Abstract
An effective human immunodeficiency virus (HIV) vaccine will likely need to reduce mucosal transmission and, if infection occurs, control virus replication. To determine whether our best simian immunodeficiency virus (SIV) vaccine can achieve these lofty goals, we vaccinated eight Indian rhesus macaques with SIVmac239Δnef and challenged them intrarectally (i.r.) with repeated low doses of the pathogenic heterologous swarm isolate SIVsmE660. We detected a significant reduction in acquisition of SIVsmE660 in comparison to that for naïve controls (log rank test; P = 0.023). After 10 mucosal challenges, we detected replication of the challenge strain in only five of the eight vaccinated animals. In contrast, seven of the eight control animals became infected with SIVsmE660 after these 10 challenges. Additionally, the SIVsmE660-infected vaccinated animals controlled peak acute virus replication significantly better than did the naïve controls (Mann-Whitney U test; P = 0.038). Four of the five SIVsmE660 vaccinees rapidly brought virus replication under control by week 4 postinfection. Unfortunately, two of these four vaccinated animals lost control of virus replication during the chronic phase of infection. Bulk sequence analysis of the circulating viruses in these animals indicated that recombination had occurred between the vaccine and challenge strains and likely contributed to the increased virus replication in these animals. Overall, our results suggest that a well-designed HIV vaccine might both reduce the rate of acquisition and control viral replication.
The goals of any human immunodeficiency virus (HIV) vaccine are both to prevent infection and, if infection occurs, to control virus replication. If vaccinated individuals who become infected are able to reduce virus replication to extremely low or undetectable levels, they will live longer, healthier lives and will be less likely to transmit the virus to others (7, 16, 41). An HIV vaccine that successfully meets these two goals will therefore have a significant impact on slowing the spread of HIV (3).
Live-attenuated simian immunodeficiency virus (SIV) vaccines have proven to be universally effective at protecting macaques against homologous virus challenges, regardless of the route of transmission (10, 21, 33, 36, 50, 51). For this reason, live-attenuated SIV vaccines are considered the “gold standard” of protection in the SIV/rhesus macaque model of HIV infection (25). Previously, we and others showed that SIVmac239Δnef-vaccinated animals can reduce plasma virus replication after intravenous (i.v.) inoculation with the uncloned heterologous swarm virus SIVsmE660 (43, 50). This vaccine-induced effect was most pronounced, particularly during acute infection, in animals expressing major histocompatibility complex (MHC) class I alleles (Mamu-A*01, -B*08, and -B*17) previously associated with control of pathogenic SIVmac239 replication (29, 38, 43, 52, 54). Despite these encouraging results for this subset of animals, and in contrast to previous studies using homologous virus challenges, most of the vaccinated animals failed to maintain control of virus replication of the challenge strain during the chronic phase of infection.
There are several potential explanations for why SIVmac239Δnef vaccination was not as effective against i.v. exposure to the heterologous challenge virus (1, 43, 50). First, sequence variation between the vaccine and infecting strains may have rendered the vaccine-induced immune responses ineffective at controlling chronic-phase virus replication. Second, unlike the case in homologous SIVmac239 challenge studies using cloned viral stocks, the heterologous SIVsmE660 isolate contains many quasispecies within the inoculum (23, 49). Third, the heterologous challenges were administered i.v., thereby bypassing any potentially protective vaccine-induced immune responses at mucosal surfaces. All of the SIVsmE660 quasispecies in the inoculum therefore had the potential to infect cells and to establish a reservoir of viral diversity. This broad spectrum of viral diversity may have contributed to the decreased efficacy of SIVmac239Δnef-induced immune responses in protecting against heterologous virus replication after a high-dose i.v. challenge.
Since a large i.v. dose of multiple quasispecies of heterologous virus might overwhelm any potentially protective vaccine-induced immune responses, we tested the possibility that SIVmac239Δnef vaccination may be more efficacious against a more physiologically relevant low-dose challenge. In the SIV/rhesus macaque model of HIV infection, repeated low doses of pathogenic SIV more accurately reflect human sexual transmission than a single high-dose i.v. challenge does (32). Keele et al. recently established that one to three virus strains typically cross mucosal barriers to establish HIV infections (22). We and others observed similar results using repeated-dose mucosal challenge of macaques (23, 49). This model therefore facilitates the testing of vaccines in a more physiologically relevant manner.
MATERIALS AND METHODS
Animals and viruses.
Indian rhesus macaques (Macaca mulatta) from the Wisconsin National Primate Research Center were housed and cared for according to the regulations set forth in the Guide for the Care and Use of Laboratory Animals of the National Research Council (38a), as approved by the University of Wisconsin Institutional Animal Care and Use Committee. Eight macaques were inoculated intravenously with 0.5 ng p27 of SIVmac239Δnef, provided by R. Desrosiers (New England Primate Research Center, Southborough, MA), and were challenged approximately 7 months later. All animals were challenged intrarectally (i.r.) with up to five inoculations of 6 × 106 copies of SIVsmE660 (225 50% tissue culture infective doses [TCID50]), on a weekly basis. If an animal remained uninfected after these five challenges, up to an additional five inoculations with 1.2 × 107 copies of SIVsmE660 (450 TCID50) were administered on a weekly basis. Challenges were stopped once an animal tested positive for SIVsmE660 infection by quantitative real-time PCR (QRT-PCR).
IFN-γ ELISPOT assays.
Fresh peripheral blood mononuclear cells (PBMC) isolated from EDTA-anticoagulated blood were used in enzyme-linked immunospot (ELISPOT) assays for the detection of gamma interferon (IFN-γ)-secreting cells as previously described (28), with the exception of how positive responses were determined. Test wells were run with two replicates, while control wells were run with replicates of 2, 4, or 6, depending on the assay. Responses comprising <50 spot-forming cells (SFC) per 1 × 106 cells were considered negative and were not tested statistically. Positive responses were determined using a one-tailed t test and an alpha level of 0.05, where the null hypothesis was that the background level would be greater than or equal to the treatment level. If determined to be positive statistically, the values were reported as the average of the test wells minus the average of all negative-control wells. Peptides used in these assays were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
Additionally, we examined CD4+ T-cell responses by depleting PBMC of CD8+ cells by use of a CD8 microbead kit for nonhuman primates (Miltenyi Biotec Inc., Bergisch Gladbach, Germany) according to the manufacturer's instructions.
When possible, we mapped pool-specific responses down to the individual 15-mer(s) responsible for eliciting the positive response, using IFN-γ ELISPOT assays. We included whole PBMC or PBMC depleted of CD8+ cells, along with the individual 15-mers comprising the pool in the assay. The 15-mer peptides eliciting positive responses in these assays were used to monitor anamnestic immune responses postchallenge. We classified responses as being mediated by either CD8+ or CD4+ T cells, based on which population of cells the responses were detected in. Responses detected in whole PBMC but not PBMC depleted of CD8+ cells were classified as CD8+ T-cell responses. Conversely, responses detected in assays performed with PBMC depleted of CD8+ cells were classified as being mediated by CD4+ T cells.
