the second inoculation and then remained at a similar magnitude following further boosting (Fig. 1B). In contrast, the neutralizing Ab activity against MN increased after subsequent immunizations, suggesting that boosting stimulated selected Ab specificities (Fig. 1B, right panel). There was no significant difference in the neutralizing Ab titers against SF162 and MN between the wt, 368, or 423/425/431 trimer-inoculated animals at any of the time points measured. However, CoRbs-directed Abs, as defined using an HIV-2 pseudovirus assay (10), were elicited only in the wt trimer-immunized animals and not in the animals immunized with the CD4-binding-defective 368 or 423/425/431 trimers (13) (Fig. 1C). These data are consistent with our original observation that in vivo CD4 binding by soluble Env is a requirement for Env to undergo the conformational change necessary for elicitation of CoRbs-directed Abs (14). These results illustrate clearly that the ability of an Ab to bind an antigen (antigenicity) is not equivalent to the ability of the antigen to de novo elicit Abs against the same epitope (immunogenicity).
FIG. 1.
Immunogenic profiles of Env immunogens. (A) Schematic presentation of immunization, bleeds, and challenge of the Env trimer-immunized monkeys. (B) Pseudovirus neutralization of SF162 and MN as shown by plasma ID50 titer. Shown is neutralization at 2 weeks after immunizations 1 to 5. Error bars indicate standard errors of the means. (C) Coreceptor binding site Abs present in plasma samples from the immunized groups, as determined by the HIV-2 pseudovirus neutralization assay 2 weeks after immunization 5. (D) Log10 OD50 binding titers in plasma to the YU2 variable region 3 peptide, TRPNNNTRKSINIGPGRALYTTG, at 2 weeks after immunization 5.
To further investigate if in vivo CD4 binding affects the elicited Ab response, we assessed the levels of variable region 3 (V3)-directed Abs elicited by each of the three immunogens. Both SF162 and MN are sensitive to YU2 trimer-elicited V3-directed NAbs (43), and several independent studies suggest that CD4 binding may influence the exposure of V3 (21, 44, 64). By measuring V3-directed Abs in plasma collected after the last immunization by direct binding to a YU2-derived V3 peptide, we observed no significant difference (P > 0.05) in the OD50 binding titers between animals inoculated with the CD4-binding-competent and CD4-binding-defective Env trimers (Fig. 1D).
Rhesus macaques immunized with CD4-binding-competent and CD4-binding-defective Env trimers demonstrate similar reductions of viremia upon challenge.
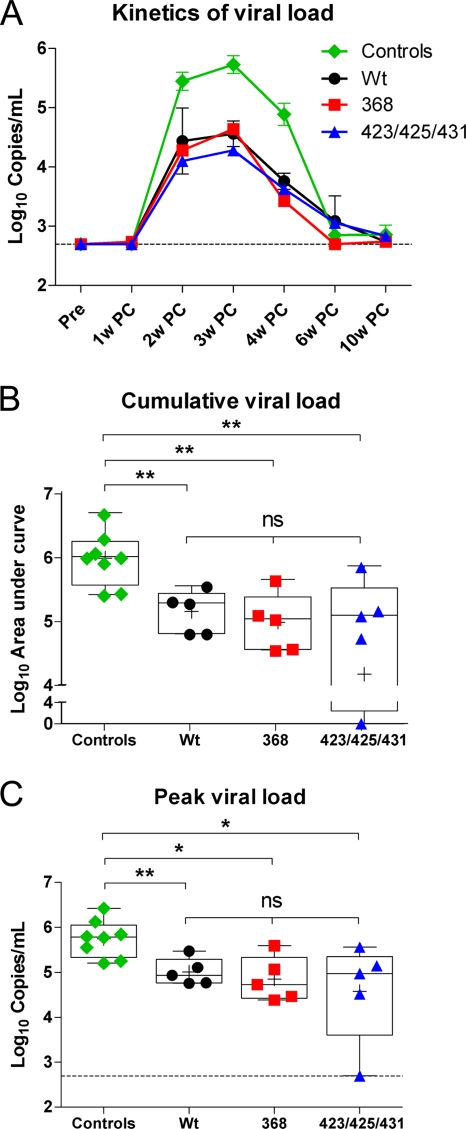
The potential impact of in vivo interactions between CD4-binding-competent Env immunogens and primate CD4, including the generation of CoRbs-directed Ab responses, for protection against HIV-1 or SHIV infection remains unclear. To investigate this, we inoculated wt, 368, and 423/425/431 trimer-immunized animals via the intravenous route with SHIV-SF162P4. A control group of eight Env-naïve animals was similarly challenged. Viral replication in plasma was monitored for 10 weeks after the challenge, at which time the viral loads were below the limit of detection in all control animals (Fig. 2 A). There was a reduction in viral load (copies/ml) in the plasma of all vaccinated animals, independent of which of the three Env immunogens the animals were inoculated with. The enhanced control in vaccinated animals relative to that in the control animals was apparent at all time points measured, suggesting that the vaccine-induced response contributed to the early control of the virus. Furthermore, there was a significant decrease in the cumulative (Fig. 2B) and peak (Fig. 2C) viral loads in all Env trimer-immunized animals compared to the control animals.
FIG. 2.
SHIV-SF162P4 challenge. At 4 weeks after immunization 5, all three immunized animals (wt, 368, and 423/425/431; n = 5 per group) and a control group (n = 8) of naïve rhesus macaques were challenged intravenously with SHIV-SF162P4. (A) Kinetics of viral load. Viral loads were measured in plasma both prior to challenge (Pre) and at 1 to 10 weeks postchallenge (PC). The lower detection limit was set to 500 copies/ml. (B and C) Cumulative viral load as calculated from area under the curve (AUC) (B) and peak viral load (C) were determined for each animal. The data are shown for individual monkeys and as box plots. Statistical significance was evaluated with the Mann-Whitney U test. ns, not significant.
