Abstract
Memory CD4 T cells specific for influenza virus are generated from natural infection and vaccination, persist long-term, and recognize determinants in seasonal and pandemic influenza virus strains. However, the protective potential of these long-lived influenza virus-specific memory CD4 T cells is not clear, including whether CD4 T-cell helper or effector functions are important in secondary antiviral responses. Here we demonstrate that memory CD4 T cells specific for H1N1 influenza virus directed protective responses to influenza virus challenge through intrinsic effector mechanisms, resulting in enhanced viral clearance, recovery from sublethal infection, and full protection from lethal challenge. Mice with influenza virus hemagglutinin (HA)-specific memory CD4 T cells or polyclonal influenza virus-specific memory CD4 T cells exhibited protection from influenza virus challenge that occurred in the presence of CD8-depleting antibodies in B-cell-deficient mice and when CD4 T cells were transferred into lymphocyte-deficient RAG2−/− mice. Moreover, the presence of memory CD4 T cells mobilized enhanced T-cell recruitment and immune responses in the lung. Neutralization of gamma interferon (IFN-γ) production in vivo abrogated memory CD4 T-cell-mediated protection from influenza virus challenge by HA-specific memory T cells and heterosubtypic protection by polyclonal memory CD4 T cells. Our results indicate that memory CD4 T cells can direct enhanced protection from influenza virus infection through mobilization of immune effectors in the lung, independent of their helper functions. These findings have important implications for the generation of universal influenza vaccines by promoting long-lived protective CD4 T-cell responses.
Influenza virus poses substantial threats to world health due to the emergence of new pandemic strains through viral mutation and reassortment, including the 2009 H1N1 pandemic strain. Developing effective vaccines that can provide immune-mediated protection to multiple influenza virus strains remains a major challenge, as current vaccines generate neutralizing antibodies directed against the highly variable hemagglutinin (HA) and neuraminidase (NA) surface viral glycoproteins (18). These vaccines are only partially effective at protecting individuals from succumbing to seasonal strains and are largely ineffective at protecting individuals from new pandemics. In contrast, T lymphocytes have the potential to provide long-term cross-strain protection, through their recognition of invariant viral determinants (3, 9), generation of effector responses to coordinate both cellular and humoral immunity, and development of memory populations that persist for decades (34). In humans, influenza virus-specific CD4 and CD8 T cells recognize internal polymerase, matrix, and nucleoprotein components of influenza virus which are conserved in multiple strains (3). Influenza virus-specific memory T cells generated from virus exposure and vaccines can be detected readily in the peripheral blood of healthy older children and adults (16, 30). Elucidating the protective capacities of memory T cells in antiviral immunity and their underlying mechanisms is therefore crucial to understanding clinical responses to influenza and to developing strategies to boost T-cell-mediated immunity for the next emerging pandemic.
The potent cytolytic responses of virus-specific CD8 T cells and their roles in antiviral primary and secondary responses have been well established (58); however, considerably less is known about the function of memory CD4 T cells in antiviral immunity. Memory CD4 T cells have the potential to play more diverse roles in coordinating secondary responses than those of memory CD8 T cells via their ability to “help” or promote cellular and humoral immunity, and also through direct effector functions. Compared to CD8 T-cell responses, memory CD4 T-cell responses in humans were found to recognize a more diverse array of influenza virus-specific epitopes (46-48) and to exhibit cross-reactivities with new pandemic strains, including avian H5N1 and 2009 H1N1 “swine flu” strains (23, 28, 36, 48). In addition, antiviral memory CD4 T cells generated as a result of influenza vaccination (22) were found to persist longer than CD8 T cells in vivo following smallpox vaccination (29). These findings suggest that memory CD4 T-cell responses could be potential targets for boosting long-term cellular immunity following vaccination, although their protective capacity remains undefined.
The role of CD4 T cells in anti-influenza virus immunity has been elucidated mainly for primary responses, and less is known about the protective potential and mechanisms underlying memory CD4 T-cell-directed secondary responses. In primary influenza virus infection, CD4 T cells promote antibody production by B cells necessary for complete viral clearance (2, 17, 19, 39, 40, 57) and also promote the generation of memory CD8 T cells (4). Whether memory CD4 T cells have a similar helper-intensive role in promoting B cells and CD8 T cells in secondary influenza responses or whether effector responses predominate is not known. In this study, we investigated the mechanisms by which memory CD4 T cells mediate secondary responses and promote recovery from influenza virus infection in the clinically relevant scenario of a persisting CD4 T-cell response but no preexisting antibody response to a new influenza virus strain. We demonstrate that both influenza virus HA-specific and polyclonal influenza virus-specific memory CD4 T cells direct rapid lung viral clearance and protect from lethality via secondary antiviral responses in the absence of CD8 T cells, B cells, or any lymphocytes. Unlike primary responses to influenza virus, which can mediate protection independent of gamma interferon (IFN-γ), memory CD4 T-cell-mediated protection in the lung is dependent on secreted IFN-γ and is associated with localized interactions with lung airways and foci of T-cell-directed responses. Our findings reveal that memory CD4 T cells drive antiviral protection in the lung through a qualitatively distinct mechanism and have important implications for exploiting the protective role of persisting memory CD4 T cells in vaccines and immunotherapies.
MATERIALS AND METHODS
Mice.
BALB/c, C57BL/6, and B6.Ly5.2 mice (8 to 16 weeks of age) were obtained from the National Cancer Institute Biological Testing Branch. Congenic BALB/c(Thy1.1) mice were bred as homozygotes, and HA-TCR transgenic mice expressing a transgene-encoded T-cell receptor (TCR; clonotype 6.5) specific for an influenza virus HA peptide (110-119) and I-Ed (35) were bred as heterozygotes onto BALB/c(Thy1.2) or BALB/c(Thy1.1) hosts. JhD−/− mice were purchased from Taconic Farms, Inc. (Germantown, NY), and RAG2−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained in the animal facility at the University of Maryland School of Medicine (Baltimore, MD). All animal procedures using mice were approved by and conducted in accordance with guidelines set forth by the Institutional Animal Care and Use Committee (IACUC) at the University of Maryland, Baltimore, MD.
Reagents.
The following purified antibodies were purchased from BioXcell (West Lebanon, NH): anti-CD8 (TIB105), anti-CD4 (GK1.5), anti-I-Ad (212.A1), and anti-Thy1 (TIB238). An anti-clonotype 6.5 antibody directed against the HA-TCR (35) was purified and conjugated to biotin (Pierce, Rockford, IL). Fluorescently conjugated antibodies directed against CD62L, CD90.1, CD90.2, CD4, CD8α (clone 53-6.7), and CD8β (clone 53-5.8) were purchased from BD Pharmingen (San Diego, CA). Influenza virus HA peptide (110-120 [SFERFEIFPKE]) was synthesized by the Biopolymer Laboratory at the University of Maryland School of Medicine.
Influenza virus infection.
Influenza virus (A/PR/8/34) was grown in the allantoic fluid of 10-day-old embryonated chicken eggs as described previously (52). Determination of influenza viral titers in viral stocks or lung homogenates was accomplished by a 50% tissue culture infective dose (TCID50) assay as described previously (50), with titers expressed as the reciprocal of the dilution of lung extract that corresponded to 50% virus growth in Madine Darby canine kidney (MDCK) cells, as calculated by the Reed-Muench method. For in vivo infection, mice were anesthetized with isoflurane, 30 μl PR8 influenza virus was administered intranasally at 500 TCID50 for a sublethal dose or 5,000 TCID50 as a lethal dose, and weight loss morbidity was monitored. Lethally infected mice either died or were sacrificed after losing more than 25% of their starting weight, according to our animal protocol. All infected mice were housed in the biocontainment suite at the animal facility of the University of Maryland, Baltimore, where tissue harvest from infected mice was also performed.
