Abstract
JC virus (JCV) is latent in the kidneys and lymphoid organs of healthy individuals, and its reactivation in the context of immunosuppression may lead to progressive multifocal leukoencephalopathy (PML). Whether JCV is present in the brains or other organs of healthy people and in immunosuppressed patients without PML has been a matter of debate. We detected JCV large T DNA by quantitative PCR of archival brain samples of 9/24 (38%) HIV-positive PML patients, 5/18 (28%) HIV-positive individuals, and 5/19 (26%) HIV-negative individuals. In the same samples, we detected JCV regulatory region DNA by nested PCR in 6/19 (32%) HIV-positive PML patients, 2/11 (18%) HIV-positive individuals, and 3/17 (18%) HIV-negative individuals. In addition, JCV DNA was detected in some spleen, lymph node, bone, and kidney samples from the same groups. In situ hybridization data confirmed the presence of JCV DNA in the brains of patients without PML. However, JCV proteins (VP1 or T antigen) were detected mainly in the brains of 23/24 HIV-positive PML patients, in only a few kidney samples of HIV-positive patients, with or without PML, and rarely in the bones of HIV-positive patients with PML. JCV proteins were not detected in the spleen or lymph nodes in any study group. Furthermore, analysis of the JCV regulatory region sequences showed both rearranged and archetype forms in brain and extraneural organs in all three study groups. Regulatory regions contained increased variations of rearrangements correlating with immunosuppression. These results provide evidence of JCV latency in the brain prior to severe immunosuppression and suggest new paradigms in JCV latency, compartmentalization, and reactivation.
JC virus (JCV) is the etiologic agent of the often fatal brain-demyelinating disease progressive multifocal leukoencephalopathy (PML) (23a). JCV remains latent in the kidneys, lymph nodes, and bone marrow of healthy and immunosuppressed individuals without PML (2, 21, 24) and, upon reactivation, can cause a lytic infection of oligodendrocytes in the brain, leading to PML (14). Although JCV is often found in the urine of healthy individuals (12, 18), it is not usually detected in the blood of patients without PML (15). The pathway leading to viral reactivation and replication in the brains of immunosuppressed individuals is not well defined. Molecular analysis of JCV has prompted hypotheses on how the virus emerges from latency and becomes pathogenic. JCV has a double-stranded, circular DNA of 5,130 bp. While the coding region is well conserved, the noncoding regulatory region (RR) of JCV is hypervariable. The kidneys and urine usually contain JCV with a well-conserved, nonpathogenic RR which is called the “archetype” (30). The JCV RR detected in the brains and the cerebrospinal fluid (CSF) of PML patients usually has duplications, tandem repeats, and deletions and has been called “rearranged” compared to the archetype. Although it is not clear which form of JCV RR is propagated at the time of primary infection, it has been hypothesized that JCV with the archetype RR remains confined in the kidneys of most healthy individuals and that rearrangements which confer neurotropism need to occur prior to viral migration to the brain to destroy the myelin-producing glial cells. Whether JCV can reach the brain and establish latency in the central nervous systems (CNS) of otherwise-healthy individuals are matters of debate. While some investigators detected JCV DNA in 28 to 68% of frozen (8, 27) and 18 to 71% of formalin-fixed, paraffin-embedded (FFPE) (4, 7, 20) brain samples of patients without PML, others reported negative results (3, 6, 10, 23). Clearly, characterizing JCV sites of latency is imperative in the prevention of viral reactivation and PML. Recently, a group of PML patients has emerged among those treated with monoclonal antibodies, including natalizumab (13, 17, 26), efalizumab (16, 19a), and rituximab (5), for multiple sclerosis, psoriasis, hematological malignancies, and rheumatologic diseases. Mechanisms of JCV reactivation in these patients has yet to be defined. To better understand JCV organ tropism and characterize the types of JCV RRs in different compartments, we used archival pathology samples to detect JCV DNA and proteins and to analyze JCV RRs in various organ systems in HIV-positive individuals with and without PML and in HIV-negative subjects.
MATERIALS AND METHODS
Specimens.
Formalin-fixed, paraffin-embedded (FFPE) samples of brain, kidney, vertebral bone, spleen, and lymph nodes from HIV-positive patients with or without PML and HIV-negative patients without PML were obtained from the National NeuroAIDS Tissue Consortium (NNTC). All brain samples in the HIV-positive and HIV-negative groups were sections from the cerebrum. Brain sections containing PML lesions were studied from the HIV-positive PML group. Of the 24 samples, 13 were from the cerebellum and 11 from the cerebrum.
Samples of brain, brain stem, kidney, spleen, and lymph nodes from a PML patient were obtained at autopsy. Half of the tissues were frozen for further analysis, and the other half were fixed in formalin and paraffin embedded for further analysis. Prior consent was obtained for the study protocol, approved by the Beth Israel Deaconess Medical Center Institutional Review Board of Human Studies.
DNA extraction from formalin-fixed, paraffin-embedded samples.
Ten 5-μm-thick slices from FFPE blocks were collected into an Eppendorf tube, and a new microtome blade was used for each block. DNA was extracted after deparaffinization in 100% xylene for 10 min and then in 50% xylene and 50% ethanol for 10 min, followed by 100% ethanol for 10 min twice, all performed at 56°C. The dried sample was dissolved in 200 μl of tissue lysis solution, part of the Qiagen DNeasy blood and tissue kit (Qiagen). DNA was extracted by following the instructions of the manufacturer.
DNA extraction from frozen samples.
Freshly frozen autopsy samples were kept frozen at −80°C until use. DNA extraction was performed with a Qiagen DNeasy blood and tissue kit (Qiagen).
qPCR.