Apparent sterilizing protection was achieved in one monkey (F77) inoculated with 423/425/431 trimers. This animal did not display detectable viral loads at any of the time points measured (Fig. 2B and C). The protection was supported by the lack of an anamnestic Ab response in this animal, in contrast to all other vaccinated animals (data not shown). Interestingly, no difference in cumulative or peak viral loads was observed between the wt, 368, or 423/425/431 trimer-inoculated animals, suggesting that the CoRbs-directed Abs present prior to challenge in the wt trimer-immunized animals did not add any additional protective effect against the SHIV-SF162P4 challenge. Overall, the results demonstrate that the capacity of the soluble Env trimers to interact with primate CD4 in vivo did not affect the capacity of the elicited immune response to control viremia in the SHIV-SF162P4 challenge model.
Potent anamnestic Ab responses to Env following SHIV-SF162P4 challenge.
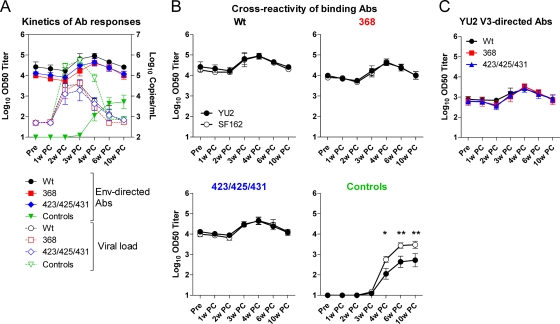
To examine whether the animals immunized with wt, 368, or 423/425/431 trimers differed in their anamnestic Ab response postchallenge, we measured HIV-1 Env binding titers in immunized and control animals following SHIV-challenge (Fig. 3 A). One week after the viral loads reached peak levels, anamnestic Ab responses were observed in all immunized animals except animal F77. An increase in Env-specific Ab titers of approximately 1 order of magnitude over a 2-week period was observed. This increase coincided with a decrease in systemic plasma viral loads. Compared to the anamnestic response in the Env-immunized groups, the Env-specific Ab response in the control animals was delayed by approximately 1 week.
FIG. 3.
Anamnestic antibody responses. (A) Graph illustrating the anamnestic Ab responses to gp120 (left axis, solid lines) in comparison to viral load (right axis, dotted lines). (B) YU2 (black)- and SF162 (white)-specific Ab responses were determined for each group before challenge (Pre) and 1 to 10 weeks after challenge (PC). (C) Variable loop 3-directed Ab responses were evaluated as for gp120. Shown are means ± standard errors of the means of log10 OD50 binding titers (n = 5 for immunized animals and n = 8 for controls) Statistical significance was determined with repeated-measures two-way ANOVA.
To evaluate the quality of the Env-specific Ab response following challenge, the plasma was assayed for binding to YU2 gp120 (the immunogen strain) and SF162 gp120 (the challenge strain) by ELISA (Fig. 3B). Equal levels of binding were seen for YU2 and SF162 in all Env-immunized animals, indicating that a high level of cross-reactive Abs was elicited by the YU2 trimeric immunogens. The observed cross-reactivity in the Env-immunized groups was sustained even when the anamnestic Abs were boosted by the SF162 challenge virus. When the cross-reactive Abs induced by the SHIV-SF162P4 challenge virus alone (in the control animals) were analyzed, 70% of the Abs were found to be specific for SF162 and did not bind YU2 at 10 weeks following challenge (P ≤ 0.01) (Fig. 3B, lower right panel). To determine whether V3-directed Abs were boosted similarly in the groups of animals receiving the three immunogens, plasma was assayed for YU2 V3 peptide binding Abs by ELISA. The V3-specific Ab levels were similar during the course of the SHIV infection in all immunized groups (P > 0.05) (Fig. 3C), and the kinetics of the anamnestic response was similar to that of total gp120-reactive Abs.
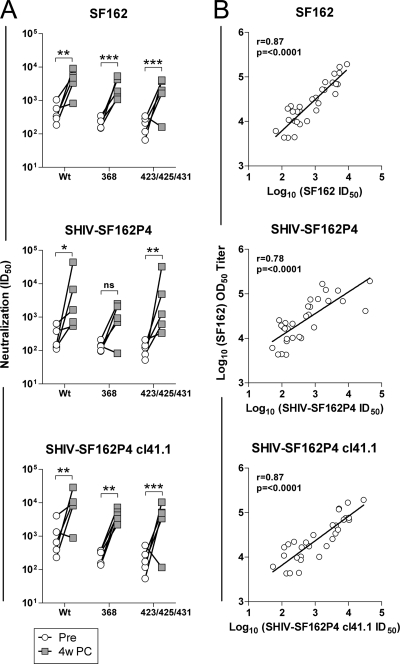
The effect of the anamnestic response on the neutralizing Ab titers was next investigated. Plasma samples collected before and 4 weeks after the SHIV-SF162P4 challenge were analyzed for neutralizing activity against the parental SF162 pseudovirus, the SHIV-SF162P4 virus stock, and a pseudovirus generated from a clone derived from the SHIV-SF162P4 stock (clone 41.1; see Methods). A significant increase in neutralizing activity against all three SHIV viruses was observed in the samples collected postchallenge from animals inoculated with both wt and the CD4-binding-defective Env trimers (368 or 423/425/431 trimers) (Fig. 4 A). Furthermore, the increase in neutralizing Ab activity against SF162, the SHIV-SF162P4 stock, and SHIV-SF162P4 clone 41.1 was highly correlated to the increase in postchallenge SF162 binding Abs (Fig. 4B).
FIG. 4.