Generation of influenza virus-specific memory CD4 T cells.
Generation of HA-specific memory CD4 T cells in congenic BALB/c(Thy1.1) hosts was accomplished as previously described (6, 38). CD4 T cells purified from spleens of HA-TCR mice were primed in vitro by culture with 5.0 μg/ml HA peptide and with mitomycin C-treated, T-cell-depleted BALB/c splenocytes, as antigen-presenting cells (APC), in complete Clicks medium (1) (Irvine Scientific, Irvine, CA) for 3 days at 37°C. The resultant activated HA-specific effector cells were transferred into congenic BALB/c(Thy1.1) hosts (5 × 106 cells/mouse) to yield “HA-memory” mice with a stable population of HA-specific memory CD4 T cells (6, 38, 43).
Polyclonal memory CD4 T cells specific for influenza virus were generated by infecting C57BL/6 mice intranasally with 500 TCID50 of PR8 influenza virus. CD4 T cells from the spleen and lungs were harvested at 4 to 8 weeks postinfection and were purified by negative selection as described above. The relative frequencies of influenza virus-specific IFN-γ-secreting memory CD4 T cells in response to stimulation with a conserved influenza virus NP peptide (311-325) in the presence of 1 μg/ml anti-CD28 antibody and monensin (BD Pharmingen) for 6 h were determined as previously described (14), and cytokine production was determined by intracellular cytokine staining as described previously (11).
Antibody-mediated CD8 T-cell depletion and IFN-γ neutralization.
Anti-CD8 (TIB105), anti-murine IFN-γ antibody (clone XMG1.2), and IgG1 isotype control antibodies were purchased from BioXcell (West Lebanon, NH). For CD8 T-cell depletion, 200 μg of TIB105 antibody was administered by intraperitoneal injection on days −2, 0, and 2 postinfection. For IFN-γ neutralization, mice were treated on days −2, 0, 2, and 4 postinfection with 500 μg/mouse of XMG1.2 or IgG1 isotype control antibody by intraperitoneal injection and, in some cases, with an additional 100 μg of XMG1.2 or IgG1 via intranasal administration in a 20-μl volume on days 0 and 2 postinfection.
Flow cytometry.
Cells were stained with fluorochrome-conjugated antibodies as described previously (42), fixed and acquired using an LSRII flow cytometer (BD, San Jose, CA) with a minimum acquisition of 100,000 events, and analyzed using FACSDiva (BD, San Jose, CA) and FlowJo software.
Histopathology.
Lungs from saline-perfused PR8-infected HA memory CD4 T cells or X31-infected mice with polyclonal influenza virus-specific memory CD4 T cells were inflated with formalin through the trachea. Five-micrometer sections were cut, and antigen retrieval was performed with sodium citrate buffer (pH 6.0) in a pressure cooker for 20 min. Endogenous peroxide was quenched with 1% H2O2 in phosphate-buffered saline (PBS) for 10 min. Tissues were blocked with PBS containing 10% donkey serum for 30 min, followed by avidin-biotin blocking per the manufacturer's instructions (Thermo Scientific). CD3 T cells were stained with a rat anti-mouse/human CD3 antibody (Serotec clone MA1471) overnight at 4°C, followed by a biotin-conjugated anti-rat antibody (Jackson Immunoresearch) and then streptavidin-horseradish peroxidase (KPL). The signal was developed using DAB substrate (KPL) followed by counterstaining with contrast green (KPL).
Statistics.
The results are expressed as mean values for individual groups ± standard errors of the means (SEM [indicated with error bars]) unless designated otherwise. The significance of differences between experimental groups was determined by two-tailed Student t tests and also by one-way analysis of variance (ANOVA) for comparison of multiple groups, assuming a normal distribution for all groups.
RESULTS
Memory CD4 T-cell-mediated protective responses to influenza virus challenge.
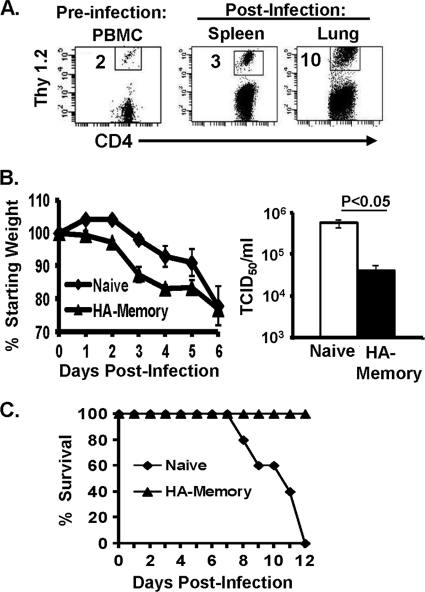
We examined mechanisms for secondary responses mediated by influenza virus-specific memory CD4 T cells by using an in vivo system, extensively validated in the lab, in which naive BALB/c mice contain a heterogeneous population of influenza virus HA-specific memory CD4 T cells and are designated “HA-memory” mice (56). HA-memory mice were generated by in vitro priming of HA-specific CD4 T cells from HA-TCR transgenic mice and subsequent adoptive transfer into BALB/c congenic hosts (6, 38, 56), resulting in a stable population of HA-specific memory CD4 T cells comprising 0.5 to 2% of total endogenous CD4 T cells (6) (Fig. 1A). HA-specific memory CD4 T cells persist in lymphoid and multiple nonlymphoid tissues sites and exhibit both phenotypic and functional properties of in vivo-primed polyclonal memory CD4 T cells, including rapid production of IFN-γ as the predominant effector cytokine (1, 6, 42-44). Infection of HA-memory mice with a sublethal dose of PR8 influenza virus resulted in similar virus-induced morbidity to that of infected naive mice, as assessed by continuous weight loss over a 6- to 7-day period (Fig. 1B, left panel); however, HA-memory mice exhibited increased clearance of influenza virus from the lungs compared to naive mice (Fig. 1B, right panel). This viral clearance was associated with an increased frequency of HA-specific memory CD4 T cells in the spleen and lungs following infection (Fig. 1A), indicating virus-driven expansion of the persisting memory population.
FIG. 1.
Influenza virus-specific memory CD4 T cells mediate secondary responses to influenza virus challenge. (A) Influenza virus infection induces expansion of HA-specific memory CD4 T cells. Flow cytometry plots show the frequencies of CD4+ Thy1.2+ HA-specific memory CD4 T cells in the blood 1 day before infection (left) and in the spleen and lungs 6 days following influenza virus challenge (right), with the percentage of HA-specific Thy1.2+ memory CD4 T cells among total CD4 T cells indicated in each plot. (B) Naive or HA-memory mice were infected intranasally with 500 TCID50 of PR8 influenza virus (sublethal dose) and monitored for 6 days. (Left) Daily weight loss, expressed as a percentage of starting weight (100%), in naive and HA-memory mice following influenza virus challenge (the average weight loss for naive mice was 23%, and that for HA-memory mice was 24%). (Right) Lung viral titers determined by TCID50 assay (see Materials and Methods) of lung homogenates harvested at 6 days postinfection, with a P value of <0.05 for the difference in titers between naive and HA-memory mice (four mice per group). Results are representative of 6 independent experiments. (C) Naive BALB/c or HA-memory mice were infected with a lethal dose of PR8 influenza virus and then monitored daily. The graph shows the percentage of mice surviving each day after infection, with five mice per group. The results are representative of two independent experiments.