We used quantitative JCV PCR (qPCR) to detect and quantify JCV DNA in all samples as previously described (22). The limit of detection of the assay was 10 copies of JCV/μg of cellular DNA. All samples underwent two qPCR analyses with the same primers and conditions. The amount of DNA used per reaction ranged from 6 to 500 ng, and the results are expressed in numbers of copies of JCV/μg of cellular DNA. In the first qPCR analysis, we tested all samples in duplicate, and a sample was considered positive if at least one of the two wells had detectable DNA. In the second qPCR analysis, we tested the same samples in triplicate and considered positive those for which at least two of the three wells had detectable DNA. We then combined data from both analyses and considered positive those samples where JCV DNA could be detected in both the duplicate- and the triplicate-qPCR analysis.
Cloning and sequencing of the JCV RR.
We amplified the JCV RR sequences by nested PCR using outer primers JRE1 (nucleotides [nt] 4989 to 5009) and LP2 (nt 537 to 518) and inner primers RREV (nt 310 to 291) and RFOR (nt 5085 to 5104) as described elsewhere (19). For each PCR, 20 pmol of primers was used in 30 cycles of amplification, with an annealing temperature of 63°C. The amount of DNA used in the reaction mixture ranged from 2 to 789 ng. The PCR products corresponding to the expected size were cloned with a TOPO-TA cloning kit (Invitrogen, Carlsbad, CA). Up to 10 clones for each PCR product were analyzed by restriction enzyme digestion and electrophoresis. We sequenced up to 10 clones of amplified fragments with the expected length. The DNA sequence was obtained on an ABI 3730xl sequencer (Applied Biosystems). Sequence analyses were performed using the Lasergene software MegAlign 7.1 (DNAStar, Madison, WI).
IHC.
The expression of JCV proteins was determined by immunohistochemistry (IHC) staining using the anti-JCV antibody VP1 PAB597 (a generous gift from Walter Atwood) and the anti-large T antigen (T Ag) (simian virus 40 [SV40] Tag v-300; Santa Cruz Biotechnology), which cross-reacts with JCV, as previously described (28).
ISH.
The presence of JCV DNA was determined by in situ hybridization (ISH), using a biotinylated JCV DNA probe (Enzo Life Sciences) as described previously (29), with modifications of the tissue preparation with antigen-retrieval solution (Dako) at 95°C for 20 minutes, followed by a 0.3% hydrogen peroxidase wash (Sigma) at room temperature for 60 minutes and then by proteinase K (50 ng/ml; Enzo Life Sciences) digestion at room temperature for 5 min. GenPoint (Dako), a catalyzed-signal amplification system, was used for staining.
Histological examination.
All slides were stained with hematoxylin and Luxol fast blue solution to determine cellular structures and PML lesions. After deparaffinization and hydration in up to 95% ethanol, slides were placed in Luxol fast blue solution at 56°C overnight.
Statistical analysis.
Correlation between JCV DNA detection in each patient and organ group was determined using Fisher's two-tailed exact test.
RESULTS
JCV DNA detection in FFPE tissues compared to frozen tissues.
To determine the sensitivity of qPCR in detecting JCV large T DNA in formalin-fixed, paraffin-embedded (FFPE) tissues, we analyzed several organs from a PML patient that were either FFPE or frozen at −80°C (Table 1). All of the organs tested, including the brain, brain stem, kidney, spleen, and lymph nodes of this PML patient, contained detectable JCV DNA in samples obtained from both types of tissue preparations. Brain stem, spleen, and lymph node extractions had comparable quantities of detected JCV DNA in both preparations. However, the JCV DNA extracted from brain and kidney of FFPE tissues had 6-log- and 2-log-smaller viral loads, respectively, than their frozen counterparts. These results indicate that while FFPE tissues can be used for qualitative assessment of JCV DNA in tissues, quantitative measures may underestimate the actual viral burden in these specimens.
TABLE 1.
Quantification of JCV DNA by qPCR in the brain, brain stem, kidney, spleen, and lymph node extracted from a PML patient
| Sample typea | No. of JCV copies/μg of DNA in: |
||||
|---|---|---|---|---|---|
| Brain | Brain stem | Kidney | Spleen | Lymph node | |
| FFPE | 194 | 6 × 108 | 249 | 725 | 1.41 × 103 |
| Frozen | 1.00 × 108 | 4.70 × 109 | 1.10 × 104 | 8,309 | 1.57 × 103 |
Frozen, frozen at −80°C; FFPE, formalin fixed, paraffin embedded.
JCV DNA detection in brain and other organs.
To determine the prevalence of JCV in different body compartments, we first performed qPCR detection of JCV large T DNA in the brain tissue of HIV-positive PML patients, as well as of HIV-positive and HIV-negative subjects (Table 2). We detected JCV large T DNA in 9/24 (38%) of the HIV-positive PML group, compared to 5/18 (28%) of the HIV-positive and 5/19 (26%) of the HIV-negative groups (P = 0.78). The JC viral loads ranged between 38 and 3,464 (mean, 910) copies/μg of DNA in the HIV-positive PML group, 111 and 9,254 (mean, 3,033) copies/μg in the HIV-positive group, and 377 and 1,457 (mean, 768) copies/μg in the HIV-negative group.
TABLE 2.
Detection of JCV DNA in the large T and regulatory regions (RR) of HIV-positive PML, HIV-positive, and HIV-negative patients’ brains, kidneys, bones, spleens, and lymph nodes
| DNA type | Group | No. positive/total no. of specimens tested (%) |
||||
|---|---|---|---|---|---|---|
| Brain | Kidney | Bone | Spleen | Lymph node | ||
| Large T Ag | HIV positive/PML | 9/24 (38) | 1/11 (9) | 0/15 | 4/10 (40) | 1/4 (25) |
| HIV positive | 5/18 (28) | 0/11 | 2/11 (18) | 1/9 (11) | 3/7 (43) | |
| HIV negative | 5/19 (26) | 0/12 | 1/12 (8) | 2/9 (22) | NAa | |
| RR | HIV positive/PML | 6/19 (32) | 2/5 (40) | 3/4 (75) | 4/10 (40) | 2/2 (100) |
| HIV positive | 2/11 (18) | 0/3 | 3/4 (75) | 7/7 (100) | 4/6 (67) | |
| HIV negative | 3/17 (18) | 0/1 | 0/5 | 3/9 (33) | NA | |
NA, not available.