Effect of anamnestic antibody response on neutralization. (A) Neutralization titers (ID50) of the parental SF162 and SHIV-SF162P4 clone 41.1 pseudoviruses and SHIV-SF162P4 replication-competent stock before challenge (Pre) and 4 weeks after challenge (PC). Shown are results for individual monkeys. Statistical significance was determined with two-way ANOVA. Monkey F77 was excluded from all postchallenge analysis as it did not become infected by the challenge virus. (B) Correlation between SF162 binding Abs and neutralization to SF162, SHIV-SF162P4 clone 41.1, and the SHIV-SF162P4 stock following the anamnestic response. Correlations were determined with the Spearman test.
V3-directed Ab-mediated neutralization and MAb characterization of SHIV-SF162P4.
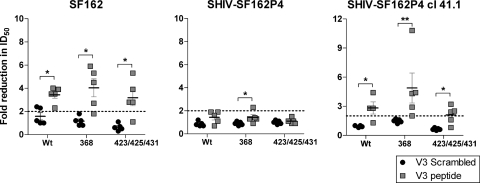
We previously showed that virus pseudotyped with the parental SF162 Env is sensitive to neutralization by YU2 trimer-elicited V3-directed Abs (43). To investigate whether YU2 trimer-elicited V3-directed Abs contribute to the neutralization of SHIV-SF162P4, we performed a YU2 V3 peptide competition neutralization assay on the plasma samples after five immunizations. The data obtained from the parental SF162 Env-pseudotyped virus, the SHIV-SF162P4 challenge stock, and the SHIV-SF162P4-derived clone 41.1 pseudovirus are presented as fold reduction of neutralization (ID50) (Fig. 5). In the presence of the V3 peptide, there was a potent reduction in the neutralization ID50 for the parental SF162 and the SHIV-derived 41.1 Env-pseudotyped viruses, while little or no reduction in the neutralization ID50 against the uncloned SHIV stock was observed. The mean fold reduction in ID50, compared to a V3 scrambled peptide, observed for SF162 was 3.5-fold (71%), and that for SHIV-SF162P4 clone 41.1 was 3.3-fold (70%). In contrast, only a minor difference between the V3 peptide and the V3 scrambled peptide was observed for the SHIV-SF162P4 stock, with a mean reduction in ID50 of 1.4-fold (29%). No significant difference was observed between the animals immunized with CD4-binding-competent or CD4-binding-deficient trimers (P > 0.05) against either the parental SF162 Env-pseudotyped virus, the SHIV-SF162P4 challenge stock, or the SHIV-SF162P4-derived clone 41.1 pseudovirus. This is consistent with the data shown in Fig. 1D, which indicated that each of the Env trimers elicited similar levels of anti-V3 Abs.
FIG. 5.
Characterization of SHIV-SF162P4 neutralization sensitivity. Neutralizing titers (ID50) against the pseudoviruses SF162 and SHIV-SF162P4 clone 41.1 and the replication-competent SHIV-SF162P4 stock in plasma samples from immunized animals (wt, 368, and 423/425/431; n = 5 per group) was determined 2 weeks after immunization 5. Plasma was preincubated with YU2 V3 peptide or scrambled control peptide before being added to the neutralization assay mixture. Neutralization due to V3-directed Abs was calculated as fold reduction of ID50 in comparison to that for mock infection. Significance was evaluated with the Mann-Whitney U test.
As the SHIV-SF162P4 stock was more resistant to YU2-trimer elicited V3-mediated neutralization than the parental SF162 Env-pseudotyped virus and the SHIV-SF162P4-derived clone 41.1 pseudotyped virus, we characterized the neutralization sensitivities of these viruses in further detail using a panel of well-described MAbs and sCD4. The panel included two CD4bs-directed MAbs (b12 and F105), two CoRbs-directed MAbs (17b and 48d), two V3-directed MAbs (447 and 39F), the glycan-sensitive MAb (2G12), and two membrane proximal external region (MPER)-directed MAbs (4E10 and 2F5). Neither the pseudoviruses nor the SHIV stock was sensitive to the two CoRbs-directed Abs 17b and 48d (Table 2), similarly to what has been shown for SHIV-SF162P3 (22). In contrast, all three viruses were neutralized by the two V3-directed MAbs, 447 and 39F, although the SHIV-SF162P4 stock was about three times more resistant to these V3-directed Abs than the parental SF162 and SHIV-SF162P4 clone 41.1. These data may be related to the difference observed in the V3 peptide neutralization competition assay (Fig. 5). Both the pseudoviruses and the SHIV stock were sensitive to the CD4bs MAb b12 and soluble CD4 but not to the CD4bs MAb F105. The parental SF162 and SHIV-SF162P4 clone 41.1 pseudoviruses showed some degree of sensitivity (50% inhibitory concentration [IC50], <4 μg/ml) to the glycan-directed Ab 2G12 and to the MPER-directed Abs 4E10 and 2F5, while the SHIV-SF162P4 stock was relatively resistant (IC50, >15 μg/ml). In general, the SHIV-SF162P4 stock was more resistant to all MAbs tested than the parental SF162 and SHIV-SF162P4-derived clone 41.1 pseudotyped viruses.
TABLE 2.