Based on the findings that memory CD4 T cells can mediate enhanced viral clearance to a sublethal infection, we asked whether memory CD4 T cells could also mediate protection from lethal influenza virus infection by challenging HA-memory mice with a lethal influenza virus dose leading to death in 100% of naive mice (Fig. 1C). We found that HA-memory mice were fully protected from lethal influenza virus infection, revealing the potential of memory CD4 T cells to mediate protective immunity to high viral doses (Fig. 1C). Together, these results indicate that influenza virus-specific memory CD4 T cells can direct a classical secondary immune response to influenza virus challenge, with early control of virus replication, resulting in complete protection from a lethal virus challenge.
Depletion of CD8 T cells does not inhibit memory CD4 T-cell-directed protection from influenza virus challenge.
In primary responses to influenza virus, the critical immune effectors that mediate virus clearance are the CD8 effector response and B-cell-derived influenza virus-specific antibodies (5), which are both promoted by CD4 T cells. We hypothesized that the increased viral clearance following influenza virus infection in the presence of memory CD4 T cells could be due to increased helper responses and could require the participation of CD8 T cells and/or B cells. We investigated these cellular requirements for memory CD4 T-cell-mediated protection by using depletion strategies and genetically deficient mouse models.
To examine the CD8 T-cell requirement for memory CD4 T-cell-mediated protection, we depleted CD8 T cells in vivo in naive and HA-memory mice by using a well-defined anti-CD8 depleting antibody (40, 65) prior to and throughout the course of influenza virus infection and then analyzed the resultant effects on morbidity and viral load. Antibody-mediated CD8 depletion resulted in a substantial reduction in the proportion of CD8 T cells in peripheral blood, with no remaining cells staining positive with anti-CD8α antibodies and only a small proportion staining weakly with anti-CD8β antibodies (Fig. 2A). The frequency of CD4 T cells was unaltered by the treatment (Fig. 2A). Following infection, there was a significant and specific reduction in the proportion of CD8 T cells in the spleen (>90%) and the lungs (>75%) at 6 days postinfection, while CD4 T cells predominated in this system (Fig. 2B). Despite the reduction in CD8 T cells, we found no differences in virus-induced weight loss morbidity in naive and HA-memory mice following influenza virus infection, with both control and anti-CD8-treated HA-memory mice losing more weight than infected naive groups early after influenza virus challenge (Fig. 2C). Moreover, depletion of CD8 T cells in HA-memory mice did not inhibit the enhanced viral clearance at 6 days postinfection and also did not alter the course of infection in naive mice (Fig. 2D). These results suggest that influenza virus-specific memory CD4 T cells can mediate enhanced viral clearance in the absence of significant contributions by CD8 T cells.
FIG. 2.
Memory CD4 T-cell-mediated viral clearance in hosts depleted of CD8 T cells. Naive BALB/c and HA-memory mice were left untreated (control) or administered anti-CD8 depleting antibody 1 day prior to and every other day throughout the course of infection. (A) CD8 and CD4 T-cell frequencies in peripheral blood following anti-CD8 depletion in uninfected mice. Plots show CD3ɛ versus CD8β or CD8α in control or anti-CD8-treated mice. (B) Frequencies of CD4 and CD8 T cells in the spleens and lungs of control and CD8-depleted mice 6 days after influenza virus infection. Results are graphed as means ± standard deviations (SD) for five mice per group and are representative of two experiments. Significant differences between control and treated groups were found for CD8 T cells in the spleen (***, P < 0.001) and lungs (**, P < 0.01). (C) Daily weight loss, expressed as a percentage of starting weight (100%), following influenza virus challenge in naive BALB/c or HA-memory mice treated with anti-CD8 depleting antibody or with PBS (control). (D) Lung viral titers determined by TCID50 assay (see Materials and Methods) of lung homogenates harvested at 6 days postinfection. Results were compiled from two independent experiments (n = 8 to 10 mice/group). Significant differences in titers were found between PBS-treated naive and HA-memory mice (*, P < 0.05) and between anti-CD8-treated naive and HA-memory mice (**, P < 0.01).
Antiviral memory CD4 T cells protect in the absence of B cells.
We examined whether the presence of influenza virus-specific memory CD4 T cells would direct enhanced viral clearance within B-cell-deficient hosts. In primary responses, B-cell-deficient mice display delayed viral clearance and increased mortality after influenza virus infection compared to wild-type mice (25, 26). We generated HA-specific memory CD4 T cells in hosts devoid of B cells by transfer of primed HA-specific CD4 T cells, as described for Fig. 1, into syngeneic, JhD−/−, B-cell-deficient hosts, resulting in “JhD-memory” mice. HA-specific memory CD4 T cells persisted in similar frequencies in diverse tissue sites in JhD-memory mice and wild-type HA-memory mice (Fig. 3A and data not shown). Influenza virus challenge of JhD-memory hosts resulted in substantial frequencies of HA-specific memory CD4 T cells in the spleen and, particularly, in the lungs (Fig. 3A), similar to their accumulation after infection of wild-type HA-memory hosts (Fig. 1A).
FIG. 3.

Memory CD4 T cells mediate protective responses to influenza virus challenge in the absence of B cells. (A) Influenza virus infection induces expansion of HA-specific memory CD4 T cells in B-cell-deficient (JhD−/−) hosts. Flow cytometry plots show the frequencies of CD4+ Thy1.1+ HA-specific memory CD4 T cells in the blood 1 day before infection (left) and in the spleen and lungs 6 days following influenza virus challenge (right), with the percentage of HA-specific Thy1.1+ memory CD4 T cells among total CD4 T cells indicated in each plot. (B) JhD-memory, JhD naive, and control wild-type naive and HA-memory mice were infected intranasally with 500 TCID50 of PR8 influenza virus and monitored for up to 10 days postinfection. The graph shows daily weights, expressed as percentages of starting weight (100%), for all infected groups. (C) Kinetics of viral clearance in JhD-memory, JhD naive, and control wild-type naive and HA-memory mice at 6 and 10 days postinfection. On day 10, all groups except for JhD naive mice exhibited viral loads below the detection limit of the assay (bd.). Significant differences in titers were found between all groups (P < 0.001) by one-way ANOVA. **, P = 0.005, comparing JhD naive to JhD-memory mice; ***, P = 0.001, comparing naive BALB/c to JhD-memory mice. Results are representative of two independent experiments (7 to 10 mice/group). bd., below detection.
We analyzed the kinetics of weight loss morbidity and protection in influenza virus-challenged JhD-memory and JhD naive mice, and also in wild-type naive and HA-memory mice, as controls. We found that the presence of memory CD4 T cells in both wild-type and B-cell-deficient hosts could direct enhanced recovery from virus-induced illness compared to that of infected naive mice. Both JhD-memory and HA-memory groups exhibited early weight loss which continued until day 8 postinfection, when mice started to regain weight, exhibiting full recovery by day 10 postinfection. In contrast, naive mice infected with influenza virus started to recover at day 10 postinfection, while JhD naive mice continued to lose weight through day 10 postinfection, consistent with their worse outcome for primary influenza virus infection (26). For protection, both HA-memory and JhD-memory mice exhibited lower lung viral loads than those in wild-type and JhD naive mice at day 6 postinfection. At day 10 postinfection, both memory groups and wild-type naive mice exhibited complete viral clearance (Fig. 3C), while JhD naive mice maintained significant lung viral loads, consistent with delayed viral clearance in primary responses observed previously in B-cell-deficient mice (57). These results indicate that HA-specific memory CD4 T cells can mediate protective responses independent of B cells. Thus, CD4 T cells have disparate roles in primary and secondary responses: in primary responses, they promote protection through help for antibody production, whereas in secondary responses, their protective capacities are uncoupled from their B-cell helper functions.