Among the five HIV-positive patients who had detectable JCV large T DNA in their brain by qPCR, four had normal brain histology and one had a poorly differentiated metastatic tumor on pathological examination. Three of these patients had plasma HIV loads of 54,439, >75,000, and <400 copies/ml and corresponding CD4 counts of 8, 13, and 663 cells/μl. One other patient had a CD4 count of 25 cells/μl. Among the five HIV-negative subjects with detectable JCV DNA, three had amyotrophic lateral sclerosis (ALS) and the other two did not have any brain pathology on examination.
JCV large T DNA was detected in the kidney of only one HIV-positive PML patient and in the bone samples of one HIV-positive and one HIV-negative subject. JCV DNA was detected in the spleen and lymph nodes of few individuals in all available study groups. To further clarify JCV DNA detection in these FFPE tissues, we also amplified the JCV RR by nested PCR with available samples (Table 2). Detection of the amplified RR product by gel electrophoresis was confirmed by cloning and sequencing. In the brain tissues, detection of the JCV RR was comparable to detection of JCV large T, with prevalences of 6/19 (32%) in HIV-positive PML patients and 2/11 (18%) and 3/17 (18%) in HIV-positive and HV-negative patients, respectively (P = 0.67), but in the kidney, bone, spleen, and lymph nodes, RR detection by nested PCR exceeded that of large T DNA. However, only 1/11 brain, 1/5 kidney, 2/6 bone, and 1/26 spleen samples with detectable RRs by nested PCR had no large T detection by qPCR in any of the runs. The rest of the samples had at least one qPCR run that was positive for large T but did not meet the criteria for a positive qPCR result.
In a separate analysis, the large T and RR DNA detections showed no significant differences in the rates of detection of JCV DNA in the brains of HIV-positive PML patients compared to the HIV-positive and HIV-negative groups. Detection of large T DNA in the brain was not significantly more frequent than in the extraneural organs combined for any of the three groups (P = 0.07, 0.26, and 0.12). Similarly, the detection of regulatory region DNA in the brains of HIV-positive patients with PML and HIV-negative patients was also not significantly more frequent than in the extraneural organs combined (P = 0.22 and 1). In the HIV-positive group, however, the JCV regulatory region DNA was more frequently found in the combined extraneural organs than in the brain (P = 0.009). These results showed that JCV DNA is detectable in multiple organs and that JCV DNA is present in the brains of patients both with and without immunosuppression.
JCV VP1 and T Ag protein detection in brain and other organs.
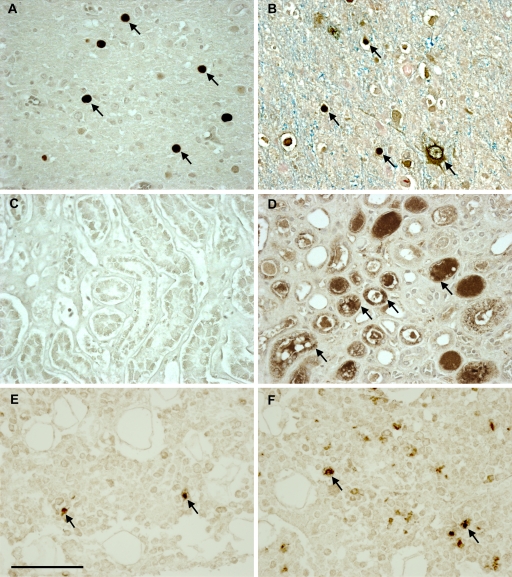
We performed IHC on all available tissues. We found that JCV large T antigen (T Ag), a regulatory protein expressed early in the viral life cycle, was detected in the brains of 21 of the 24 HIV-positive PML patients (Table 3) and that the major capsid protein VP1, indicating the presence of mature viral particles, was expressed in the lesions of 20/24 (83%) HIV-positive PML patient brain samples. All together, 23 of the 24 HIV-positive PML brain samples expressed either one or both of the JCV proteins tested. One brain sample showed extensive demyelination but did not stain for VP1 or T Ag protein. We also found T Ag in one brain sample each from the HIV-positive and -negative control groups. As expected, JCV proteins were significantly more frequently expressed in the brains of PML patients than in the HIV-positive and HIV-negative groups (P = <0.001). Furthermore, T Ag were detected at low levels in kidney samples from the HIV-positive PML and HIV-positive groups. T Ag and VP1 were rarely detected in the bone samples of the HIV-positive PML group only. T Ag was not detected in the extraneural organs of the HIV-negative group, and VP1 protein was not detected in any organs of this group (Fig. 1).
TABLE 3.
Detection of JCV large T Ag and capsid protein VP1 in brain, kidney, bone, spleen, and lymph node FFPE samples from HIV-positive PML, HIV-positive, and HIV-negative individuals
| Sample type | Group | No. positive/total no. of samples tested (%)a |
||
|---|---|---|---|---|
| Large T Ag | Capsid protein VP1 | T Ag or VP1 | ||
| Brain | HIV positive/PML | 21/24 (88) | 20/24 (83) | 23/24 (96) |
| HIV positive | 1/18 (6) | 0/18 | 1/18 (6) | |
| HIV negative | 1/19 (5) | 0/19 | 1/19 (5) | |
| Kidney | HIV positive/PML | 1/11 (9) | 0/11 | 1/11 (9) |
| HIV positive | 3/11 (27) | 0/11 | 3/11 (27) | |
| HIV negative | 0/12 | 0/12 | 0/12 | |
| Bone | HIV positive/PML | 1/13 (8) | 1/13 (8) | 2/13 (15) |
| HIV positive | 0/11 | 0/11 | 0/11 | |
| HIV negative | 0/12 | 0/12 | 0/12 | |
| Spleen | HIV positive/PML | 0/9 | 0/9 | 0/9 |
| HIV positive | 0/9 | 0/9 | 0/9 | |
| HIV negative | 0/9 | 0/9 | 0/9 | |
| Lymph node | HIV positive/PML | NA | NA | NA |
| HIV positive | 0/7 | 0/7 | 0/7 | |
| HIV negative | NA | NA | NA | |
NA, not available.