SIV neutralization assays.
We measured the neutralization of SIVsmE660 as a function of reductions in Tat-regulated Luc reporter gene expression after a single round of infection of TZM-bl cells, as previously described (37). TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program. TZM-bl cells are a CXCR4-positive HeLa cell clone engineered to express CD4, CCR5, and integrated reporter genes for firefly luciferase and Escherichia coli β-galactosidase under the control of an HIV long-terminal repeat sequence (40, 48). Briefly, TZM-bl cells were plated at 1 × 104 cells per well with a 1:4 dilution of the virus mixed with DEAE-dextran at 20 μg/ml in a 96-well flat-bottomed plate (Corning Inc., Corning, NY) and then were incubated for 24 h at 37°C in 5% CO2. Heat-inactivated sera were serially diluted along with the positive control, CD4 IgG2. One hundred microliters of medium containing virus yielding approximately 100,000 relative light units (RLU) was mixed with serially diluted sera, incubated for 1 h at 37°C, added to the cells, and incubated for a further 48 h. The wells were aspirated and washed once with phosphate-buffered saline (PBS), followed by addition of 60 μl of luciferase cell culture lysis reagent (Promega, Madison, WI). The lysate was mixed by pipetting, 50 μl was transferred to a round-bottomed plate (Corning), and the plate was centrifuged at 1,800 × g for 10 min at 4°C. Twenty microliters was transferred to an opaque assay plate (Corning), and the luciferase activity was measured on a luminometer (EG&G Berthold LB 96V; Perkin Elmer, Gaithersburg, MD), using luciferase assay reagent (Promega).
To measure neutralizing activity against SIVmac239, we used a single-round infectious SIVmac239 pseudovirus, with TZM-bl cells again serving as targets for infection. Pseudoviruses were generated in 293T cells as described previously (55). Briefly, pseudoviruses producing approximately 100,000 RLU were incubated for 1 h at 37°C with serially diluted sera and control CD4 IgG2. TZM-bl cells were resuspended in medium containing a final concentration of dextran of 20 μg/ml, washed, counted, and plated at 1 × 104 cells per well over the incubated solution of virus and sera for an additional 48 h. The luciferase activity was measured in the same manner as that described above for the TZM-bl cell assay. The percentage of virus neutralization at a given antibody concentration was determined by calculating the reduction in luciferase activity in the presence of antibody relative to that in virus-only wells.
Viral load determination.
Levels of circulating plasma virus were determined using a previously described QRT-PCR assay which amplifies both SIVmac239Δnef and SIVsmE660 (13).
The challenge strain SIVsmE660 was differentiated from SIVmac239Δnef in vaccinated animals via a second QRT-PCR assay run on the same viral RNA (vRNA) used for quantitating virus concentrations, as previously described (43).
Sequencing of plasma viral RNA.
vRNAs were extracted from plasma samples as previously described (13). Samples were sequenced at 6 weeks post-SIVsmE660 infection for animal 02016 and at 4 months post-SIVsmE660 infection for animals 03004 and 02127. Sequencing was performed as previously described (43).
Statistics.
Kaplan-Meier survival analyses were used to test whether vaccinated (n = 8) and control (n = 8) animals differed in the number of challenges required until infection. To determine whether sex and age were important covariates, we also ran Cox regressions for these comparisons, with a nominal alpha value of 0.05. Neither sex nor age was significant, while the treatment effect (control versus vaccinated) was significant, so the nonparametric Kaplan-Meier analysis was retained. All analyses were performed in SAS 9.2 (SAS Institute, NC).
To test whether SIVmac239Δnef-vaccinated and unvaccinated animals had different maximum viral loads, we started with a general linear model (using sex and age as covariates) in Minitab 14 (Minitab Inc., PA). Since normality of the standardized raw maximum viral load was rejected (Anderson-Darling test) for both data sets, the maximum viral load was log transformed and retested (P = 0.372). Homoscedasticity of transformed raw measurements was not rejected (Levene's test; P = 0.111). With a nominal alpha value of 0.05, we found no support for either sex or age being statistically significant. While we found no evidence for the assumptions of a standard general linear model being violated, we opted to report the more conservative Mann-Whitney U test results due to the inherent difficulty (low power) of testing such assumptions with a small sample size.
To test whether viral loads (log transformed) were significantly different between vaccinated and control groups during the first 20 weeks after infection with SIVsmE660, we first ran a repeated-measures analysis of variance (ANOVA) with an autoregressive covariance structure. Initially, we included sex and age as covariates, but these were not significant, so they were removed from the model. We employed multiple contrasts, using ANOVA, to test whether vaccinated and control individuals differed in their viral loads each week. The model fit was ascertained by examining the Student and Pearson residual plots in SAS 9.2. As a sensitivity analysis, we also ran four Mann-Whitney U tests (Minitab 14).
RESULTS
Immunizing macaques with SIVmac239Δnef.
To address whether an HIV vaccine can affect both the acquisition of infection and viral replication after infection, we used a well-described macaque AIDS model of protective immunity. We immunized eight rhesus macaques with the live-attenuated SIV strain SIVmac239Δnef (Table 1). None of the vaccinated animals expressed MHC class I alleles previously associated with control of SIVmac239 (Mamu-A*01, -B*08, or -B*17) (29, 38, 52, 54). The animals had a peak of virus replication 2 weeks after inoculation with SIVmac239Δnef of between 2.3 × 104 and 1.1 × 106 vRNA copy equivalents (Eq)/ml of plasma. Six of the eight animals resolved SIVmac239Δnef replication to undetectable or nearly undetectable levels by 7 months postinoculation (see Fig. S1 in the supplemental material). The two remaining animals had plasma virus levels of between 2 × 103 and 1.2 × 104 vRNA copy Eq/ml. This level of replication is relatively high but, in our experience, not uncommon for SIVmac239Δnef-immunized animals.
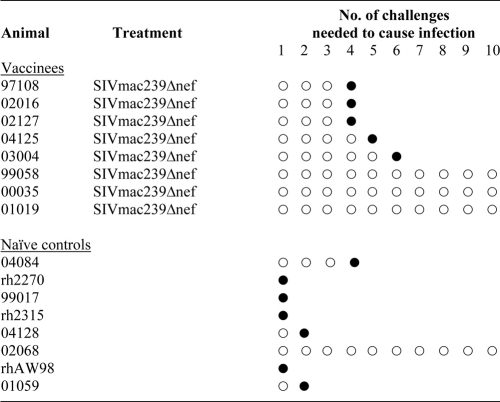
TABLE 1.