Polyclonal influenza virus-specific memory CD4 T cells can direct protective immunity in lymphocyte-deficient hosts.
We investigated whether early viral clearance mediated by TCR-transgenic HA-specific memory CD4 T cells in BALB/c mice in the absence of accessory lymphocytes was applicable to polyclonal systems. We previously found that transfer of polyclonal influenza virus-specific memory CD4 T cells into naive mice resulted in enhanced viral clearance following influenza virus challenge [56]. We generated polyclonal influenza virus-specific memory CD4 T cells as described previously (42, 56) by infecting unmanipulated C57BL/6 mice with a sublethal dose of PR8 influenza virus and letting them recover and persist for 4 to 8 weeks postinfection, resulting in “flu-memory” mice. We harvested total CD4 T cells from spleens and lungs of flu-memory mice and determined the frequency of influenza virus-specific memory CD4 T cells by enzyme-linked immunospot (ELISPOT) assay (42, 56) and intracellular cytokine staining (Fig. 4A), by which we detected a measurable frequency of memory CD4 T cells specific for a conserved major histocompatibility complex (MHC) class II (I-Ab)-restricted epitope of influenza virus nucleoprotein (NP311-325) (14). We examined the intrinsic protective capacities of polyclonal CD4 T-cell populations by transferring equal numbers of total CD4 T cells from naive C57BL/6 and flu-memory mice into lymphocyte-deficient RAG2−/− hosts, generating RAG+Naive and RAG+Memory hosts, respectively. The resultant T-cell-reconstituted RAG2−/− naive and memory recipients had similar numbers of total CD4 T cells (data not shown), enabling a direct comparison of CD4 T-cell-directed responses in the absence of CD8 T cells or B cells. RAG+Naive, RAG+Memory, and unmanipulated RAG2−/− hosts were challenged with PR8 influenza virus, and morbidity and viral clearance were monitored. While all groups exhibited similar weight loss morbidities for up to 7 days postinfection (Fig. 4B), there was a significant reduction in lung viral loads of RAG+Memory mice compared to those in infected RAG2−/− and RAG+Naive mice (Fig. 4C). Thus, polyclonal memory CD4 T cells generated in response to influenza virus infection can promote efficient viral clearance in response to influenza virus challenge in lymphocyte-deficient hosts, consistent with the helper-independent clearance mediated by HA-specific memory CD4 T cells.
FIG. 4.

Protection mediated by polyclonal influenza virus-specific memory CD4 T cells occurs in lymphocyte-deficient hosts. C57BL/6 mice were infected with 500 TCID50 of PR8 influenza virus and monitored for infection, and CD4 T cells were purified from the spleens and lungs of these “flu-memory” mice at 4 to 8 weeks postinfection. (A) Frequencies of influenza virus NP-specific CD4 T cells 8 weeks after infection, as determined by intracellular cytokine staining following stimulation with 1 μg/ml anti-CD28 (medium alone), 1 μg/ml NP311-325 plus 1 μg/ml anti-CD28 (NP311-325), or phorbol myristate acetate-ionomycin (PMA/Iono). These results are representative of two experiments, each with five mice per group. (B) CD4 T cells purified from the spleens and lungs of naive C57BL/6 mice or C57BL/6 flu-memory mice were transferred (8 × 106 total cells, comprising 4 × 106 cells from the spleen and 4 × 106 cells from the lungs) intravenously into RAG2−/− recipients, which were subsequently infected with 500 TCID50 of PR8. The graph shows weight loss morbidities of the influenza virus-infected RAG2−/−, RAG2+Naive, and RAG2+Memory groups up until day 7 postinfection. Weight loss differences between naive and memory groups were not significant. (C) Lung viral titers at day 7 postinfection in RAG2−/−, RAG2+Naive, and RAG2+Memory mice, expressed as the average for 10 mice per group, compiled from two independent experiments with 5 mice per group (representative of three independent experiments). The significance of differences in viral titers between the RAG+Memory, RAG+Naïve, and RAG−/− groups was determined by one-way ANOVA (P = 0.002).
Memory CD4 T cells mobilize an enhanced T-cell immune response in the lung.
The ability of CD4 T cells from mice previously infected with influenza virus to direct protective responses independent of B cells or CD8 T cells suggested that memory CD4 T cells mediated a quantitatively and qualitatively distinct response in the lungs compared to the primary response. We examined the in situ lung T-cell response to influenza virus infection mediated by polyclonal naive or memory CD4 T cells in both RAG2−/− and intact C57BL/6 hosts. Thus, total CD4 T cells from naive or flu-memory mice (Fig. 4) were transferred into C57BL/6 or RAG2−/− recipients, which were subsequently challenged with a sublethal dose of PR8 influenza virus. Lung sections of influenza virus-infected mice were isolated at 7 days postinfection, stained for CD3 expression, and analyzed by immunohistochemistry (Fig. 5A). In the primary response mediated by naive CD4 T cells in both C57BL/6 and RAG2−/− hosts, there was a diffuse pattern of T-cell staining in the lung and throughout the alveolar spaces (Fig. 5A, second row) that was not present in uninfected control mice (Fig. 5A, top panel). In contrast, lungs from influenza virus-challenged C57BL/6 and RAG2−/− recipients of CD4 T cells from flu-memory mice exhibited increased T-cell staining and clustering in alveolar spaces and around airways (Fig. 5A, bottom row). These clustered T-cell infiltrates in secondary influenza virus responses in the lung were interspersed with clear areas of healthy lung tissue, suggesting that the memory CD4 T-cell-directed response prevents the spread and dissemination of virus throughout the lung. Quantitative analysis of T-cell infiltration into the lungs of C57BL/6 hosts following infection revealed a substantially greater T-cell lung infiltrate for memory than for primary responses (Fig. 5B), comprised mainly of CD4 T cells, as determined by flow cytometry (56; data not shown). These results reveal enhanced recruitment and mobilization of the T-cell infiltrates in memory versus primary responses, suggesting that memory CD4 T cells act directly within the lung tissue to mediate viral clearance.
FIG. 5.

Localized memory CD4 T-cell-directed responses in the lung are distinct from diffuse T-cell distribution during primary infection. (A) Localization of CD3+ T cells in the lungs of uninfected C57BL/6 mice (top) and influenza virus-infected C57BL/6 mouse recipients of total CD4 T cells from naive (middle left) or C57BL/6 flu-memory (lower left) mice 7 days after infection with X31, using immunohistochemistry. Similar CD3 immunohistochemistry analysis was performed on lungs from PR8-infected RAG2−/− mice that received total CD4 T cells from naive (middle right) or C57BL/6 flu-memory (lower right) mice (see Fig. 6). CD3+ T cells stain brown, as indicated by arrows, and images for representative animals of five mice per group are shown at a magnification of ×20. (B) CD3 T cells were enumerated by counting the total number of cells staining for CD3 and taking the average for five 1-mm2 sections of lung per animal. Each section counted had a similar lung architecture. Data shown are the average counts for five animals per group. ***, P < 0.001, representing the difference in CD3 numbers between groups, as determined by ANOVA.