FIG. 1.
Immunohistochemical staining of JCV proteins Vp1 and T Ag in brain, kidney, and bone in HIV-positive PML patients. Numerous cells (arrows) in the brain stained positive for VP1 (A) and TAg (B). While no VP1 protein was detected in the kidney (C), JCV-infected kidney tubular epithelium cells (arrows) stained positive for T Ag (D). In the bone, rare cells (arrows) stained positive for both VP1 (E) and T Ag (F). All the images are magnified 40-fold, and the scale bar is 100 μm.
All five HIV-positive patients with detectable JCV DNA in the brain had negative staining for VP1, and the patient with an undifferentiated tumor had a brain sample which stained positive for T Ag. All five HIV-negative patients with detectable JCV DNA in the brain showed no expression of VP1 protein, and one of the five with normal brain pathology had rare cells expressing T Ag.
In situ hybridization of JCV DNA.
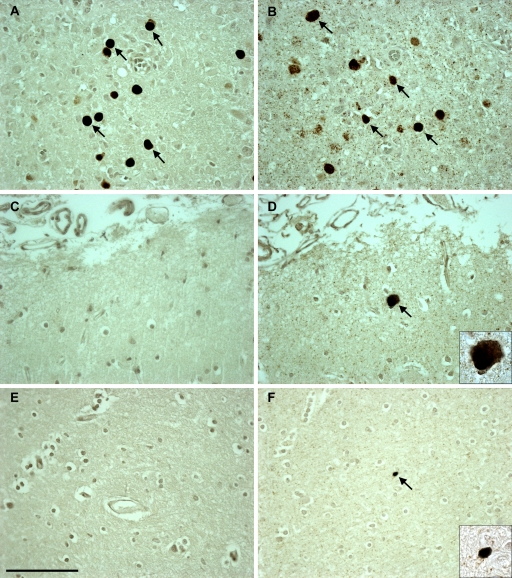
To further clarify the discrepant results between JCV DNA detection by qPCR and JCV protein detection by IHC, we performed in situ hybridization (ISH) for JCV DNA on a subset of the FFPE tissue sections (Fig. 2). In the HIV-positive control group, the brain tissue sections of 3 of the 5 patients who were positive for JCV by qPCR exhibited rare cells (1 to 2 cells per tissue section) which also stained JCV positive by ISH. As described above, only 1 of these 5 patients had rare cortical cells expressing T Ag, and the ISH was also positive in this case. Serving as controls, 5 of 5 other HIV-positive patients with negative qPCR and IHC results also showed negative ISH results (data not shown). In the HIV-negative control group, 3 of the 5 patients with positive qPCR results also had 1 to 10 cells per tissue section that stained positive by ISH, including one with positive IHC T Ag staining in the same distribution. Furthermore, three additional HIV-negative control patients with undetectable JCV DNA by qPCR and proteins detected by IHC also had negative ISH results (data not shown). In HIV-positive patients with PML, samples that had detectable JCV DNA by qPCR and JCV proteins by IHC all had positive ISH results. Finally, there were 15/24 brain samples in this group with discrepant results; each sample had at least one cell staining positive for JCV protein by IHC staining but did not meet the criteria for qPCR positivity. Of those 15 samples, 8 were positive for JCV DNA by ISH; therefore, compared to that of IHC, the sensitivity of ISH to detect JCV in PML lesions was 8/15 (53%). The one HIV-positive PML patient brain sample with demyelinated lesions, but no VP1 or T Ag IHC staining and undetectable JCV DNA, was positive by ISH. Lastly, in all ISH-positive cases, JCV-infected cells were located in the brain parenchyma and not inside identifiable blood vessels.
FIG. 2.
In situ hybridization (ISH) detection of JCV-positive cells in the brain samples of HIV-positive PML, HIV-positive, and HIV-negative individuals compared to the immunohistochemical staining detection of JCV VP1 proteins in the same patients. Positive VP1 protein staining in JCV-infected cells (arrows) in an HIV-positive PML individual (A) corresponds to positive ISH for JCV DNA (arrows) in the same individual (B). (C) JCV VP1 protein is not detected in the brain of an HIV-positive individual. (D) However, ISH detected rare cells harboring JCV DNA (arrow) in this patient. (E) JCV VP1 protein is not detected in an HIV-negative individual. (F) However, ISH showed rare JCV DNA-positive cells (arrow). The images are magnified 40-fold, and the insets are magnified 100-fold. Scale bar = 100 μm.
JCV regulatory region analysis.
Samples that were positive for JCV DNA on the first duplicate analysis by qPCR were selected to be tested for the JCV regulatory region (RR) in a nested PCR assay (Table 4). We obtained RR sequences from 39 samples (11 brains, 14 spleens, 6 bones, 6 lymph nodes, 2 kidneys) from 27 patients (12 PML, 10 HIV-positive, and 5 HIV-negative). Whenever possible, 10 clones from each nested-PCR-positive RR sample were sequenced, with an average of 8 clones per sample (311 clones total).
TABLE 4.