Challenge results for rhesus macaques used in this studya
○, challenge that did not result in detectable SIVsmE660 infection; •, challenge that resulted in detectable SIVsmE660 infection.
Vaccine-induced immune responses.
Throughout the course of the study, we monitored the development of vaccine-induced immune responses. We identified CD8+ and CD4+ T-cell responses by using whole PBMC and PBMC depleted of CD8+ cells, respectively, in IFN-γ ELISPOT assays containing pools of peptides corresponding to the entire SIVmac239 proteome. When possible, we broke down the pool-specific responses to the individual 15-mer peptides by using IFN-γ ELISPOT assays (see Table S1 in the supplemental material). Although none of the animals in the study expressed the MHC class I molecules Mamu-A*01, -B*08, and -B*17, one of the vaccinated animals (03004) expressed Mamu-A*02, for which minimal optimal epitopes have previously been identified (30, 44, 47). Additionally, three of the vaccinated animals expressed Mamu-B*48 (animals 97108, 02127, and 04125), which binds an immunodominant epitope in Gag (Gag276-283RI8) (44). Therefore, we also included peptides corresponding to these known epitopes in the IFN-γ ELISPOT assays.
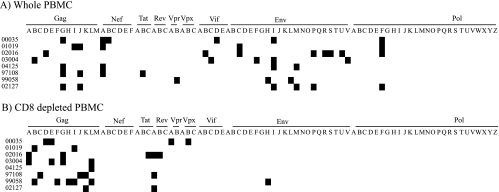
We detected a broad number of CD8+ T-cell responses directed against several viral proteins (Fig. 1 A; see Table S1 in the supplemental material). We identified an average of five, ranging from three to eight, peptide pool-specific CD8+ T-cell responses per animal. Additionally, we detected one to six CD4+ T-cell responses per animal, with a mean of 3.5 pool-specific responses (Fig. 1B; see Table 1 in the supplemental material). These CD4+ T-cell responses, however, were directed primarily against the Gag viral protein. Collectively, the cellular immune responses we detected are consistent with those in previous studies investigating live-attenuated SIV vaccines (15, 31, 43).
FIG. 1.
Vaccine-induced cellular immune responses. Cellular immune responses against pools of peptides, detected using whole PBMC (A) or PBMC depleted of CD8+ cells (B) in IFN-γ ELISPOT assays during the vaccine phase of the study. For each animal, the black boxes indicate positive responses against specific pools.
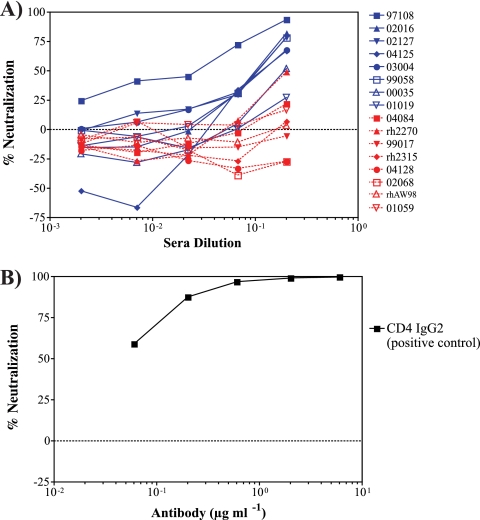
Since vaccine-induced antibodies might be important in blocking infection or controlling virus replication, we also assessed the levels of neutralizing antibodies induced by SIVmac239Δnef vaccination. Sera collected from the vaccinated animals on the day of the first SIVsmE660 challenge displayed weak neutralizing activity against the parental SIVmac239 strain (Fig. 2), with the highest titers detected in animal 97108. Sera isolated from the naïve controls at the same time point displayed little to no neutralizing activity against SIVmac239 (Fig. 2). Additionally, we found no neutralizing activity against SIVsmE660 in the sera from any of the vaccinated animals on the day of the first SIVsmE660 challenge (data not shown).
FIG. 2.
Weak in vitro neutralization of SIVmac239 by vaccine-induced antibodies. (A) Percent neutralization of SIVmac239, after a single round of replication in a luciferase reporter assay using a SIVmac239-pseudotyped virus, by sera from SIVmac239Δnef-vaccinated animals (blue) or naïve controls (red) collected on the day of first challenge with SIVsmE660. (B) Representative data for CD4 IgG2, which was used as a positive control for each assay. Percent neutralization is presented as the reduction of luciferase activity at the end of the assay in comparison to that for cells infected with the pseudotyped virus alone. Negative values represent an enhancement of infection.
Repeated low-dose SIVsmE660 challenge.
To test the protective efficacy of SIVmac239Δnef-induced immune responses against a physiologically relevant model, we challenged the eight vaccinees, along with eight naïve controls also not expressing Mamu-A*01, -B*08, or -B*17, with repeated low-dose mucosal exposures of SIVsmE660 approximately 7 months after vaccination with SIVmac239Δnef. SIVsmE660 differs from SIVmac239 by approximately 15% of its amino acids (43). This correlates well with the estimated difference between consensus clade-based HIV vaccines and circulating strains within that clade (14). The animals were first challenged with 6 × 106 vRNA copy Eq of SIVsmE660, but if an animal remained uninfected after the fifth inoculation, the challenge dose was increased to 1.2 × 107 vRNA copy Eq for an additional five inoculations. We monitored SIV infection on a weekly basis, using SIV-specific primers in a QRT-PCR assay. Additionally, to distinguish between SIVmac239Δnef and SIVsmE660 replication in the vaccinated animals, we designed SIVsmE660-specific primers targeting the region of nef deleted in SIVmac239Δnef. However, for consistency and ease of comparison to previous SIV challenge studies, all plasma virus concentrations are reported for the QRT-PCR assay for SIV Gag that enumerates both SIVmac239Δnef and SIVsmE660 (9, 13). Challenges were terminated once an animal tested positive for SIVsmE660.
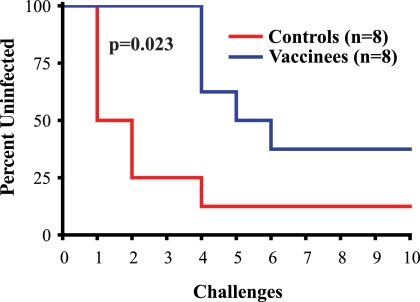
Surprisingly, the SIVmac239Δnef-vaccinated animals showed a reduced rate of SIVsmE660 acquisition after repeated low-dose mucosal challenges. We infected seven of the eight control animals after 10 exposures to SIVsmE660. Of the seven infected animals in the control group, four were infected with a single challenge and two more were infected after the second challenge (Table 1). In contrast, SIVmac239Δnef vaccination resulted in a significant delay in the acquisition of SIVsmE660 infection (log rank test; chi square = 5.1968; df = 1; P = 0.023) (Fig. 3). We detected SIVsmE660 replication in only five of the eight vaccinated macaques after 10 challenges, and it took until the fourth dose to infect the first animals (Table 1; Fig. 3).