Enhanced viral clearance and protection mediated by memory CD4 T cells are dependent on IFN-γ.
We hypothesized that the ability of influenza virus-specific memory CD4 T cells to direct localized lung responses depends on their effector function. Because the predominant effector cytokine produced by both polyclonal and HA-specific memory CD4 T cells is IFN-γ (6, 42), we hypothesized that IFN-γ may be required for memory CD4 T-cell-mediated protection from influenza virus. We examined the requirement for IFN-γ in both HA-specific and polyclonal systems by administration of a neutralizing anti-IFN-γ antibody (XMG1.2) both intravenously and intranasally, as this approach was previously shown to be efficacious in eliminating secreted IFN-γ in lung homogenates without altering the cytokine profile of resident T cells (32, 54). Neutralization of IFN-γ in naive or HA-memory mice following influenza virus infection did not affect weight loss for up to 5 days postinfection for either group (Fig. 6A), although HA-memory mice treated with anti-IFN-γ exhibited a low-level mortality (<10%) at day 5 postinfection with this sublethal dose (data not shown). In addition, neutralization of IFN-γ had differential effects on viral clearance, specifically in treated HA-memory mice. In naive mice infected with influenza virus, IFN-γ neutralization did not alter lung viral loads for 5 days post-influenza virus infection compared to those in control infected naive mice (Fig. 6B), consistent with previous reports that IFN-γ production is dispensable for viral clearance in the primary response (27, 59). In contrast, in HA-memory mice, neutralization of IFN-γ significantly inhibited the enhanced viral clearance observed in the control-treated memory group (Fig. 6B), indicating that during a secondary immune response driven by memory CD4 T cells, enhanced viral clearance is dependent on IFN-γ secretion.
FIG. 6.
Memory CD4 T-cell-mediated protection is dependent on IFN-γ. Naive and HA-memory mice were treated intraperitoneally and intranasally with control IgG and anti-IFN-γ antibody (see Materials and Methods) and then challenged with 500 TCID50 of PR8 influenza virus. (A) Daily weight loss, measured as described previously. (B) Lung viral titers at 5 days post-influenza virus challenge, determined by the TCID50 method as described previously. Results are representative of 2 independent experiments (8 to 10 mice/group). *, P < 0.05, comparing HA-memory mice treated with XMG1.2 and IgG1. (C) Absolute numbers of HA-specific memory CD4 T cells in spleen and lung tissue, calculated from flow cytometry analysis and microscopic cell counts. Data are shown as the total number of Thy1.2+ memory CD4 T cells in each tissue. (D) IFN-γ neutralization does not skew cytokine production of HA-memory CD4 T cells after influenza virus infection. HA-specific memory CD4 T cells were isolated at 5 days postinfection from the spleen and lungs of influenza virus-infected HA-memory mice and stimulated ex vivo with PMA-ionomycin for 5 h. The graphs show percentages of Thy1.2+ memory CD4 T cells producing IFN-γ, IL-4, and IL-17 in both spleen and lung tissues.
IFN-γ signaling has been shown to be important for memory T-cell differentiation, stability, and protective capacity (63, 64), and it was therefore important to determine whether in vivo IFN-γ neutralization altered the expansion or function of memory CD4 T cells. We found that IFN-γ neutralization in HA-memory hosts did not inhibit the accumulation of HA-specific memory CD4 T cells in lung or spleen tissues after infection (Fig. 6C). In both the spleen and lungs following infection, HA-specific memory CD4 T cells exhibited a Th1-like profile, with predominant IFN-γ producers and some interleukin-4 (IL-4) producers in the lungs, with minimal IL-17 and IL-10 production (Fig. 6D). This memory CD4 T-cell-specific cytokine profile was unaltered in mice treated with anti-IFN-γ neutralizing antibodies (Fig. 6D). In addition, memory CD4 T-cell-mediated production of the cytokines IFN-γ, IL-4, IL-10, and IL-17 in both spleen and lung tissues was not affected by treatment with IFN-γ-neutralizing antibodies, demonstrating that IFN-γ neutralization in vivo did not skew HA-memory CD4 T-cell cytokine production to a Th2 or Th17 profile. The reduced memory CD4 T-cell-mediated viral clearance in the presence of neutralizing anti-IFN-γ antibodies did not appear to result from altered T-cell migration or function and was therefore dependent on the secretion of IFN-γ.
Heterosubtypic memory CD4 T-cell-mediated protection from influenza virus is IFN-γ dependent.
T-cell-directed responses can uniquely mediate protection against heterosubtypic strains of influenza virus displaying altered HA and NA sequences. We investigated whether memory CD4 T-cell-mediated protection from heterosubtypic influenza virus challenge required IFN-γ. We transferred CD4 T cells from flu-memory mice previously infected with PR8 influenza virus into intact B6.Ly5.2 congenic naive mouse hosts, challenged the resultant hosts with the heterosubtypic influenza virus strain X31 (H3N2), and monitored antiviral responses in groups receiving control or IFN-γ-neutralizing antibodies. We found increased viral loads in mouse memory hosts treated with IFN-γ-neutralizing antibody compared to those in mice treated with control antibody (Fig. 7B), although morbidity was not altered (Fig. 7A). In addition, neutralization of IFN-γ exacerbated mononuclear cell infiltrates in mice with memory CD4 T cells (Fig. 7C). At 7 days postinfection, lungs from influenza virus-infected memory hosts exhibited regions of concentrated cellular infiltrates interspersed with regions of healthy lung (Fig. 7C, compare middle panel to healthy lung in first panel), as also observed by CD3 immunohistochemistry (Fig. 6). In contrast, lungs of influenza virus-infected memory hosts treated with neutralizing anti-IFN-γ antibody had greatly exacerbated lung pathology, with blood leakage into alveolar spaces and other hallmarks of lung damage induced by lethal viral doses (61) or highly pathogenic influenza virus strains (33). Taken together, the heightened viral load and worse lung pathology in the presence of IFN-γ neutralization indicate that secreted IFN-γ is required for local lung protective responses by polyclonal memory CD4 T cells and for curtailing the spread of virus in the lung.
FIG. 7.
Heterosubtypic protection mediated by memory CD4 T cells requires IFN-γ secretion. CD4 T cells containing influenza virus-specific memory CD4 T cells were purified from the spleens and lungs of C57BL/6 mice infected with 500 TCID50 of PR8 influenza virus 6 weeks previously, as described in the legend to Fig. 5A, and 8 × 106 CD4 T cells were transferred into B6.Ly5.2 congenic hosts. The resultant hosts of polyclonal memory CD4 T cells were challenged with 10,000 TCID50 of the heterosubtypic strain HK-X31 and then administered control IgG or anti-IFN-γ (XMG1.2) antibody at day 0 and every other day throughout infection. (A) Weight losses of differentially treated mouse memory recipients were plotted as percentages of starting weights. (B) Viral titers were determined by MDCK assay at 7 days postinfection and are shown as averages for five mice per group, with the P value of <0.001 representing a difference in titers between memory mice treated with either control antibody or anti-IFN-γ antibody. The results are representative of three independent experiments. (C) Lung histology was determined by hematoxylin and eosin staining at 7 days postinfection. An image for a representative animal is shown for each group (five mice per group) and for a lung from an uninfected animal.