Characterization of MAb neutralization of SF162 viral variants
| Antibody | SF162 |
SHIV-SF162P4 |
||||
|---|---|---|---|---|---|---|
| Clone 41.1 |
||||||
| IC50 (μg/ml) | IC80 (μg/ml) | IC50 (μg/ml) | IC80 (μg/ml) | IC50 (μg/ml) | IC80 (μg/ml) | |
| sCD4 | 0.35 | 1.39 | 0.40 | 1.90 | 0.16 | 0.62 |
| b12 | 0.05 | 0.27 | 0.14 | 0.73 | 0.05 | 0.16 |
| F105 | 26.3 | >50 | 31.2 | >50 | 5.9 | >50 |
| 17b | 25.4 | >50 | 28.0 | >50 | 5.7 | >50 |
| 48d | >50 | >50 | >50 | >50 | >50 | >50 |
| 2G12 | 0.46 | 3.29 | 16.8 | >50 | 1.80 | 16.0 |
| 447 | 0.10 | 1.90 | 0.35 | 5.59 | 0.11 | 1.21 |
| 39F | 0.03 | 0.36 | 0.07 | 1.03 | 0.03 | 0.26 |
| 4E10 | 1.4 | 14.0 | 18.1 | >50 | 3.7 | 22.0 |
| 2F5 | 0.85 | 5.44 | 31.8 | >50 | 1.52 | 6.4 |
| HIV Ig | 16.1 | 81.4 | 79.7 | 376 | 16.1 | 83.8 |
DISCUSSION
CD4-binding-competent HIV-1 Env-based immunogens clearly interact with cells expressing primate CD4 in vivo. We recently investigated the influence of this interaction on the immune responses elicited by soluble Env trimer immunization in rhesus macaques and showed that similar magnitudes of total Env-binding Ab and T cell responses were elicited by wt and CD4-binding-defective trimers. However, only wt trimers stimulated the production of CoRbs-directed Abs (13). In the current study, the distinct difference in the elicitation of CoRbs Abs between animals immunized with wt and CD4-binding-defective trimer variants prompted us to investigate the role of CoRbs-directed Abs for protection against SHIV-SF162P4 and to ask whether Env-CD4 in vivo interactions during Env trimer immunization of primates affect the protective response to SHIV challenge in any other measurable way.
The potential role of CoRbs-directed Abs for virus neutralization in vivo and protection against HIV-1 or SHIV infection remains unclear. Access of intact Ab molecules to the conserved CoRbs on the HIV-1 functional virus spike is thought to be sterically restricted (31), while smaller Ab subunits can access this region and interfere with viral entry (7). Small ligands targeting this conserved region may therefore have potential for prevention and therapeutic intervention (28). However, as a target for vaccine-induced Ab responses, the CoRbs may be less desirable unless Abs from this class that are different from and/or more potent than the Abs currently identified are elicited. Abs against the CoRbs are abundantly generated during natural HIV-1 infection in humans and following experimental SHIV challenge of naïve NHPs (12, 17, 41, 55), suggesting that the virus evolves and replicates efficiently in the presence of such humoral responses. The detection of CoRbs-directed Abs prior to autologous virus neutralization in HIV-1-infected patients also suggests that the CoRbs is nonneutralizing (17). This is supported by data from the phase III Vaxgen clinical trial (VAX04), where no protection against acquisition of infection was observed (52) despite high titers of CoRbs-directed Abs in all sera from a randomly selected but representative subset (14). In contrast, CoRbs-directed Abs were suggested to be associated with more rapid viral clearance in macaques immunized with covalently linked gp120-CD4 complexes and then challenged with SHIV-SF162P3 in one study (12). In the current study, we observed a similar reduction of viremia after intravenous SHIV-SF162P4 challenge in all immunized animals, but this was independent of the presence or absence of detectable CoRbs-directed Ab responses. These results show that CoRbs-directed Abs, at the titers elicited by the soluble YU2 Env trimers used here, did not contribute to the control of heterologous SHIV-SF162P4 infection in the current study. Additional studies are needed to determine if CoRbs-directed Abs with improved neutralization capabilities can be elicited by immunization, perhaps by using Env immunogens that better mimic the native HIV-1 Env spike. There is at present limited information about different subspecificities of CoRbs-directed Abs, and we cannot exclude that more potent neutralizing CoRbs-directed Abs can be generated by other vaccination protocols or by natural HIV-1 infection.
The SHIV-SF162P4 challenge virus is genetically related to the SHIV-SF162P3 virus, but the SHIV-SF162P4 Env is more neutralization sensitive and closely resembles the parental SF162 Env clone. The SHIV-SF162P4 stock was generated by in vivo passage at peak viremia, likely in the absence of effective host-mediated immune pressure (1, 62). In contrast, the SHIV-SF162P3 stock was generated by in vivo passage after prolonged replication in rhesus macaques, forcing the virus to evolve under the pressure of the host adaptive immune response (9, 20, 40). Despite the frequent use of the SHIV-SF162P4 challenge virus, little is known about its sensitivity to well-characterized neutralizing MAbs. Here, we characterized the SHIV-SF162P4 challenge virus and a clone derived thereof (clone 41.1) for their sensitivities to known MAbs and the immune sera from monkeys immunized with the YU2-based Env trimers. We show that the SHIV-SF162P4 stock is less sensitive to several MAbs, including the two CoRbs-directed MAbs 17b and 48d and the two MPER-directed MAbs 2F5 and 4E10, than the parental SF162 virus. It is, however, highly sensitive to the CD4bs Ab b12 and the V3-directed 447 and 39F MAbs. Despite its sensitivity to these two V3-directed MAbs, markedly higher concentrations of the MAbs were required to neutralize the uncloned SHIV-SF162P4 stock compared to viruses pseudotyped with the parental SF162 Env or the SHIV-SF162P4-derived 41.1 Env. However, since the SHIV-SF162P4 clone generated here was not derived from single-genome amplification (57), it is possible that there are other variants in the stock exhibiting a more resistant phenotype. The cell type from which the viruses were generated, C8166-CCR5 cells for the uncloned SHIV stock and 293 cells for the pseudotyped virus, may also affect the neutralization sensitivity (39, 63).