FIG. 3.
Kaplan-Meier rate of infection after repeated low-dose inoculations with SIVsmE660. The percentage of animals vaccinated with SIVmac239Δnef or control animals remaining uninfected after each of the 10 inoculations with SIVsmE660 is shown. The statistical significance of the difference between SIVmac239Δnef-vaccinated and control animals was determined by the log rank test.
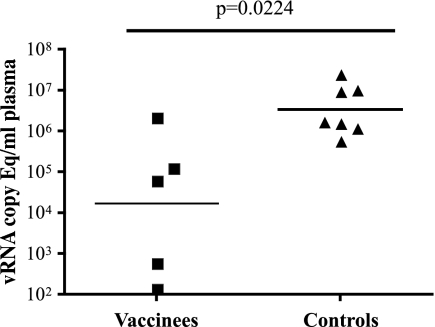
Acute plasma virus replication.
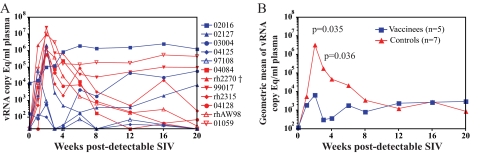
A secondary goal of any HIV vaccine should be to reduce virus replication if infection occurs. We therefore monitored the effect that SIVmac239Δnef vaccination had on plasma virus replication after repeated mucosal challenges with SIVsmE660. The vaccinated animals showed significantly lowered peak acute virus replication in comparison to the infected controls (Mann-Whitney U test; W = 19.0; P = 0.038) (Fig. 4). The vaccinated animals had peaks of virus replication during the first 3 weeks of SIVsmE660 infection ranging from 140 to 2.2 × 106 vRNA copies/ml plasma (Fig. 5 A). We detected SIVsmE660 replication on successive weeks in animal 04125, but the concentration of virus in the plasma was never higher than 140 vRNA copies/ml and returned to undetectable levels by week 3 postinfection (p.i.). Three of the four remaining vaccinated animals rapidly brought virus replication under control, to 500 vRNA copies/ml plasma or less, by week 4 p.i. As a group, the vaccinated animals had significantly lower plasma virus levels at weeks 2 (F test; P = 0.035) and 3 (P = 0.036) p.i. than those of the SIVsmE660-infected controls (Fig. 5B). The only vaccinated animal that did not appreciably reduce acute-phase SIVsmE660 replication was animal 02016. This animal also had the highest levels of SIVmac239Δnef replication prior to challenge.
FIG. 4.
Peak acute plasma virus concentrations after infection with SIVsmE660. A scatter plot comparison of the peak plasma virus replication occurring at any point during the first 3 weeks of infection with SIVsmE660 is shown for the SIVmac239Δnef-vaccinated macaques and their controls. The line represents the geometric mean for each group. The differences between the vaccinated and control animals are statistically significant (Mann-Whitney U test).
FIG. 5.
Plasma virus levels after detectable SIVsmE660 replication. (A) vRNA copies per ml of plasma for the individual vaccinated (blue) and control (red) animals in the weeks following the first detection of SIVsmE660. The week prior to detectable virus replication is labeled week 0. (B) Geometric means of vRNA copy Eq/ml for the vaccinated (blue) and control (red) groups during the first 20 weeks after detectable SIVsmE660 replication. The differences between the geometric means of the plasma virus concentrations of the vaccinees and controls were significant at weeks 2 and 3 (Mann-Whitney U test). †, animal rh2270 was euthanized at day 17 p.i. due to an underlying medical condition not directly related to SIV infection.
Despite control of acute SIVsmE660 replication by four of the five vaccinated macaques, two of these animals (03004 and 02127) displayed gradually increasing plasma virus levels during the chronic phase of infection (Fig. 5A). Bulk sequencing of circulating viruses from animals 03004, 02127, and 02016 indicated that recombination occurred between the challenge and vaccine strains of the virus (data not shown). Recombination occurred rapidly in animal 02016, as we found evidence for recombination at week 6 p.i. with SIVsmE660. We screened for and detected recombination at 4 months p.i. in animals 03004 and 02127. However, the recombination events between the two viral strains likely occurred earlier during infection. Previous studies have indicated that recombination between live-attenuated and pathogenic strains of SIV can result in the outgrowth of more-virulent strains within the vaccinated animals (17, 35, 43). Therefore, recombination may have contributed to the loss of viral control during the chronic phase of infection for three of the vaccinated animals, in particular animals 03004 and 02127.
Anamnestic cellular immune responses.
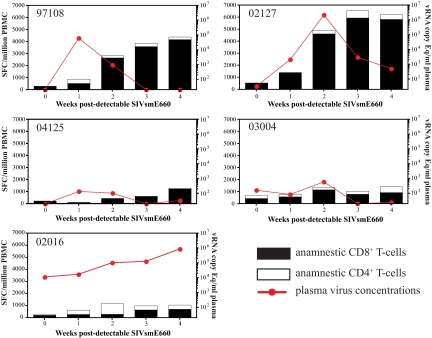
Successful control of acute virus replication by the vaccinated animals after SIVsmE660 infection might have been due to rapid expansion of anamnestic cellular immune responses. To assess this possibility, we monitored vaccine-induced cellular immune responses on a weekly basis for up to 4 weeks after detectable infection with SIVsmE660. We used IFN-γ ELISPOT assay mixtures containing freshly isolated PBMC and peptides identified during the vaccine phase of the study (see Table S1 in the supplemental material). Due to constraints on cell numbers, we were unable to use PBMC depleted of CD8+ cells to monitor CD4+ T-cell responses. However, peptides identified as eliciting virus-specific CD4+ T-cell responses during the vaccine phase of the study were included in our weekly screen of anamnestic immune responses in whole PBMC.
We detected expansion of anamnestic cellular immune responses in the PBMC of the SIVmac239Δnef-vaccinated animals during the acute phase of SIVsmE660 infection (Fig. 6). The sum of the peptide-specific responses at least doubled for each animal during the acute phase of infection in comparison to the presumed day of infection. Additionally, for all of the animals except for animal 04125, we detected an increase in the number of SFC in the PBMC directed against peptides identified during the vaccine phase as inducing CD4+ T-cell responses. The most dramatic expansion of anamnestic responses was observed in animals 02127 and 97108, and these responses were associated with a rapid decline in plasma virus levels (Fig. 6). Both animals 02127 and 97108 expressed the MHC class I molecule Mamu-B*48, which restricts the immunodominant epitope Gag276-283RI8. A large fraction of the responding cells in these two animals were directed against this single epitope, which is conserved between SIVmac239Δnef and SIVsmE660. We detected less-dramatic expansions of anamnestic responses in animals 03004 and 04125, despite effective control of SIVsmE660 replication, to below 600 vRNA copy Eq/ml plasma, in these animals during the acute phase of infection. The reduced antigen burdens in these animals, however, likely contributed to the relatively modest expansion of anamnestic responses in comparison to those in animals 02127 and 97108, which had much higher peaks of plasma virus replication. It is noteworthy that animal 04125 also expressed Mamu-B*48 and that responses directed against Gag276-283RI8 constituted virtually all of the anamnestic responses detected in this animal.