DISCUSSION
Protecting the population from the next major influenza pandemic remains a major public health challenge that cannot be met by current vaccines, which generate strain-specific protective immunity. Memory CD4 T cells arise following vaccination and infection, exhibit long-term persistence, recognize a broad array of influenza virus epitopes, and have the potential to coordinate multiple aspects of adaptive immunity through enhanced helper and effector functions. However, the intrinsic protective capacity of memory CD4 T cells is not well defined. We demonstrate here that memory CD4 T cells can direct protective responses to influenza virus infection, as manifested by early lung viral clearance, enhanced recovery, and protection from lethal challenge. Memory CD4 T-cell-mediated antiviral responses were independent of helper functions, as B-cell-deficient, CD8 T-cell-depleted, and even RAG2−/− mice displayed enhanced virus clearance in the presence of memory CD4 T cells. Memory CD4 T-cell-mediated enhanced viral clearance was dependent on IFN-γ secretion and was associated with increased T-cell infiltration in the lung. These results demonstrate that memory CD4 T cells can drive protective immunity to a respiratory virus infection through an IFN-γ-dependent effector mechanism which targets immune infiltrates to the lung. Our results reveal the potential of memory CD4 T cells to mediate efficacious protection from respiratory virus infection via vaccines and also to mediate the clinical response to infection.
Viral clearance after influenza virus infection and protection from subsequent infection are generally associated with the production of high-affinity antibodies by activated B cells, which both neutralize infectivity of the virus and invoke effector mechanisms for complete viral clearance (for a review, see reference 24). In addition, the cytolytic function of effector CD8 T cells contributes significantly to protection in both primary and secondary influenza virus challenges (13, 20, 58). CD4 T cells promote viral clearance in primary responses to influenza virus predominantly through their indirect helper functions for the production of high-affinity influenza virus-specific antibodies (39-41). In contrast to naive CD4 T cells, which prevail in primary responses, memory CD4 T cells exhibit rapid effector responses, enhanced migration to nonlymphoid tissue, and an increased ability to be activated by different APC, independent of certain costimulatory pathways (55, 60), suggesting that memory CD4 T cells could mediate protection in antiviral secondary responses by distinct mechanisms. We demonstrate here that influenza virus-specific memory CD4 T cells mediate rapid viral clearance in the lung that does not require B-cell-mediated antibody production or the presence of CD8 T cells.
The B-cell independence of the memory CD4 T-cell-mediated antiviral responses identified here can be distinguished from the results of previous studies showing that secondary or effector CD4 T-cell-mediated protection from influenza virus requires B cells (8, 45, 57). Studies examining secondary responses directly in B-cell-deficient mice found impaired protection (45, 57); however, B-cell-deficient mice were also shown to exhibit impaired generation of memory CD4 T cells (31, 62), so these studies did not examine a wild-type memory CD4 T-cell response in the context of B-cell deficiency. Furthermore, a study by Brown et al. (8) demonstrated impaired protection by transfer of activated effector CD4 T cells into B-cell-deficient hosts. Effector CD4 T cells differ from the resting memory CD4 T cells examined here in their survival, propensity for activation-induced cell death, and expansion capacity (34). In response to influenza virus challenge, transferred effector CD4 T cells were detected only briefly in vivo and were essentially absent from the lung at 6 days postinfection (8). In contrast, in vivo-generated memory CD4 T cells in our system expanded upon secondary virus challenge, and significant numbers were found in the lung 6 days after infection in both wild-type and B-cell-deficient hosts (Fig. 1 and 3). The different B-cell requirements in the two systems are therefore likely due to the continued and robust presence of CD4 T cells in the lung during memory CD4 T-cell-mediated secondary responses versus their transient presence in effector CD4 T-cell transfers.
We also found that memory CD4 T cells could provide protection under conditions of antibody-induced or congenital CD8 T-cell depletion, indicating that protection by memory CD4 T cells was not driven by CD8 T-cell effector responses. In addition, we did not find that the presence of memory CD4 T cells resulted in enhanced CD8 T-cell recruitment to the lung (56; data not shown), indicating that memory CD4 T cells do not promote CD8 T-cell priming and effector differentiation.
We showed that memory CD4 T-cell-mediated viral clearance is abrogated in the presence of IFN-γ neutralization, whereas primary responses are not similarly dependent on secreted IFN-γ. Previous studies found unimpaired primary responses to influenza virus infection in IFN-γ-deficient mice (27), consistent with our findings. In contrast, heterosubtypic immunity in mouse models required IFN-γ production (7), but this protection was attributed mainly to CD8 T cells. Our findings that memory CD4 T-cell-directed responses require IFN-γ are consistent with recent findings that influenza virus protection is associated with transfer of Th1 effector or resting effector cells bearing phenotypic properties of memory T cells (53). Moreover, elevated numbers of memory T cells capable of rapid IFN-γ production were correlated with protection from influenza virus infection in both mice and humans (15, 21). In other viral systems, IFN-γ production during memory CD4 T-cell-mediated secondary responses was also found to direct the outcome of viral disease. In lethal coronavirus infection, IFN-γ was required for memory CD4 T-cell-mediated protection (49), similar to our findings, whereas in respiratory syncytial virus (RSV) infection, IFN-γ production during secondary responses was shown to be dispensable for viral clearance but to exacerbate systemic disease (10).
Our findings further suggest that memory CD4 T cells coordinate enhanced CD4 T-cell responses and recruitment in the lung and set up foci of immune responses around the airways. The ability of CD4 T cells to promote T-cell responses in the lung has been observed in mouse models of severe acute respiratory syndrome (SARS) (12) and may represent an additional mechanism by which CD4 T cells function in antiviral immunity. We propose that IFN-γ produced by memory CD4 T cells and/or additional recruited effector CD4 T cells in the lung can mediate viral clearance by several different mechanisms, including direct effects on the infected cells by stimulating the production of antiviral molecules (51) and activation of macrophages or other innate immune cells for subsequent pathogen destruction (37). In addition, such early lung responses mediated by memory CD4 T cells can lead to immunopathology, as manifested by enhanced T-cell recruitment and also a consistent pattern of early weight loss in memory compared to naive mice. Understanding the factors which balance out the memory immune response would be essential for optimizing these aspects of secondary antiviral T-cell responses.
The ability of memory CD4 T cells to mobilize lung immune responses and effector functions has important implications for optimizing human immune responses to influenza virus. There is increasing evidence that influenza virus-specific memory CD4 T cells can be generated by natural infection and/or vaccination, can persist in healthy individuals, and can cross-react with determinants from pandemic strains (28, 30, 36, 48). These findings suggest that memory CD4 T cells may constitute a first-line defense against infection with new influenza virus strains and subtypes and may likewise provide protection against severe morbidity and mortality. Taken together with our findings that memory CD4 T cells coordinate protective influenza immunity, targeting the generation of IFN-γ-producing memory CD4 T cells specific for invariant influenza virus determinants could be a viable strategy for promoting cross-strain protective immunity to the next pandemic.
Acknowledgments
We thank Daniel Popkin for a critical review of the manuscript.
This work was supported by Public Health Service grant U19 AI083022 from the National Institute of Allergy and Infectious Diseases. D.V. was supported by an NIH LRP fellowship.
Footnotes
Published ahead of print on 30 June 2010.