Types of JCV RRs found in brain, kidney, bone, spleen, and lymph node FFPE samples from HIV-positive PML, HIV-positive, and HIV-negative individuals
| Sample type | Group | No. of patients | No. of clones | No. of RRs of the indicated type/no. of clones sequenced (%)a |
|||
|---|---|---|---|---|---|---|---|
| I-S | I-R | II-S | II-R | ||||
| Brain | HIV positive/PML | 6 | 28 | 3/28 (11) | 13/28 (46) | 12/28 (43) | 0 |
| HIV positive | 2 | 19 | 4/19 (21) | 15/19 (79) | 0 | 0 | |
| HIV negative | 3 | 21 | 9/21 (43) | 12/21 (57) | 0 | 0 | |
| Kidney | HIV positive/PML | 2 | 19 | 0 | 19/19 (100) | 0 | 0 |
| HIV positive | 0 | 0 | NA | NA | NA | NA | |
| HIV negative | 0 | 0 | NA | NA | NA | NA | |
| Bone | HIV positive/PML | 3 | 20 | 10/20 (50) | 10/20 (50) | 0 | 0 |
| HIV positive | 3 | 25 | 0 | 6/25 (24) | 9/25 (36) | 10/25 (40) | |
| HIV negative | 0 | 0 | NA | NA | NA | NA | |
| Spleen | HIV positive/PML | 4 | 32 | 2/32 (6) | 16/32 (50) | 14/32 (44) | 0 |
| HIV positive | 7 | 65 | 10/65 (15) | 43/65 (66) | 12/65 (18) | 0 | |
| HIV negative | 3 | 30 | 0 | 0 | 30/30 (100) | 0 | |
| Lymph node | HIV positive/PML | 2 | 19 | 0 | 9/19 (47) | 10/19 (53) | 0 |
| HIV positive | 4 | 33 | 3/33 (9) | 26/33 (79) | 4/33 (12) | 0 | |
| HIV negative | NA | NA | NA | NA | NA | NA | |
NA, not available.
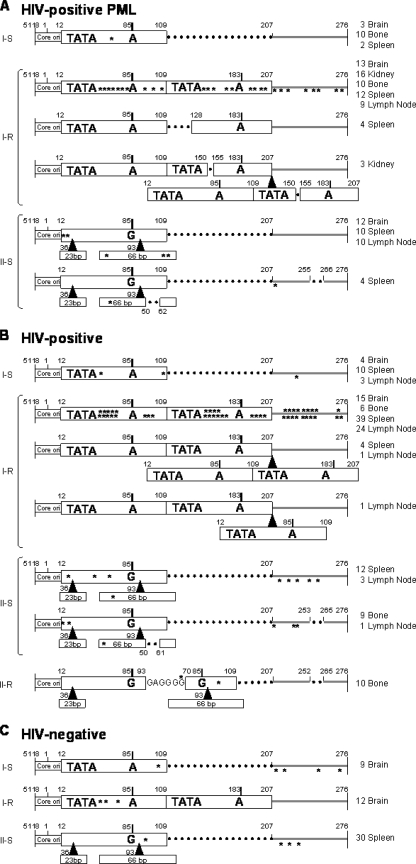
The RR forms were classified into four types as previously described (11). Type I-S has a single 98-base-pair unit, and type I-R has repeats of this 98-base-pair unit, with various deletions, as seen in the JCV Mad-1 and Mad-4 strains; both of these types have no inserts. Conversely, type II-S has a single 98-base-pair unit and one 23- and one 66-base-pair insert, as seen in the archetype, and type II-R has repeats of this 98-base-pair unit and inserts with various mutations, also called rearranged RRs. In the brains of HIV-positive PML patients, the RRs were significantly more frequently of the types I-R and II-S (P = 0.007) (Fig. 3). All the RRs from the kidneys of two HIV-positive PML patients were of type I-R. While the bone RRs were of all four types, the greatest numbers of patients and clones were obtained from the spleen. Finally, in the HIV-negative group, only one sequence representation was detected in each of the three RR types (I-S, I-R, II-S), whereas in both the HIV-positive PML and HIV-positive groups, both type I-R and type II-S clones with several different sequence rearrangements were detected. This indicates that JCV RR rearrangements occur more frequently with increased immunosuppression.
FIG. 3.
Sequencing results of the JCV RRs from the brains, kidneys, bones, spleens, and lymph nodes of HIV-positive patients with PML (A), HIV-positive patients (B), and HIV-negative patients (C). The nucleotide numbers are based on the prototype MAD-1 sequence. Each 98-bp unit is represented by an open box. The TATA box is represented by TATA. The 23-bp insert and 66-bp insert characteristic of archetype (II-S) sequences are represented as open boxes labeled “23 bp” and “66 bp,” respectively. Gray lines represent the region downstream of the 98-bp units. Dotted lines represent deletions or regions not present. Asterisks represent single nucleotide point mutations or deletions. Letters indicate nucleotides. MAD-1 contains an adenine at positions 85 and 183, compared with all other sequences, which contain guanine at these positions. The types of RRs are indicated. These numbers represent total numbers of clones from all patients in the category and may include multiple clones of the type from a single patient. ori, origin of replication. The black triangles indicate the locations of the drawn fragment insert sites.
In 9 of the 27 patients, we were able to obtain RR sequences from more than one organ. We cloned and sequenced the RRs from three organs in three patients; in an HIV-positive patient with PML, we detected the I-S type in the brain and bone, the I-R type in the brain, and the II-S type in the lymph node. In an HIV-positive patient, we found type II-S in the bone and a mixture of types I-S and I-R in both the spleen and lymph node. Another HIV-positive individual had type II-R in the bone and both types I-R and II-S in the spleen and lymph nodes. We obtained sequences from two organs in six patients, including three HIV-positive PML patients. In one HIV-positive patient with PML, we found type I-R in both the brain and kidney. In two other HIV-positive PML cases, we detected RR sequences in the brain and spleen, including types I-R and II-S in the brain and type II-S in the spleen in one of them and type I-R in the brain and types I-S and I-R in the spleen in the other. We obtained sequences from two organs in two HIV-positive control patients. In both cases, we found JCV RRs in spleen and lymph node, with one patient having types I-R and II-S in both organs and the other having types I-R and I-S in both organs. Finally, RR sequences from two organs in one HIV-negative control patient showed types I-S and I-R in the brain and type II-S in the spleen. In all cases where both spleen and lymph node sequences were obtained from the same patient, the same types of RRs were present in both organs.