FIG. 6.
Expansion of anamnestic cellular immune responses after detectable infection with SIVsmE660. The sum of SFC in IFN-γ ELISPOT assays using 15-mer peptides identified as inducing positive responses during the vaccine phase of the study is shown for each animal (see Table S1 in the supplemental material). Whole PBMC were used in the assays, but peptides stimulating CD8+ T cells are represented by black boxes and peptides stimulating CD4+ T cells are represented by white boxes. The responses are plotted with the plasma virus concentrations during the first 4 weeks after detectable SIVsmE660 infection. Week 0 is the week prior to detectable SIVsmE660 infection.
In contrast to the other vaccinated animals, animal 02016 had high plasma virus levels and minimal expansion of anamnestic cellular immune responses. The sum of the vaccine-induced CD8 T-cell responses increased from only 215 SFC/million PBMC on the day of challenge to 715 SFC/million PBMC by week 4 after detectable SIVsmE660 infection. This expansion of responses pales in comparison to those we observed in animals 02127 and 97108, even though all three animals had similar peak acute plasma virus levels. Note that animal 02016 had the largest expansion of CD4+ T-cell responses of any of the SIVmac239Δnef-vaccinated animals. As noted earlier, we detected recombination between the vaccine and challenge strains of the virus in animal 02016 early after infection with SIVsmE660. It is difficult, therefore, to determine what caused the failure of this vaccinated animal to control SIVsmE660 replication at any time point postinfection.
We also monitored the expansion of anamnestic cellular immune responses on a weekly basis for the SIVmac239Δnef-vaccinated animals that did not become productively infected with SIVsmE660. We did not detect any significant increases in the levels of circulating virus-specific cellular immune responses during the challenge phase of the study for any of these animals (data not shown). However, it is possible that cellular immune responses located at the site of inoculation may have contributed to these animals being protected from becoming infected productively with SIVsmE660. Additionally, at the end of the 10 challenges, we performed an IFN-γ ELISPOT assay with whole PBMC and peptide pools corresponding to the deleted and out-of-frame region of Nef. The amino acid sequences of the peptides in these pools corresponded to those in SIVsmE543, a molecular clone closely related to SIVsmE660. We did not detect any de novo responses against this region of Nef (data not shown), which suggests that these animals were not occultly infected with SIVsmE660.
At the end of the 10 challenges, we assessed whether SIV-specific immune responses were generated by the only naïve control, animal 02068, to remain uninfected with SIVsmE660. We did not detect cellular immune responses by using freshly isolated PBMC in IFN-γ ELISPOT assays containing pools of peptides corresponding to the SIVsmE543 proteome. We also did not detect evidence of the induction of humoral immune responses by Western blotting with SIVmac239 proteins or neutralizing antibodies against SIVmac239 (data not shown). These results are consistent with a previous study investigating naïve animals that resisted infection after repeated mucosal SIV challenges (26).
DISCUSSION
The implications of this study are that a properly designed HIV vaccine can affect both the acquisition of HIV and viral replication. This is in contrast to recent findings from preclinical vaccine studies of macaques with the repeated-low-dose challenge model. These previous studies, using a DNA prime-adenovirus 5 (Ad5) boost regimen or recombinant rhesus cytomegalovirus (RhCMV) to immunize macaques, either protected against infection or controlled acute and chronic viral replication, but not both (18, 49). Using a DNA prime-Ad5 boost regimen with a vaccine encoding all of the SIV proteins except the viral envelope (Env), we recently achieved long-term control of virus replication in six of eight vaccinated animals (49). However, this DNA prime-Ad5 boost vaccine regimen had no effect in delaying acquisition of SIVsmE660 after repeated low-dose challenges. This suggests that there are fundamental differences between the SIVmac239Δnef and DNA prime-Ad5 boost vaccine regimens. SIVmac239Δnef contains Env and might have induced antibody responses important for eliminating virus particles at the portal of entry (19, 20). It is also possible that the continuously replicating live-attenuated SIVmac239Δnef vaccine, like the RhCMV vaccine, may have induced the correct type of cellular immune responses of sufficient magnitude at the portal of entry to rapidly eliminate virally infected cells (27). However, the RhCMV-based vaccine did not control virus replication in the vaccinated animals that became infected. Seemingly, there are essential components present in live-attenuated SIV vaccines that allow them to be effective against infection and in limiting virus replication.
Recent results emerging from a Thai vaccine trial suggest that a reduction in the acquisition of HIV is possible (42). Even the modest protection against HIV infection observed in that study is an improvement over previous large-scale HIV vaccine efficacy trials that either failed to protect against HIV entirely or possibly even enhanced the rate of HIV infection (6, 12, 39). Unfortunately, the Thai vaccine failed to have an effect on virus replication in vaccinated individuals (42). Therefore, the long-term value of the Thai vaccine trial will likely come from determining the precise correlates of protection. However, dissecting out these mechanisms in humans, given the marginally effective nature of the vaccine, will be daunting. Live-attenuated SIV vaccination in the nonhuman primate model of HIV infection may provide an alternative and useful tool for determining the correlates of protection of an effective anti-AIDS virus vaccine.
The mechanisms underlying the protection afforded by live-attenuated SIV vaccination still need to be elucidated fully, particularly at the portal of entry. We know that virus-specific CD8+ T cells induced by live-attenuated SIV likely play an important role in controlling pathogenic SIV infection (11, 21, 33, 43, 46). Probably the strongest evidence supporting this view is that transient depletion of peripheral CD8+ cells during chronic infection leads to a recrudescence of plasma virus replication in live-attenuated SIV-vaccinated animals (33, 43). However, the depleting anti-CD8 antibody used in these studies also removes NK cells, which may play an important supportive role in suppression of virus replication, which complicates this analysis. In the current study, we detected broad but modest (in magnitude) vaccine-induced cellular immune responses prior to the initiation of challenges, consistent with previous studies investigating live-attenuated SIV vaccines (31, 43). In the vaccinated animals that became infected productively with SIVsmE660, we observed an expansion of anamnestic cellular immune responses during the acute phase of infection. Expansion of anamnestic responses was associated with a decline in plasma virus concentrations in four of the five SIVsmE660-infected vaccinated animals. This provides further evidence that vaccine-induced cellular immune responses contribute to controlling heterologous virus infection.