REFERENCES
- 1.Ahmadzadeh, M., and D. L. Farber. 2002. Functional plasticity of an antigen-specific memory CD4 T cell population. Proc. Natl. Acad. Sci. U. S. A. 99:11802-11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan, W., Z. Tabi, A. Cleary, and P. C. Doherty. 1990. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J. Immunol. 144:3980-3986. [PubMed] [Google Scholar]
- 3.Assarsson, E., H. H. Bui, J. Sidney, Q. Zhang, J. Glenn, C. Oseroff, I. N. Mbawuike, J. Alexander, M. J. Newman, H. Grey, and A. Sette. 2008. Immunomic analysis of the repertoire of T-cell specificities for influenza A virus in humans. J. Virol. 82:12241-12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belz, G. T., D. Wodarz, G. Diaz, M. A. Nowak, and P. C. Doherty. 2002. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J. Virol. 76:12388-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender, B. S., T. Croghan, L. Zhang, and P. A. Small, Jr. 1992. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J. Exp. Med. 175:1143-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bingaman, A. W., D. S. Patke, V. R. Mane, M. Ahmadzadeh, M. Ndejembi, S. T. Bartlett, and D. L. Farber. 2005. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur. J. Immunol. 35:3173-3186. [DOI] [PubMed] [Google Scholar]
- 7.Bot, A., S. Bot, and C. A. Bona. 1998. Protective role of gamma interferon during the recall response to influenza virus. J. Virol. 72:6637-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, D. M., A. M. Dilzer, D. L. Meents, and S. L. Swain. 2006. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J. Immunol. 177:2888-2898. [DOI] [PubMed] [Google Scholar]
- 9.Bui, H. H., B. Peters, E. Assarsson, I. Mbawuike, and A. Sette. 2007. Ab and T cell epitopes of influenza A virus, knowledge and opportunities. Proc. Natl. Acad. Sci. U. S. A. 104:246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castilow, E. M., M. R. Olson, D. K. Meyerholz, and S. M. Varga. 2008. Differential role of gamma interferon in inhibiting pulmonary eosinophilia and exacerbating systemic disease in fusion protein-immunized mice undergoing challenge infection with respiratory syncytial virus. J. Virol. 82:2196-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandran, S. S., D. Verhoeven, J. R. Teijaro, M. J. Fenton, and D. L. Farber. 2009. TLR2 engagement on dendritic cells promotes high frequency effector and memory CD4 T cell responses. J. Immunol. 183:7832-7841. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J., Y. F. Lau, E. W. Lamirande, C. D. Paddock, J. H. Bartlett, S. R. Zaki, and K. Subbarao. 2010. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 84:1289-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen, J. P., P. C. Doherty, K. C. Branum, and J. M. Riberdy. 2000. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J. Virol. 74:11690-11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowe, S. R., S. C. Miller, D. M. Brown, P. S. Adams, R. W. Dutton, A. G. Harmsen, F. E. Lund, T. D. Randall, S. L. Swain, and D. L. Woodland. 2006. Uneven distribution of MHC class II epitopes within the influenza virus. Vaccine 24:457-467. [DOI] [PubMed] [Google Scholar]
- 15.Deliyannis, G., K. Kedzierska, Y. F. Lau, W. Zeng, S. J. Turner, D. C. Jackson, and L. E. Brown. 2006. Intranasal lipopeptide primes lung-resident memory CD8+ T cells for long-term pulmonary protection against influenza. Eur. J. Immunol. 36:770-778. [DOI] [PubMed] [Google Scholar]
- 16.Di Fabio, S., I. N. Mbawuike, H. Kiyono, K. Fujihashi, R. B. Couch, and J. R. McGhee. 1994. Quantitation of human influenza virus-specific cytotoxic T lymphocytes: correlation of cytotoxicity and increased numbers of IFN-gamma producing CD8+ T cells. Int. Immunol. 6:11-19. [DOI] [PubMed] [Google Scholar]
- 17.Doherty, P. C., D. J. Topham, R. A. Tripp, R. D. Cardin, J. W. Brooks, and P. G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105-117. [DOI] [PubMed] [Google Scholar]
- 18.Doherty, P. C., S. J. Turner, R. G. Webby, and P. G. Thomas. 2006. Influenza and the challenge for immunology. Nat. Immunol. 7:449-455. [DOI] [PubMed] [Google Scholar]
- 19.Eichelberger, M. C., M. L. Wang, W. Allan, R. G. Webster, and P. C. Doherty. 1991. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J. Gen. Virol. 72:1695-1698. [DOI] [PubMed] [Google Scholar]
- 20.Epstein, S. L., C. Y. Lo, J. A. Misplon, and J. R. Bennink. 1998. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J. Immunol. 160:322-327. [PubMed] [Google Scholar]
- 21.Forrest, B. D., M. W. Pride, A. J. Dunning, M. R. Capeding, T. Chotpitayasunondh, J. S. Tam, R. Rappaport, J. H. Eldridge, and W. C. Gruber. 2008. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin. Vaccine Immunol. 15:1042-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galli, G., D. Medini, E. Borgogni, L. Zedda, M. Bardelli, C. Malzone, S. Nuti, S. Tavarini, C. Sammicheli, A. K. Hilbert, V. Brauer, A. Banzhoff, R. Rappuoli, G. Del Giudice, and F. Castellino. 2009. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc. Natl. Acad. Sci. U. S. A. 106:3877-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge, X., V. Tan, P. L. Bollyky, N. E. Standifer, E. A. James, and W. W. Kwok. 2010. Assessment of seasonal influenza A virus-specific CD4 T-cell responses to 2009 pandemic H1N1 swine-origin influenza A virus. J. Virol. 84:3312-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerhard, W. 2001. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 260:171-190. [DOI] [PubMed] [Google Scholar]
- 25.Gerhard, W., K. Mozdzanowska, M. Furchner, G. Washko, and K. Maiese. 1997. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 159:95-103. [DOI] [PubMed] [Google Scholar]
- 26.Graham, M. B., and T. J. Braciale. 1997. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J. Exp. Med. 186:2063-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham, M. B., D. K. Dalton, D. Giltinan, V. L. Braciale, T. A. Stewart, and T. J. Braciale. 1993. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J. Exp. Med. 178:1725-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenbaum, J. A., M. F. Kotturi, Y. Kim, C. Oseroff, K. Vaughan, N. Salimi, R. Vita, J. Ponomarenko, R. H. Scheuermann, A. Sette, and B. Peters. 2009. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. U. S. A. 106:20365-20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammarlund, E., M. W. Lewis, S. G. Hansen, L. I. Strelow, J. A. Nelson, G. J. Sexton, J. M. Hanifin, and M. K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131-1137. [DOI] [PubMed] [Google Scholar]
- 30.He, X. S., T. H. Holmes, C. Zhang, K. Mahmood, G. W. Kemble, D. B. Lewis, C. L. Dekker, H. B. Greenberg, and A. M. Arvin. 2006. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J. Virol. 80:11756-11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homann, D., A. Tishon, D. P. Berger, W. O. Weigle, M. G. von Herrath, and M. B. Oldstone. 1998. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J. Virol. 72:9208-9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou, S., X. Y. Mo, L. Hyland, and P. C. Doherty. 1995. Host response to Sendai virus in mice lacking class II major histocompatibility complex glycoproteins. J. Virol. 69:1429-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itoh, Y., K. Shinya, M. Kiso, T. Watanabe, Y. Sakoda, M. Hatta, Y. Muramoto, D. Tamura, Y. Sakai-Tagawa, T. Noda, S. Sakabe, M. Imai, Y. Hatta, S. Watanabe, C. Li, S. Yamada, K. Fujii, S. Murakami, H. Imai, S. Kakugawa, M. Ito, R. Takano, K. Iwatsuki-Horimoto, M. Shimojima, T. Horimoto, H. Goto, K. Takahashi, A. Makino, H. Ishigaki, M. Nakayama, M. Okamatsu, D. Warshauer, P. A. Shult, R. Saito, H. Suzuki, Y. Furuta, M. Yamashita, K. Mitamura, K. Nakano, M. Nakamura, R. Brockman-Schneider, H. Mitamura, M. Yamazaki, N. Sugaya, M. Suresh, M. Ozawa, G. Neumann, J. Gern, H. Kida, K. Ogasawara, and Y. Kawaoka. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251-262. [DOI] [PubMed] [Google Scholar]