DISCUSSION
Our results suggest that JCV can spread throughout the body in immunosuppressed and immunocompetent individuals alike and that it is present in the brains of individuals without PML. Using FFPE tissues allowed us access to archival material from PML patients suitable for our study. However, to estimate the yields of JCV DNA extracted from FFPE samples, we first compared JCV DNA detection in freshly frozen tissues to that in FFPE tissues obtained at autopsy from the same PML patient seen at our institution. These experiments indicated that although the PCR results were concordant, JC viral load was 1 to 6 logs lower in FFPE tissues than in freshly frozen tissues. However, the main demyelinating lesions were located in the brain stem of this PML patient. The FFPE tissues from the brain stem showed only a 1-log decrease in JC virus load from that in the frozen sample, whereas the FFPE tissues from the brain had a 6-log decrease compared with the viral load in the frozen sample. This may be due to inhomogeneous spread of the virus within the brain areas sampled for frozen and FFPE tissue analysis.
Although the availability of FFPE tissues allowed us to test a large number of cases, the frequency of DNA detection was likely underestimated. This was demonstrated by finding either JCV large T Ag or RR DNA in only 50% of PML cases, while JCV proteins could be detected in 96% of them. The archival samples used in the study groups were available autopsy samples from patients with various lengths of survival after the diagnosis of PML. Therefore, the lower frequency of JCV DNA detection by PCR can be due to the fact that the viral burden in the lesions decreased in time, commensurate with the destruction of glial cells and demyelination. Another possibility is that many of these autopsy samples had been in storage for as long as 13 years and had been incubated for extended periods of time in formalin prior to being embedded, which affects the integrity and quality of the extracted DNA. Using archival samples is not ideal, but it was the only way to study a rare and deadly disease such as PML.
The samplings of tissues used in our study represent a small portion of the organ, thus subjecting our analysis to sampling bias. Specifically, prior studies demonstrated detection of JCV DNA in the urine of up to 30% of healthy individuals (12, 18), far greater than the detection of JCV DNA in the kidneys (9% in the HIV-positive group with PML and none in the HIV-positive and HIV-negative groups without PML) in our study. This discrepancy is due to the fact that urine represents a sampling of the entire kidney instead of focal sites, as demonstrated by others (21). However, this bias is balanced by comparing only focal samples in all organs. Future studies should determine whether JCV spread is homogeneous in each organ.
We took several measures to ensure the integrity of our data and to eliminate the possibility of contaminations. All laboratory procedures were performed only after working surfaces were cleaned with DNase. DNA extractions from all specimens were performed in laboratory space entirely separate from areas of PCR amplification. All samples were deidentified and processed in batches by researchers. We also performed two separate qPCR experiments on each sample. In the first qPCR experiment, we analyzed the samples in duplicate. Samples were considered positive if one out of two tubes had detectable JCV DNA. We then repeated the qPCR experiment, analyzing all of the same samples in triplicate. In this experiment, samples were considered positive if two out of the three tubes had detectable JCV DNA. Combining the results of two separate qPCR experiments, we set the stringent criteria that a sample was considered to have detectable JCV DNA only if it was considered positive in both qPCR experiments and thus had JCV DNA in at least 3 of 5 replicates. These criteria may have contributed to a lower qPCR sensitivity. Furthermore, several PML patient lesions had extensive demyelination and only a few remaining JCV-infected cells, which may explain some of the negative PCR results. In these cases, ISH seemed to be a more robust method than PCR to demonstrate the presence of rare cells harboring JCV DNA in FFPE specimens from PML patient lesions. We therefore employed ISH as an additional technique to help us decipher JCV DNA detection in these tissues. Our data showed that stringent qPCR criteria for the detection of JCV DNA may have in some cases assigned negative results to samples. It is also likely that the JCV DNA from rare positive cells detected by ISH was diluted within genomic DNA and dropped below the detection level of the qPCR assay.
While ISH confirmed the results for all the qPCR-positive samples in the HIV-positive PML group, only 3 of the 5 large T DNA-positive brains from the HIV-positive and HIV-negative groups were positive by ISH staining. As shown in Fig. 2, ISH detected rare positive cells in the latter two groups. In the brains of PML patients, the higher sensitivity of ISH may have been due to the fact that JCV DNA was more abundant in these samples and could readily be detected by ISH. In the brain of non-PML patients, some qPCR-positive cases were ISH negative. Since the piece of tissue used to extract DNA for qPCR is not the same as the sections used for ISH, this apparent discrepancy may be caused by sampling in areas of low JCV burden. This most likely contributed to the lower sensitivity of ISH in the HIV-positive and HIV-negative groups than in the HIV-positive PML group.
We did not expect to detect JCV DNA in the brains of a third of the HIV-positive and negative patients without PML and therefore performed ISH to verify these findings. ISH confirmed the presence of rare JCV-infected cells in most of these cases. However, only one subject in each HIV-positive and HIV-negative group without PML had expression of JCV T Ag, but not VP1, in the brain. These results suggest that JCV DNA may remain latent in a limited number of cells in many immunocompetent and immunosuppressed individuals alike, which may lead to an abortive infection with occasional expression of the T Ag only, without the virus undergoing a full replicative cycle. We have recently reported 38 cases of PML occurring in individuals with minimal or occult immunosuppression (9). It is therefore possible that in those patients, JCV was already latent and reactivated from within the brain.
The similar prevalences of JCV DNA in the brains of all three study groups indicate that the presence of JCV DNA in the brain is not associated with immunosuppression. A prior study has also shown that JCV DNA is present in 44% of brain samples of HIV-positive patients without PML and 33% of brain samples of HIV-negative patients without PML (25).
To determine the extent of JCV spread in the body, we also tested kidney, bone, spleen, and lymph node samples. PCR analysis, including detection of JCV large T and RR DNA, indicated that JC virus may spread throughout the body in both immunocompetent and immunosuppressed individuals with and without PML. However, JCV protein expression was seldom found in the kidneys of HIV-positive patients, with or without PML, and only occasionally in bone samples from HIV-positive PML patients. Taken together, these data demonstrate that although JCV DNA is present in various organs, active JCV replication and protein expression occur preferentially in the brains of patients who develop PML.