The plasma NAb titers after five immunizations measured against the SHIV-SF162P4 challenge virus were comparable to or slightly lower than the titers reported following immunization with homologous SF162-derived gp140 oligomers (3, 4, 11). In the study by Barnett et al. (3), macaques immunized with homologous SF162ΔV2 Env were protected from infection in response to vaginal challenge with SHIV-SF162P4. Similar results were observed in a study by Bogers et al. (4), where three out of four SF162ΔV2 Env protein-boosted macaques were protected from rectal challenge with SHIV-SF162P4. The SF162-derived gp140 oligomers used in these studies were previously shown to induce high levels of V1-specific Abs (11, 61), and V1-directed Abs are known to be highly potent but also strain specific (8, 32, 36, 51, 56). Recent attempts to compete out the neutralizing activity elicited by SF162-derived gp140 oligomers against SHIV-SF162P4 using unconstrained V1 or V3 peptides suggested that these specificities were not responsible for most of the activity (2). Nevertheless, Abs specific for variable regions of SF162, not detected in these peptide competition assays, may be an important subset contributing to the protection from infection with homologous SHIV-SF162P4. V1-directed Abs have been implied to be important for control of SHIV-SF162P3 infection, as variants escaping from immune pressure during infection with this virus exhibit mutations in this region (9).
As shown in our previous study, Env-specific CD4 and CD8 T cell responses prior to challenge were similar in the groups receiving wt, 368, or 423/425/431 trimers (13). These responses did not correlate with the reduction in cumulative viral loads in the current SHIV challenge experiment (data not shown). The initial control of SHIV-SF162P4 replication after immunization has been shown to be independent of CD8 T cells (5), and although infection induces a strong T cell response, this apparently does not correlate to viral clearance in SHIV-SF162P3 infection (47). In the present study we observed enhanced control of SHIV-SF162P4 viremia in all YU2 trimer-vaccinated animals, suggesting that immune responses that are partially protective against heterologous SHIV challenge are elicited by this vaccine regimen. Furthermore, one monkey (F77) was apparently protected from infection, with no detectable viral loads. Immune parameters that contributed to protection in this animal could not be identified, as the titers of total gp120 binding Abs, NAbs, and T cell responses in this animal were of similar magnitude to those in the other animals (13). We did not investigate other factors that may have influenced the acquisition of infection, such as early innate immune responses (33), major histocompatibility complex (MHC) haplotypes (16), or polymorphisms in genetic resistance factors (37), as part of the current effort, since this was not the main focus of the study.
An interesting additional observation from the current study was that the anamnestic NAb response stimulated by the SHIV challenge was increased by 1 order of magnitude over the prechallenge NAb titers. There was a strong correlation between the titers of HIV-1 Env binding Abs and the neutralizing Abs against the challenge virus. Since the anamnestic Abs reacted equally well with YU2- and SF162-derived gp120, the increase in NAb titers stimulated by the challenge virus likely resulted from efficient boosting of vaccine-induced specificities rather than elicitation of new SHIV Env-specific B cell responses by the heterologous infection. This shows that there is room to further improve the overall magnitude of the Ab responses elicited by the protein in the adjuvant vaccine regimen used here.
In conclusion, we show that immunization with soluble Env trimers in adjuvant elicited immune responses that resulted in improved control of the heterologous SHIV-SF162P4 virus. There was no difference between the animals immunized with CD4-binding-competent or CD4-binding-defective Env timers, suggesting that Env-CD4 in vivo interactions and the elicitation of CoRbs Abs did not influence the level of protection achieved. Follow-up studies are required to dissect the immune correlates of protection in this model. Further studies will also determine whether the response elicited by the YU2 Env trimers might be more effective in blunting infection of heterologous SHIV challenge via the vaginal route of administration or if the quality of the elicited responses can be enhanced by different prime-boost regimens, improved adjuvants, or modified immunogen design.
Acknowledgments
We thank Mats Spångberg, Helene Fredlund, and the personnel at Astrid Fagraeus laboratory at the Swedish Institute for Infectious Disease Control for expert assistance with the rhesus macaques; Eva Hansson-Pihlainen for help with reverse transcriptase assays; and Linda Stertman and Karin Lövgren Bengtsson at Isconova AB for the generous gift of Abisco-100. We thank James Robinson for providing MAbs 17b, 48d, F91, 1.5E, E51, and 2.1C; Susan Zolla-Pazner for MAb 447-52D; Marshal Posner for MAb F105; and Dennis Burton for the b6 and b12 MAbs. We also thank Leonidas Stamatatos for providing the SF162 Env cDNA and Dana Gabuzda for murine leukemia virus.
This study was supported by grants from the International AIDS Vaccine Initiative and the Swedish International Development Agency (Sida)/Department of Research Cooperation (SAREC), the National Institute of Allergy and Infectious Diseases, the National Institutes of Health intramural research program, and the Karolinska Institutet.
Footnotes
Published ahead of print on 7 July 2010.