- 35.Kirberg, J., A. Baron, S. Jakob, A. Rolink, K. Karjalainen, and H. von Boehmer. 1994. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 180:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, L. Y., D. L. Ha, C. Simmons, M. D. de Jong, N. V. Chau, R. Schumacher, Y. C. Peng, A. J. McMichael, J. J. Farrar, G. L. Smith, A. R. Townsend, B. A. Askonas, S. Rowland-Jones, and T. Dong. 2008. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Invest. 118:3478-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacMicking, J. D. 2009. Recognizing macrophage activation and host defense. Cell Host Microbe 5:405-407. [DOI] [PubMed] [Google Scholar]
- 38.Moulton, V. R., N. D. Bushar, D. B. Leeser, D. S. Patke, and D. L. Farber. 2006. Divergent generation of heterogeneous memory CD4 T cells. J. Immunol. 177:869-876. [DOI] [PubMed] [Google Scholar]
- 39.Mozdzanowska, K., M. Furchner, K. Maiese, and W. Gerhard. 1997. CD4+ T cells are ineffective in clearing a pulmonary infection with influenza type A virus in the absence of B cells. Virology 239:217-225. [DOI] [PubMed] [Google Scholar]
- 40.Mozdzanowska, K., M. Furchner, D. Zharikova, J. Feng, and W. Gerhard. 2005. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J. Virol. 79:5943-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mozdzanowska, K., K. Maiese, and W. Gerhard. 2000. Th cell-deficient mice control influenza virus infection more effectively than Th- and B cell-deficient mice: evidence for a Th-independent contribution by B cells to virus clearance. J. Immunol. 164:2635-2643. [DOI] [PubMed] [Google Scholar]
- 42.Ndejembi, M. P., J. R. Teijaro, D. S. Patke, A. W. Bingaman, M. R. Chandok, A. Azimzadeh, S. G. Nadler, and D. L. Farber. 2006. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J. Immunol. 177:7698-7706. [DOI] [PubMed] [Google Scholar]
- 43.Patke, D. S., M. Ahmadzadeh, A. W. Bingaman, and D. L. Farber. 2005. Anti-CD3 priming generates heterogeneous antigen-specific memory CD4 T cells. Clin. Immunol. 117:125-132. [DOI] [PubMed] [Google Scholar]
- 44.Patke, D. S., and D. L. Farber. 2005. Modulation of memory CD4 T cell function and survival potential by altering the strength of the recall stimulus. J. Immunol. 174:5433-5443. [DOI] [PubMed] [Google Scholar]
- 45.Rangel-Moreno, J., D. M. Carragher, R. S. Misra, K. Kusser, L. Hartson, A. Moquin, F. E. Lund, and T. D. Randall. 2008. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J. Immunol. 180:454-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards, K. A., F. A. Chaves, F. R. Krafcik, D. J. Topham, C. A. Lazarski, and A. J. Sant. 2007. Direct ex vivo analyses of HLA-DR1 transgenic mice reveal an exceptionally broad pattern of immunodominance in the primary HLA-DR1-restricted CD4 T-cell response to influenza virus hemagglutinin. J. Virol. 81:7608-7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards, K. A., F. A. Chaves, and A. J. Sant. 2009. Infection of HLA-DR1 transgenic mice with a human isolate of influenza A virus (H1N1) primes a diverse CD4 T-cell repertoire that includes CD4 T cells with heterosubtypic cross-reactivity to avian (H5N1) influenza virus. J. Virol. 83:6566-6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roti, M., J. Yang, D. Berger, L. Huston, E. A. James, and W. W. Kwok. 2008. Healthy human subjects have CD4+ T cells directed against H5N1 influenza virus. J. Immunol. 180:1758-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savarin, C., C. C. Bergmann, D. R. Hinton, R. M. Ransohoff, and S. A. Stohlman. 2008. Memory CD4+ T-cell-mediated protection from lethal coronavirus encephalomyelitis. J. Virol. 82:12432-12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scherle, P. A., G. Palladino, and W. Gerhard. 1992. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 148:212-217. [PubMed] [Google Scholar]
- 51.Schroder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 52.Song, H., G. R. Nieto, and D. R. Perez. 2007. A new generation of modified live-attenuated avian influenza viruses using a two-strategy combination as potential vaccine candidates. J. Virol. 81:9238-9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strutt, T. M., K. K. McKinstry, J. P. Dibble, C. Winchell, Y. Kuang, J. D. Curtis, G. Huston, R. W. Dutton, and S. L. Swain. 2010. Memory CD4+ T cells induce innate responses independently of pathogen. Nat. Med. 16:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun, K., and D. W. Metzger. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 14:558-564. [DOI] [PubMed] [Google Scholar]
- 55.Swain, S. L., J. N. Agrewala, D. M. Brown, and E. Roman. 2002. Regulation of memory CD4 T cells: generation, localization and persistence. Adv. Exp. Med. Biol. 512:113-120. [PubMed] [Google Scholar]
- 56.Teijaro, J. R., M. N. Njau, D. Verhoeven, S. Chandran, S. G. Nadler, J. Hasday, and D. L. Farber. 2009. Costimulation modulation uncouples protection from immunopathology in memory T cell responses to influenza virus. J. Immunol. 182:6834-6843. [DOI] [PubMed] [Google Scholar]
- 57.Topham, D. J., and P. C. Doherty. 1998. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J. Virol. 72:882-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topham, D. J., R. A. Tripp, and P. C. Doherty. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197-5200. [PubMed] [Google Scholar]
- 59.Turner, S. J., E. Olivas, A. Gutierrez, G. Diaz, and P. C. Doherty. 2007. Disregulated influenza A virus-specific CD8+ T cell homeostasis in the absence of IFN-gamma signaling. J. Immunol. 178:7616-7622. [DOI] [PubMed] [Google Scholar]
- 60.Verhoeven, D., J. R. Teijaro, and D. L. Farber. 2008. Heterogeneous memory T cells in antiviral immunity and immunopathology. Viral Immunol. 21:99-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verhoeven, D., J. R. Teijaro, and D. L. Farber. 2009. Pulse-oximetry accurately predicts lung pathology and the immune response during influenza infection. Virology 390:151-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitmire, J. K., M. S. Asano, S. M. Kaech, S. Sarkar, L. G. Hannum, M. J. Shlomchik, and R. Ahmed. 2009. Requirement of B cells for generating CD4+ T cell memory. J. Immunol. 182:1868-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitmire, J. K., N. Benning, and J. L. Whitton. 2005. Cutting edge: early IFN-gamma signaling directly enhances primary antiviral CD4+ T cell responses. J. Immunol. 175:5624-5628. [DOI] [PubMed] [Google Scholar]
- 64.Whitmire, J. K., B. Eam, N. Benning, and J. L. Whitton. 2007. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J. Immunol. 179:1190-1197. [DOI] [PubMed] [Google Scholar]
- 65.Williams, M. A., J. T. Tan, A. B. Adams, M. M. Durham, N. Shirasugi, J. K. Whitmire, L. E. Harrington, R. Ahmed, T. C. Pearson, and C. P. Larsen. 2001. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J. Immunol. 167:4987-4995. [DOI] [PubMed] [Google Scholar]