Recent studies indicated that one to three virus strains typically establish infection after sexual transmission of HIV, and this was recapitulated in the repeated-low-dose SIV challenge model (23, 49). Therefore, it is possible that vaccine-induced immune responses located at the site of infection can eliminate virally infected cells before they establish persistent infection. In the vaccinated animals protected from SIVsmE660 infection, we did not detect evidence of expansion of anamnestic immune responses in the PBMC that would have indicated that vaccine-induced T cells were actively interacting with virally infected cells. However, virus-specific T cells located in mucosal tissues are often effector memory cells with limited potential to expand (8, 45). Therefore, if vaccine-induced CD4+ and CD8+ T cells rapidly eliminate virally infected cells at the site of infection, we are unlikely to detect evidence of this interaction by using PBMC in IFN-γ ELISPOT assays. Further study is warranted to determine what role, if any, vaccine-induced mucosal immune responses play in protective immunity.
The role of SIV-specific antibodies in protection is less clear. Passive transfer of serum alone from a live-attenuated SIV-vaccinated animal does not protect the recipient from intravenous challenge with pathogenic SIV (2). This, along with the increase in virus replication after CD8+ T-cell depletion, suggests that neutralizing antibodies alone are not responsible for live-attenuated SIV-induced protection. However, recent evidence suggests that the threshold for neutralizing antibodies to efficiently prevent infection across mucosal surfaces may be lower than originally anticipated (20). Additionally, none of these results exclude the possibility that nonneutralizing SIV binding antibodies contribute to delayed acquisition. The few viral particles or infected lymphocytes that cross mucosal surfaces may be removed by immune complexes or by antibody-dependent cell-mediated cytotoxicity.
An unfortunate consequence of mucosal challenge with SIVsmE660 is that we and others have observed a portion of naïve animals controlling virus replication during the chronic phase of infection (23, 49, 53). We did not have any naïve animals control SIVsmE660 replication after intravenous challenge (43). Since only a few viruses establish infection after mucosal inoculations, this suggests that there may be variants within the stock of SIVsmE660 that are sensitive to neutralizing antibodies or susceptible to host cellular restriction factors that predispose animals to controlling SIVsmE660 replication. The control of SIVsmE660 replication by three of six naïve controls during the chronic phase of infection, combined with recombination between the vaccine and challenge strains of the virus, made it impossible to assess the effects of SIVmac239Δnef vaccination during the chronic phase of infection. Further investigation is needed to determine why some naïve animals have a propensity for controlling chronic SIVsmE660 replication.
In this study, we demonstrate that SIVmac239Δnef vaccination affects both the acquisition of infection and control of virus replication after repeated low-dose heterologous virus challenge. Mathematical modeling suggests that an HIV vaccine duplicating this feat can have a substantial impact on slowing the spread of HIV (3). Importantly, this effect was observed in animals that did not express the MHC class I alleles previously associated with control of SIVmac239 replication (29, 38, 52, 54). This suggests that an HIV vaccine might be effective in a diverse population of people, not just among those fortunate enough to express MHC class I alleles associated with control of HIV. Unfortunately, live-attenuated HIV vaccines themselves are unlikely to be developed because of pathogenicity fears (4, 5) and the possibility of recombination of the vaccine and infecting strains into a more pathogenic virus (17, 24, 34, 35, 43). Our study, however, supplies new evidence that live-attenuated SIV vaccines provide an ideal model with which to study effective anti-AIDS virus immunity, this time in the setting of a physiologically relevant, repeated-low-dose heterologous virus challenge. Further experiments are needed to define the precise mechanisms of protection associated with live-attenuated SIV vaccines so they can be applied to designing more effective HIV vaccines.
Supplementary Material
Acknowledgments
We thank the veterinary staff at the Wisconsin National Primate Research Center (WNPRC) for their assistance, Eva Rakasz for her helpful input and discussions, and Ronald Desrosiers for providing SIVmac239Δnef.
This research was supported by funds from the International AIDS Vaccine Initiative, National Institutes of Health (NIH) grants R01 AI049120, R01 AI076114, R24 RR016038, R24 RR015371, and R37 AI076114, and contract HHSN266200400088C to D.I.W. and by grant P51 RR000167 to the WNPRC from the National Center for Research Resources (NCRR), a component of the NIH. Additionally, this research was conducted at a facility constructed with support from the Research Facilities Improvement Program (grants RR15459-01 and RR020141-01).
This publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: SIVmac239 Vif peptides—complete set (6205), SIVmac239 Tat peptides—complete set (6207), SIVmac239 Rev (15-mer) peptides—complete set (6448), SIVmac239 Pol peptides—complete set (6443), SIVmac239 Gag peptides—complete set (6204), SIVmac239 Vpr peptides—complete set (6449), SIVmac239 Vpx peptides—complete set (6450), SIVmac239 Env peptides—complete set (6883), and SIVmac239 full-length Nef peptides—complete set (8762).
The authors have no conflicting financial interests.