Lastly, the sequencing of JCV RR from all groups contained multiple types and mutations, which further support isolation and amplification of real physiological samples and not laboratory strains. The RR of JCV, containing transcription control factor binding sites, is closely associated with pathogenesis. Although the exact mechanism of rearranging and the effects of rearrangements on pathogenesis have yet to be fully delineated, our data showed that both the archetypal and rearranged forms can be detected in both the brain and kidney. We describe novel detection of the I-S, II-S, and I-R types of RRs in the spleens of patients in all categories. Overall, the HIV-negative group had only one I-R variant and one II-S variant, while the HIV-positive group without PML had three I-R variants, three II-S variants, and one II-R variant, and the HIV-positive group with PML had three I-R variants and two II-S variants. These results suggest that active replication of JCV in immunosuppressed patients contributed to the multiple forms of the RR present in all organs. These results invite us to reappraise the classic assignment of the rearranged I-R type of RR of JCV in the brain and the archetype II-S type in the kidney. Multiple types of RRs can be detected in all organs in PML patients as well as in HIV-positive and HIV-negative patients without PML. However, HIV-negative patients had fewer RR variations detected in only two organs, indicating that immunosuppression most likely enhanced the rearrangements in the RR variations detected in multiple organs in the HIV-positive groups both with and without PML.
Our results confirm and expand the results of previous studies showing the presence of JCV DNA in the brains of individuals without PML (4, 8, 25, 27). We have performed a comprehensive study of a large number of HIV-positive PML patients as well as HIV-positive and -negative individuals using several techniques to detect the presence of JCV DNA and protein in several organs. The JCV RRs in the brains of HIV-negative patients are of the I-S and I-R types, while spleens had the II-S type. Therefore, it is possible that primary infection with JCV is carried out with a mixture of virions harboring a variety of RR types. Thereafter, JCV with the II-S type of RR may preferentially establish latency in kidney epithelium cells, while JCV with the I-R and II-R RR types establishes latency in the brain. In the context of immunosuppression, JCV reactivates both in the brain and in the kidney, producing multiple forms of RRs in all organs.
Our findings of both JCV large T DNA and regulatory region DNA in the brains of individuals without PML demonstrate that JCV is latent in the brains of patients without HIV or PML. In these people, the large T Ag is sometimes expressed but the viral capsid protein VP1 is not. It is not clear if JCV first enters the central nervous system during primary infection. Further studies are needed to show whether the latent JCV strains present in the brain can reactivate under immunosuppression and cause PML. Alternatively, PML may also result from reactivated extraneural JCV strains, which then cross the blood-brain barrier. In conclusion, by demonstrating the presence of JCV DNA in the brains of patients without PML, we have gained new insights into JCV latency, compartmentalization, and reactivation. A better understanding of JCV latency in the brain will help in devising strategies to prevent its reactivation in HIV-infected patients and those treated with immunomodulatory medications.
Acknowledgments
We are grateful to Susan Morgello, Benjamin B. Gelman, H. Aaron Aronow, Elyse Singer, and Deborah Commins for providing PML samples through the National NeuroAIDS Tissue Consortium (NNTC).
The NNTC is supported by grants R24MH59724, R24NS38841, R24MH59745, and R24MH59656 from the NIH. We acknowledge NIH grants R01 NS 041198, R01 NS 047029, and K24 NS 060950 to I.J.K., the Harvard Medical School Center for AIDS Research (CFAR), an NIH-funded program (grant P30 AI60354), NIH grant K08 NS 064215-01A1 to C.S.T., and NIH grants T32 AI007245-26 and -27 to L.C.E.
Footnotes
Published ahead of print on 7 July 2010.
REFERENCES
- 1.Reference deleted.
- 2.Atwood, W. J., K. Amemiya, R. Traub, J. Harms, and E. O. Major. 1992. Interaction of the human polyomavirus, JCV, with human B-lymphocytes. Virology 190:716-723. [DOI] [PubMed] [Google Scholar]
- 3.Buckle, G. J., M. S. Godec, J. U. Rubi, C. Tornatore, E. O. Major, C. J. Gibbs, Jr., D. C. Gajdusek, and D. M. Asher. 1992. Lack of JC viral genomic sequences in multiple sclerosis brain tissue by polymerase chain reaction. Ann. Neurol. 32:829-831. [DOI] [PubMed] [Google Scholar]
- 4.Caldarelli-Stefano, R., L. Vago, E. Omodeo-Zorini, M. Mediati, L. Losciale, M. Nebuloni, G. Costanzi, and P. Ferrante. 1999. Detection and typing of JC virus in autopsy brains and extraneural organs of AIDS patients and non-immunocompromised individuals. J. Neurovirol. 5:125-133. [DOI] [PubMed] [Google Scholar]
- 5.Carson, K. R., A. M. Evens, E. A. Richey, T. M. Habermann, D. Focosi, J. F. Seymour, J. Laubach, S. D. Bawn, L. I. Gordon, J. N. Winter, R. R. Furman, J. M. Vose, A. D. Zelenetz, R. Mamtani, D. W. Raisch, G. W. Dorshimer, S. T. Rosen, K. Muro, N. R. Gottardi-Littell, R. L. Talley, O. Sartor, D. Green, E. O. Major, and C. L. Bennett. 2009. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113:4834-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesters, P. M., J. Heritage, and D. J. McCance. 1983. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 147:676-684. [DOI] [PubMed] [Google Scholar]