REFERENCES
- 1.Balfe, P., S. Shapiro, M. Hsu, C. Buckner, J. M. Harouse, and C. Cheng-Mayer. 2004. Expansion of quasispecies diversity but no evidence for adaptive evolution of SHIV during rapid serial transfers among seronegative macaques. Virology 318:267-279. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, S. W., B. Burke, Y. Sun, E. Kan, H. Legg, Y. Lian, K. Bost, F. Zhou, A. Goodsell, J. Zur Megede, J. Polo, J. Donnelly, J. Ulmer, G. R. Otten, C. J. Miller, M. Vajdy, and I. K. Srivastava. 2010. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J. Virol. 84:5975-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett, S. W., I. K. Srivastava, E. Kan, F. Zhou, A. Goodsell, A. D. Cristillo, M. G. Ferrai, D. E. Weiss, N. L. Letvin, D. Montefiori, R. Pal, and M. Vajdy. 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22:339-348. [DOI] [PubMed] [Google Scholar]
- 4.Bogers, W. M., D. Davis, I. Baak, E. Kan, S. Hofman, Y. Sun, D. Mortier, Y. Lian, H. Oostermeijer, Z. Fagrouch, R. Dubbes, M. van der Maas, P. Mooij, G. Koopman, E. Verschoor, J. P. Langedijk, J. Zhao, E. Brocca-Cofano, M. Robert-Guroff, I. Srivastava, S. Barnett, and J. L. Heeney. 2008. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology 382:217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner, C., L. G. Gines, C. J. Saunders, L. Vojtech, I. Srivastava, A. Gettie, R. Bohm, J. Blanchard, S. W. Barnett, J. T. Safrit, and L. Stamatatos. 2004. Priming B cell-mediated anti-HIV envelope responses by vaccination allows for the long-term control of infection in macaques exposed to a R5-tropic SHIV. Virology 320:167-180. [DOI] [PubMed] [Google Scholar]
- 6.Chen, B., E. M. Vogan, H. Gong, J. J. Skehel, D. C. Wiley, and S. C. Harrison. 2005. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature 433:834-841. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W., Z. Zhu, Y. Feng, and D. S. Dimitrov. 2008. Human domain antibodies to conserved sterically restricted regions on gp120 as exceptionally potent cross-reactive HIV-1 neutralizers. Proc. Natl. Acad. Sci. U. S. A. 105:17121-17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ching, L. K., G. Vlachogiannis, K. A. Bosch, and L. Stamatatos. 2008. The first hypervariable region of the gp120 Env glycoprotein defines the neutralizing susceptibility of heterologous human immunodeficiency virus type 1 isolates to neutralizing antibodies elicited by the SF162gp140 immunogen. J. Virol. 82:949-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, H. K., J. Suschak, L. Galmin, N. Rose, and R. Pal. 2010. Characterization of a SHIV162P3 variant evolved in an infected rhesus macaque with persistent plasma viremia. Virus Res. 151:229-234. [DOI] [PubMed] [Google Scholar]
- 10.Decker, J. M., F. Bibollet-Ruche, X. Wei, S. Wang, D. N. Levy, W. Wang, E. Delaporte, M. Peeters, C. A. Derdeyn, S. Allen, E. Hunter, M. S. Saag, J. A. Hoxie, B. H. Hahn, P. D. Kwong, J. E. Robinson, and G. M. Shaw. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 201:1407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derby, N. R., Z. Kraft, E. Kan, E. T. Crooks, S. W. Barnett, I. K. Srivastava, J. M. Binley, and L. Stamatatos. 2006. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection. J. Virol. 80:8745-8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVico, A., T. Fouts, G. K. Lewis, R. C. Gallo, K. Godfrey, M. Charurat, I. Harris, L. Galmin, and R. Pal. 2007. Antibodies to CD4-induced sites in HIV gp120 correlate with the control of SHIV challenge in macaques vaccinated with subunit immunogens. Proc. Natl. Acad. Sci. U. S. A. 104:17477-17482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douagi, I., M. N. Forsell, C. Sundling, S. O'Dell, Y. Feng, P. Dosenovic, Y. Li, R. Seder, K. Lore, J. R. Mascola, R. T. Wyatt, and G. B. Karlsson Hedestam. 2010. Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates. J. Virol. 84:1683-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsell, M. N., B. Dey, A. Morner, K. Svehla, S. O'Dell, C. M. Hogerkorp, G. Voss, R. Thorstensson, G. M. Shaw, J. R. Mascola, G. B. Karlsson Hedestam, and R. T. Wyatt. 2008. B cell recognition of the conserved HIV-1 co-receptor binding site is altered by endogenous primate CD4. PLoS Pathog. 4:e1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsell, M. N., W. R. Schief, and R. T. Wyatt. 2009. Immunogenicity of HIV-1 envelope glycoprotein oligomers. Curr. Opin. HIV AIDS 4:380-387. [DOI] [PubMed] [Google Scholar]
- 16.Goulder, P. J., and D. I. Watkins. 2008. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8:619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray, E. S., P. L. Moore, I. A. Choge, J. M. Decker, F. Bibollet-Ruche, H. Li, N. Leseka, F. Treurnicht, K. Mlisana, G. M. Shaw, S. S. Karim, C. Williamson, and L. Morris. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 81:6187-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray, E. S., N. Taylor, D. Wycuff, P. L. Moore, G. D. Tomaras, C. K. Wibmer, A. Puren, A. DeCamp, P. B. Gilbert, B. Wood, D. C. Montefiori, J. M. Binley, G. M. Shaw, B. F. Haynes, J. R. Mascola, and L. Morris. 2009. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J. Virol. 83:8925-8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harouse, J. M., A. Gettie, T. Eshetu, R. C. Tan, R. Bohm, J. Blanchard, G. Baskin, and C. Cheng-Mayer. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3). J. Virol. 75:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 21.Hartley, O., P. J. Klasse, Q. J. Sattentau, and J. P. Moore. 2005. V3: HIV's switch-hitter. AIDS Res. Hum. Retroviruses 21:171-189. [DOI] [PubMed] [Google Scholar]