Footnotes
Published ahead of print on 30 June 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abdel-Motal, U. M., J. Gillis, K. Manson, M. Wyand, D. Montefiori, K. Stefano-Cole, R. C. Montelaro, J. D. Altman, and R. P. Johnson. 2005. Kinetics of expansion of SIV Gag-specific CD8+ T lymphocytes following challenge of vaccinated macaques. Virology 333:226-238. [DOI] [PubMed] [Google Scholar]
- 2.Almond, N., J. Rose, R. Sangster, P. Silvera, R. Stebbings, B. Walker, and E. J. Stott. 1997. Mechanisms of protection induced by attenuated simian immunodeficiency virus. I. Protection cannot be transferred with immune serum. J. Gen. Virol. 78:1919-1922. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R., and M. Hanson. 2005. Potential public health impact of imperfect HIV type 1 vaccines. J. Infect. Dis. 191(Suppl. 1):S85-S96. [DOI] [PubMed] [Google Scholar]
- 4.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. [DOI] [PubMed] [Google Scholar]
- 5.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 6.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler, D. M., D. M. Smith, E. R. Cachay, G. K. Hightower, C. T. Nugent, D. D. Richman, and S. J. Little. 2008. Herpes simplex virus 2 serostatus and viral loads of HIV-1 in blood and semen as risk factors for HIV transmission among men who have sex with men. AIDS 22:1667-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheroutre, H., and L. Madakamutil. 2005. Mucosal effector memory T cells: the other side of the coin. Cell. Mol. Life Sci. 62:2853-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cline, A. N., J. W. Bess, M. Piatak, Jr., and J. D. Lifson. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303-312. [DOI] [PubMed] [Google Scholar]
- 10.Cranage, M. P., A. M. Whatmore, S. A. Sharpe, N. Cook, N. Polyanskaya, S. Leech, J. D. Smith, E. W. Rud, M. J. Dennis, and G. A. Hall. 1997. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology 229:143-154. [DOI] [PubMed] [Google Scholar]
- 11.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 12.Flynn, N. M., D. N. Forthal, C. D. Harro, F. N. Judson, K. H. Mayer, and M. F. Para. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654-665. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich, T. C., L. E. Valentine, L. J. Yant, E. G. Rakasz, S. M. Piaskowski, J. R. Furlott, K. L. Weisgrau, B. Burwitz, G. E. May, E. J. Leon, T. Soma, G. Napoe, S. V. Capuano III, N. A. Wilson, and D. I. Watkins. 2007. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J. Virol. 81:3465-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaschen, B., J. Taylor, K. Yusim, B. Foley, F. Gao, D. Lang, V. Novitsky, B. Haynes, B. H. Hahn, T. Bhattacharya, and B. Korber. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296:2354-2360. [DOI] [PubMed] [Google Scholar]
- 15.Gauduin, M. C., Y. Yu, A. Barabasz, A. Carville, M. Piatak, J. D. Lifson, R. C. Desrosiers, and R. P. Johnson. 2006. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J. Exp. Med. 203:2661-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray, R. H., M. J. Wawer, R. Brookmeyer, N. K. Sewankambo, D. Serwadda, F. Wabwire-Mangen, T. Lutalo, X. Li, T. vanCott, and T. C. Quinn. 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357:1149-1153. [DOI] [PubMed] [Google Scholar]
- 17.Gundlach, B. R., M. G. Lewis, S. Sopper, T. Schnell, J. Sodroski, C. Stahl-Hennig, and K. Uberla. 2000. Evidence for recombination of live, attenuated immunodeficiency virus vaccine with challenge virus to a more virulent strain. J. Virol. 74:3537-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen, S. G., C. Vieville, N. Whizin, L. Coyne-Johnson, D. C. Siess, D. D. Drummond, A. W. Legasse, M. K. Axthelm, K. Oswald, C. M. Trubey, M. J. Piatak, J. D. Lifson, J. A. Nelson, M. A. Jarvis, and L. J. Picker. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hessell, A. J., L. Hangartner, M. Hunter, C. E. Havenith, F. J. Beurskens, J. M. Bakker, C. M. Lanigan, G. Landucci, D. N. Forthal, P. W. Parren, P. A. Marx, and D. R. Burton. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101-104. [DOI] [PubMed] [Google Scholar]
- 20.Hessell, A. J., P. Poignard, M. Hunter, L. Hangartner, D. M. Tehrani, W. K. Bleeker, P. W. Parren, P. A. Marx, and D. R. Burton. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15:951-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, R. P., R. L. Glickman, J. Q. Yang, A. Kaur, J. T. Dion, M. J. Mulligan, and R. C. Desrosiers. 1997. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J. Virol. 71:7711-7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keele, B. F., E. E. Giorgi, J. F. Salazar-Gonzalez, J. M. Decker, K. T. Pham, M. G. Salazar, C. Sun, T. Grayson, S. Wang, H. Li, X. Wei, C. Jiang, J. L. Kirchherr, F. Gao, J. A. Anderson, L. H. Ping, R. Swanstrom, G. D. Tomaras, W. A. Blattner, P. A. Goepfert, J. M. Kilby, M. S. Saag, E. L. Delwart, M. P. Busch, M. S. Cohen, D. C. Montefiori, B. F. Haynes, B. Gaschen, G. S. Athreya, H. Y. Lee, N. Wood, C. Seoighe, A. S. Perelson, T. Bhattacharya, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keele, B. F., H. Li, G. H. Learn, P. Hraber, E. E. Giorgi, T. Grayson, C. Sun, Y. Chen, W. W. Yeh, N. L. Letvin, J. R. Mascola, G. J. Nabel, B. F. Haynes, T. Bhattacharya, A. S. Perelson, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatissian, E., V. Monceaux, M. C. Cumont, M. P. Kieny, A. M. Aubertin, and B. Hurtrel. 2001. Persistence of pathogenic challenge virus in macaques protected by simian immunodeficiency virus SIVmacDeltanef. J. Virol. 75:1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koff, W. C., P. R. Johnson, D. I. Watkins, D. R. Burton, J. D. Lifson, K. J. Hasenkrug, A. B. McDermott, A. Schultz, T. J. Zamb, R. Boyle, and R. C. Desrosiers. 2006. HIV vaccine design: insights from live attenuated SIV vaccines. Nat. Immunol. 7:19-23. [DOI] [PubMed] [Google Scholar]
- 26.Letvin, N. L., S. S. Rao, V. Dang, A. P. Buzby, B. Korioth-Schmitz, D. Dombagoda, J. G. Parvani, R. H. Clarke, L. Bar, K. R. Carlson, P. A. Kozlowski, V. M. Hirsch, J. R. Mascola, and G. J. Nabel. 2007. No evidence for consistent virus-specific immunity in simian immunodeficiency virus-exposed, uninfected rhesus monkeys. J. Virol. 81:12368-12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Q., P. J. Skinner, S. J. Ha, L. Duan, T. L. Mattila, A. Hage, C. White, D. L. Barber, L. O'Mara, P. J. Southern, C. S. Reilly, J. V. Carlis, C. J. Miller, R. Ahmed, and A. T. Haase. 2009. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science 323:1726-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loffredo, J. T., B. J. Burwitz, E. G. Rakasz, S. P. Spencer, J. J. Stephany, J. P. Vela, S. R. Martin, J. Reed, S. M. Piaskowski, J. Furlott, K. L. Weisgrau, D. S. Rodrigues, T. Soma, G. Napoe, T. C. Friedrich, N. A. Wilson, E. G. Kallas, and D. I. Watkins. 2007. The antiviral efficacy of simian immunodeficiency virus-specific CD8+ T cells is unrelated to epitope specificity and is abrogated by viral escape. J. Virol. 81:2624-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loffredo, J. T., J. Maxwell, Y. Qi, C. E. Glidden, G. J. Borchardt, T. Soma, A. T. Bean, D. R. Beal, N. A. Wilson, W. M. Rehrauer, J. D. Lifson, M. Carrington, and D. I. Watkins. 2007. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 81:8827-8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loffredo, J. T., J. Sidney, C. Wojewoda, E. Dodds, M. R. Reynolds, G. Napoe, B. R. Mothe, D. H. O'Connor, N. A. Wilson, D. I. Watkins, and A. Sette. 2004. Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J. Immunol. 173:5064-5076. [DOI] [PubMed] [Google Scholar]