- 7.Delbue, S., E. Branchetti, R. Boldorini, L. Vago, P. Zerbi, C. Veggiani, S. Tremolada, and P. Ferrante. 2008. Presence and expression of JCV early gene large T antigen in the brains of immunocompromised and immunocompetent individuals. J. Med. Virol. 80:2147-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsner, C., and K. Dorries. 1992. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology 191:72-80. [DOI] [PubMed] [Google Scholar]
- 9.Gheuens, S., G. Pierone, P. Peeters, and I. J. Koralnik. 2010. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J. Neurol. Neurosurg. Psychiatry 81:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinnell, B. W., B. L. Padgett, and D. L. Walker. 1983. Distribution of nonintegrated DNA from JC papovavirus in organs of patients with progressive multifocal leukoencephalopathy. J. Infect. Dis. 147:669-675. [DOI] [PubMed] [Google Scholar]
- 11.Jensen, P. N., and E. O. Major. 2001. A classification scheme for human polyomavirus JCV variants based on the nucleotide sequence of the noncoding regulatory region. J. Neurovirol. 7:280-287. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura, T., Y. Aso, N. Kuniyoshi, K. Hara, and Y. Yogo. 1990. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J. Infect. Dis. 161:1128-1133. [DOI] [PubMed] [Google Scholar]
- 13.Kleinschmidt-DeMasters, B. K., and K. L. Tyler. 2005. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 353:369-374. [DOI] [PubMed] [Google Scholar]
- 14.Koralnik, I. J. 2006. Progressive multifocal leukoencephalopathy revisited: has the disease outgrown its name? Ann. Neurol. 60:162-173. [DOI] [PubMed] [Google Scholar]
- 15.Koralnik, I. J., D. Boden, V. X. Mai, C. I. Lord, and N. L. Letvin. 1999. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology 52:253-260. [DOI] [PubMed] [Google Scholar]
- 16.Korman, B. D., K. L. Tyler, and N. J. Korman. 2009. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch. Dermatol. 145:937-942. [DOI] [PubMed] [Google Scholar]
- 17.Langer-Gould, A., S. W. Atlas, A. J. Green, A. W. Bollen, and D. Pelletier. 2005. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353:375-381. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz, R. B., H. C. Thompson, J. F. Mueller, J. A. Cohen, and W. S. Dynan. 1993. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J. Infect. Dis. 167:13-20. [DOI] [PubMed] [Google Scholar]
- 19.Marzocchetti, A., M. Sanguinetti, S. D. Giambenedetto, A. Cingolani, G. Fadda, R. Cauda, and A. De Luca. 2007. Characterization of JC virus in cerebrospinal fluid from HIV-1 infected patients with progressive multifocal leukoencephalopathy: insights into viral pathogenesis and disease prognosis. J. Neurovirol. 13:338-346. [DOI] [PubMed] [Google Scholar]
- 19a.Molloy, E. S., and L. H. Calabrese. 2009. Therapy: targeted but not trouble-free: efalizumab and PML. Nat. Rev. Rheumatol. 5:418-419. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Liz, G., L. Del Valle, A. Gentilella, S. Croul, and K. Khalili. 2008. Detection of JC virus DNA fragments but not proteins in normal brain tissue. Ann. Neurol. 64:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randhawa, P., R. Shapiro, and A. Vats. 2005. Quantitation of DNA of polyomaviruses BK and JC in human kidneys. J. Infect. Dis. 192:504-509. [DOI] [PubMed] [Google Scholar]
- 22.Ryschkewitsch, C., P. Jensen, J. Hou, G. Fahle, S. Fischer, and E. O. Major. 2004. Comparison of PCR-Southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J. Virol. Methods 121:217-221. [DOI] [PubMed] [Google Scholar]
- 23.Stoner, G. L., C. F. Ryschkewitsch, D. L. Walker, D. Soffer, and H. D. Webster. 1986. Immunocytochemical search for JC papovavirus large T-antigen in multiple sclerosis brain tissue. Acta Neuropathol. 70:345-347. [DOI] [PubMed] [Google Scholar]
- 23a.Tan, C. S., and I. J. Koralnik. 2010. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 9:425-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan, C. S., B. J. Dezube, P. Bhargava, P. Autissier, C. Wuthrich, J. Miller, and I. J. Koralnik. 2009. Detection of JC virus DNA and proteins in the bone marrow of HIV-positive and HIV-negative patients: implications for viral latency and neurotropic transformation. J. Infect. Dis. 199:881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vago, L., P. Cinque, E. Sala, M. Nebuloni, R. Caldarelli, S. Racca, P. Ferrante, G. Trabottoni, and G. Costanzi. 1996. JCV-DNA and BKV-DNA in the CNS tissue and CSF of AIDS patients and normal subjects. Study of 41 cases and review of the literature. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:139-146. [DOI] [PubMed] [Google Scholar]
- 26.Van Assche, G., M. Van Ranst, R. Sciot, B. Dubois, S. Vermeire, M. Noman, J. Verbeeck, K. Geboes, W. Robberecht, and P. Rutgeerts. 2005. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353:362-368. [DOI] [PubMed] [Google Scholar]
- 27.White, F. A., III, M. Ishaq, G. L. Stoner, and R. J. Frisque. 1992. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J. Virol. 66:5726-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wuthrich, C., X. Dang, S. Westmoreland, J. McKay, A. Maheshwari, M. P. Anderson, A. H. Ropper, R. P. Viscidi, and I. J. Koralnik. 2009. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann. Neurol. 65:742-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wuthrich, C., S. Kesari, W. K. Kim, K. Williams, R. Gelman, D. Elmeric, U. De Girolami, J. T. Joseph, T. Hedley-Whyte, and I. J. Koralnik. 2006. Characterization of lymphocytic infiltrates in progressive multifocal leukoencephalopathy: co-localization of CD8(+) T cells with JCV-infected glial cells. J. Neurovirol. 12:116-128. [DOI] [PubMed] [Google Scholar]
- 30.Yogo, Y., T. Iida, F. Taguchi, T. Kitamura, and Y. Aso. 1991. Typing of human polyomavirus JC virus on the basis of restriction fragment length polymorphisms. J. Clin. Microbiol. 29:2130-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]