- 22.Hsu, M., C. Buckner, J. Harouse, A. Gettie, J. Blanchard, J. E. Robinson, and C. Cheng-Mayer. 2003. Antigenic variations in the CD4 induced sites of the CCR5-tropic, pathogenic SHIVsf162p3 gp120 variants. J. Med. Primatol. 32:211-217. [DOI] [PubMed] [Google Scholar]
- 23.Hsu, M., J. M. Harouse, A. Gettie, C. Buckner, J. Blanchard, and C. Cheng-Mayer. 2003. Increased mucosal transmission but not enhanced pathogenicity of the CCR5-tropic, simian AIDS-inducing simian/human immunodeficiency virus SHIV(SF162P3) maps to envelope gp120. J. Virol. 77:989-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu, M., S. H. Ho, P. Balfe, A. Gettie, J. Harouse, J. Blanchard, and C. Cheng-Mayer. 2005. A CCR5-tropic simian-HIV molecular clone capable of inducing AIDS in rhesus macaques. J. Acquir. Immune Defic. Syndr. 40:383-387. [DOI] [PubMed] [Google Scholar]
- 25.Hsu, S. T., and A. M. Bonvin. 2004. Atomic insight into the CD4 binding-induced conformational changes in HIV-1 gp120. Proteins 55:582-593. [DOI] [PubMed] [Google Scholar]
- 26.Hu, S. L., and L. Stamatatos. 2007. Prospects of HIV Env modification as an approach to HIV vaccine design. Curr. HIV Res. 5:507-513. [DOI] [PubMed] [Google Scholar]
- 27.Humbert, M., R. A. Rasmussen, R. Song, H. Ong, P. Sharma, A. L. Chenine, V. G. Kramer, N. B. Siddappa, W. Xu, J. G. Else, F. J. Novembre, E. Strobert, S. P. O'Neil, and R. M. Ruprecht. 2008. SHIV-1157i and passaged progeny viruses encoding R5 HIV-1 clade C env cause AIDS in rhesus monkeys. Retrovirology 5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khati, M., M. Schuman, J. Ibrahim, Q. Sattentau, S. Gordon, and W. James. 2003. Neutralization of infectivity of diverse R5 clinical isolates of human immunodeficiency virus type 1 by gp120-binding 2′F-RNA aptamers. J. Virol. 77:12692-12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraft, Z., N. R. Derby, R. A. McCaffrey, R. Niec, W. M. Blay, N. L. Haigwood, E. Moysi, C. J. Saunders, T. Wrin, C. J. Petropoulos, M. J. McElrath, and L. Stamatatos. 2007. Macaques infected with a CCR5-tropic simian/human immunodeficiency virus (SHIV) develop broadly reactive anti-HIV neutralizing antibodies. J. Virol. 81:6402-6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 31.Labrijn, A. F., P. Poignard, A. Raja, M. B. Zwick, K. Delgado, M. Franti, J. Binley, V. Vivona, C. Grundner, C. C. Huang, M. Venturi, C. J. Petropoulos, T. Wrin, D. S. Dimitrov, J. Robinson, P. D. Kwong, R. T. Wyatt, J. Sodroski, and D. R. Burton. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J. Virol. 77:10557-10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laird, M. E., T. Igarashi, M. A. Martin, and R. C. Desrosiers. 2008. Importance of the V1/V2 loop region of simian-human immunodeficiency virus envelope glycoprotein gp120 in determining the strain specificity of the neutralizing antibody response. J. Virol. 82:11054-11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letvin, N. L., S. S. Rao, V. Dang, A. P. Buzby, B. Korioth-Schmitz, D. Dombagoda, J. G. Parvani, R. H. Clarke, L. Bar, K. R. Carlson, P. A. Kozlowski, V. M. Hirsch, J. R. Mascola, and G. J. Nabel. 2007. No evidence for consistent virus-specific immunity in simian immunodeficiency virus-exposed, uninfected rhesus monkeys. J. Virol. 81:12368-12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Y., K. Svehla, M. K. Louder, D. Wycuff, S. Phogat, M. Tang, S. A. Migueles, X. Wu, A. Phogat, G. M. Shaw, M. Connors, J. Hoxie, J. R. Mascola, and R. Wyatt. 2009. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J. Virol. 83:1045-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, Y., K. Svehla, N. L. Mathy, G. Voss, J. R. Mascola, and R. Wyatt. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 80:1414-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim, S. Y., T. Rogers, T. Chan, J. B. Whitney, J. Kim, J. Sodroski, and N. L. Letvin. 2010. TRIM5alpha modulates immunodeficiency virus control in rhesus monkeys. PLoS Pathog. 6:e1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, J., A. Bartesaghi, M. J. Borgnia, G. Sapiro, and S. Subramaniam. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louder, M. K., A. Sambor, E. Chertova, T. Hunte, S. Barrett, F. Ojong, E. Sanders-Buell, S. Zolla-Pazner, F. E. McCutchan, J. D. Roser, D. Gabuzda, J. D. Lifson, and J. R. Mascola. 2005. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology 339:226-238. [DOI] [PubMed] [Google Scholar]
- 40.Luciw, P. A., E. Pratt-Lowe, K. E. Shaw, J. A. Levy, and C. Cheng-Mayer. 1995. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc. Natl. Acad. Sci. U. S. A. 92:7490-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malenbaum, S. E., D. Yang, and C. Cheng-Mayer. 2001. Evidence for similar recognition of the conserved neutralization epitopes of human immunodeficiency virus type 1 envelope gp120 in humans and macaques. J. Virol. 75:9287-9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mascola, J. R., M. K. Louder, C. Winter, R. Prabhakara, S. C. De Rosa, D. C. Douek, B. J. Hill, D. Gabuzda, and M. Roederer. 2002. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J. Virol. 76:4810-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morner, A., I. Douagi, M. N. Forsell, C. Sundling, P. Dosenovic, S. O'Dell, B. Dey, P. D. Kwong, G. Voss, R. Thorstensson, J. R. Mascola, R. T. Wyatt, and G. B. Karlsson Hedestam. 2009. Human immunodeficiency virus type 1 env trimer immunization of macaques and impact of priming with viral vector or stabilized core protein. J. Virol. 83:540-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Napier, K. B., Z. X. Wang, S. C. Peiper, and J. O. Trent. 2007. CCR5 interactions with the variable 3 loop of gp120. J. Mol. Model. 13:29-41. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura, Y., M. Shingai, R. Willey, R. Sadjadpour, W. R. Lee, C. R. Brown, J. M. Brenchley, A. Buckler-White, R. Petros, M. Eckhaus, V. Hoffman, T. Igarashi, and M. A. Martin. 2010. Generation of the pathogenic R5-tropic SHIVAD8 by serial passaging in rhesus macaques. J. Virol. 84:4769-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olshevsky, U., E. Helseth, C. Furman, J. Li, W. Haseltine, and J. Sodroski. 1990. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J. Virol. 64:5701-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pahar, B., X. Wang, J. Dufour, A. A. Lackner, and R. S. Veazey. 2007. Virus-specific T cell responses in macaques acutely infected with SHIV(sf162p3). Virology 363:36-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal, R., B. Taylor, J. S. Foulke, R. Woodward, M. Merges, R. Praschunus, A. Gibson, and M. Reitz. 2003. Characterization of a simian human immunodeficiency virus encoding the envelope gene from the CCR5-tropic HIV-1 Ba-L. J. Acquir. Immune Defic. Syndr. 33:300-307. [DOI] [PubMed] [Google Scholar]
- 49.Pantophlet, R., and D. R. Burton. 2006. GP120: target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 24:739-769. [DOI] [PubMed] [Google Scholar]
- 50.Phogat, S., and R. Wyatt. 2007. Rational modifications of HIV-1 envelope glycoproteins for immunogen design. Curr. Pharm. Des. 13:213-227. [DOI] [PubMed] [Google Scholar]
- 51.Pinter, A., W. J. Honnen, Y. He, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2004. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 78:5205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pitisuttithum, P., P. Gilbert, M. Gurwith, W. Heyward, M. Martin, F. van Griensven, D. Hu, J. W. Tappero, and K. Choopanya. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661-1671. [DOI] [PubMed] [Google Scholar]
- 53.Polacino, P., K. Larsen, L. Galmin, J. Suschak, Z. Kraft, L. Stamatatos, D. Anderson, S. W. Barnett, R. Pal, K. Bost, A. H. Bandivdekar, C. J. Miller, and S. L. Hu. 2008. Differential pathogenicity of SHIV infection in pig-tailed and rhesus macaques. J. Med. Primatol. 37(Suppl. 2):13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson, J. E., D. H. Elliott, E. A. Martin, K. Micken, and E. S. Rosenberg. 2005. High frequencies of antibody responses to CD4 induced epitopes in HIV infected patients started on HAART during acute infection. Hum. Antibodies 14:115-121. [PubMed] [Google Scholar]
- 56.Rong, R., B. Li, R. M. Lynch, R. E. Haaland, M. K. Murphy, J. Mulenga, S. A. Allen, A. Pinter, G. M. Shaw, E. Hunter, J. E. Robinson, S. Gnanakaran, and C. A. Derdeyn. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog. 5:e1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salazar-Gonzalez, J. F., E. Bailes, K. T. Pham, M. G. Salazar, M. B. Guffey, B. F. Keele, C. A. Derdeyn, P. Farmer, E. Hunter, S. Allen, O. Manigart, J. Mulenga, J. A. Anderson, R. Swanstrom, B. F. Haynes, G. S. Athreya, B. T. Korber, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2008. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82:3952-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheid, J. F., H. Mouquet, N. Feldhahn, M. S. Seaman, K. Velinzon, J. Pietzsch, R. G. Ott, R. M. Anthony, H. Zebroski, A. Hurley, A. Phogat, B. Chakrabarti, Y. Li, M. Connors, F. Pereyra, B. D. Walker, H. Wardemann, D. Ho, R. T. Wyatt, J. R. Mascola, J. V. Ravetch, and M. C. Nussenzweig. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636-640. [DOI] [PubMed] [Google Scholar]
- 59.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362-373. [DOI] [PubMed] [Google Scholar]
- 60.Shu, Y., S. Winfrey, Z. Y. Yang, L. Xu, S. S. Rao, I. Srivastava, S. W. Barnett, G. J. Nabel, and J. R. Mascola. 2007. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine 25:1398-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srivastava, I. K., K. VanDorsten, L. Vojtech, S. W. Barnett, and L. Stamatatos. 2003. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J. Virol. 77:2310-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan, R. C., J. M. Harouse, A. Gettie, and C. Cheng-Mayer. 1999. In vivo adaptation of SHIV(SF162): chimeric virus expressing a NSI, CCR5-specific envelope protein. J. Med. Primatol. 28:164-168. [DOI] [PubMed] [Google Scholar]
- 63.Willey, R., M. C. Nason, Y. Nishimura, D. A. Follmann, and M. A. Martin. 2010. Neutralizing antibody titers conferring protection to macaques from a simian/human immunodeficiency virus challenge using the TZM-bl assay. AIDS Res. Hum. Retroviruses 26:89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, X., A. Sambor, M. C. Nason, Z. Y. Yang, L. Wu, S. Zolla-Pazner, G. J. Nabel, and J. R. Mascola. 2008. Soluble CD4 broadens neutralization of V3-directed monoclonal antibodies and guinea pig vaccine sera against HIV-1 subtype B and C reference viruses. Virology 380:285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiang, S. H., P. D. Kwong, R. Gupta, C. D. Rizzuto, D. J. Casper, R. Wyatt, L. Wang, W. A. Hendrickson, M. L. Doyle, and J. Sodroski. 2002. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 76:9888-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang, X., J. Lee, E. M. Mahony, P. D. Kwong, R. Wyatt, and J. Sodroski. 2002. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J. Virol. 76:4634-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao, J., L. Lai, R. R. Amara, D. C. Montefiori, F. Villinger, L. Chennareddi, L. S. Wyatt, B. Moss, and H. L. Robinson. 2009. Preclinical studies of human immunodeficiency virus/AIDS vaccines: inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia. J. Virol. 83:4102-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]