- 31.Mansfield, K., S. M. Lang, M. C. Gauduin, H. B. Sanford, J. D. Lifson, R. P. Johnson, and R. C. Desrosiers. 2008. Vaccine protection by live, attenuated simian immunodeficiency virus in the absence of high-titer antibody responses and high-frequency cellular immune responses measurable in the periphery. J. Virol. 82:4135-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDermott, A. B., J. Mitchen, S. Piaskowski, I. De Souza, L. J. Yant, J. Stephany, J. Furlott, and D. I. Watkins. 2004. Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: a model for the evaluation of vaccine efficacy in nonhuman primates. J. Virol. 78:3140-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metzner, K. J., X. Jin, F. V. Lee, A. Gettie, D. E. Bauer, M. Di Mascio, A. S. Perelson, P. A. Marx, D. D. Ho, L. G. Kostrikis, and R. I. Connor. 2000. Effects of in vivo CD8(+) T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Exp. Med. 191:1921-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metzner, K. J., J. M. Binley, A. Gettie, P. Marx, D. F. Nixon, and R. I. Connor. 2006. Tenofovir treatment augments anti-viral immunity against drug-resistant SIV challenge in chronically infected rhesus macaques. Retrovirology 3:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metzner, K. J., W. J. Moretto, S. M. Donahoe, X. Jin, A. Gettie, D. C. Montefiori, P. A. Marx, J. M. Binley, D. F. Nixon, and R. I. Connor. 2005. Evaluation of CD8+ T-cell and antibody responses following transient increased viraemia in rhesus macaques infected with live, attenuated simian immunodeficiency virus. J. Gen. Virol. 86:3375-3384. [DOI] [PubMed] [Google Scholar]
- 36.Miller, C. J., M. B. McChesney, X. Lu, P. J. Dailey, C. Chutkowski, D. Lu, P. Brosio, B. Roberts, and Y. Lu. 1997. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 71:1911-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montefiori, D. C. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr. Protoc. Immunol. 2005:Chapter 12, Unit 12.11. [DOI] [PubMed]
- 38.Mothe, B. R., J. Weinfurter, C. Wang, W. Rehrauer, N. Wilson, T. M. Allen, D. B. Allison, and D. I. Watkins. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77:2736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 39.Pitisuttithum, P., P. Gilbert, M. Gurwith, W. Heyward, M. Martin, F. van Griensven, D. Hu, J. W. Tappero, and K. Choopanya. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661-1671. [DOI] [PubMed] [Google Scholar]
- 40.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, and R. H. Gray. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 42.Rerks-Ngarm, S., P. Pitisuttithum, S. Nitayaphan, J. Kaewkungwal, J. Chiu, R. Paris, N. Premsri, C. Namwat, M. de Souza, E. Adams, M. Benenson, S. Gurunathan, J. Tartaglia, J. G. McNeil, D. P. Francis, D. Stablein, D. L. Birx, S. Chunsuttiwat, C. Khamboonruang, P. Thongcharoen, M. L. Robb, N. L. Michael, P. Kunasol, and J. H. Kim. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209-2220. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds, M. R., A. M. Weiler, K. L. Weisgrau, S. M. Piaskowski, J. R. Furlott, J. T. Weinfurter, M. Kaizu, T. Soma, E. J. Leon, C. MacNair, D. P. Leaman, M. B. Zwick, E. Gostick, S. K. Musani, D. A. Price, T. C. Friedrich, E. G. Rakasz, N. A. Wilson, A. B. McDermott, R. Boyle, D. B. Allison, D. R. Burton, W. C. Koff, and D. I. Watkins. 2008. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J. Exp. Med. 205:2537-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson, S., W. A. Charini, M. H. Newberg, M. J. Kuroda, C. I. Lord, and N. L. Letvin. 2001. A commonly recognized simian immunodeficiency virus Nef epitope presented to cytotoxic T lymphocytes of Indian-origin rhesus monkeys by the prevalent major histocompatibility complex class I allele Mamu-A*02. J. Virol. 75:10179-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz, J. E., R. P. Johnson, H. M. McClure, K. H. Manson, M. S. Wyand, M. J. Kuroda, M. A. Lifton, R. S. Khunkhun, K. J. McEvers, J. Gillis, M. Piatak, J. D. Lifson, G. Grosschupff, P. Racz, K. Tenner-Racz, E. P. Rieber, K. Kuus-Reichel, R. S. Gelman, N. L. Letvin, D. C. Montefiori, R. M. Ruprecht, R. C. Desrosiers, and K. A. Reimann. 2005. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques. J. Virol. 79:8131-8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel, T. U., T. C. Friedrich, D. H. O'Connor, W. Rehrauer, E. J. Dodds, H. Hickman, W. Hildebrand, J. Sidney, A. Sette, A. Hughes, H. Horton, K. Vielhuber, R. Rudersdorf, I. P. De Souza, M. R. Reynolds, T. M. Allen, N. Wilson, and D. I. Watkins. 2002. Escape in one of two cytotoxic T-lymphocyte epitopes bound by a high-frequency major histocompatibility complex class I molecule, Mamu-A*02: a paradigm for virus evolution and persistence? J. Virol. 76:11623-11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson, N. A., B. F. Keele, J. S. Reed, S. M. Piaskowski, C. E. MacNair, A. J. Bett, X. Liang, F. Wang, E. Thoryk, G. J. Heidecker, M. P. Citron, L. Huang, J. Lin, S. Vitelli, C. D. Ahn, M. Kaizu, N. J. Maness, M. R. Reynolds, T. C. Friedrich, J. T. Loffredo, E. G. Rakasz, S. Erickson, D. B. Allison, M. J. Piatak, J. D. Lifson, J. W. Shiver, D. R. Casimiro, G. M. Shaw, B. H. Hahn, and D. I. Watkins. 2009. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J. Virol. 83:6508-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyand, M. S., K. Manson, D. C. Montefiori, J. D. Lifson, R. P. Johnson, and R. C. Desrosiers. 1999. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73:8356-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyand, M. S., K. H. Manson, M. Garcia-Moll, D. Montefiori, and R. C. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 70:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yant, L. J., T. C. Friedrich, R. C. Johnson, G. E. May, N. J. Maness, A. M. Enz, J. D. Lifson, D. H. O'Connor, M. Carrington, and D. I. Watkins. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80:5074-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh, W. W., P. Jaru-Ampornpan, D. Nevidomskyte, M. Asmal, S. S. Rao, A. P. Buzby, D. C. Montefiori, B. T. Korber, and N. L. Letvin. 2009. Partial protection of simian immunodeficiency virus (SIV)-infected rhesus monkeys against superinfection with a heterologous SIV isolate. J. Virol. 83:2686-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, Z. Q., T. M. Fu, D. R. Casimiro, M. E. Davies, X. Liang, W. A. Schleif, L. Handt, L. Tussey, M. Chen, A. Tang, K. A. Wilson, W. L. Trigona, D. C. Freed, C. Y. Tan, M. Horton, E. A. Emini, and J. W. Shiver. 2002. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J. Virol. 76:12845-12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zwick, M. B., M. Wang, P. Poignard, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J. Virol. 75:12198-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.