Abstract
Previously, we showed that overexpression of MIP-1α in mouse brain further decreased rabies virus (RABV) pathogenicity (L. Zhao, H. Toriumi, Y. Kuang, H. Chen, and Z. F. Fu, J. Virol., 83:11808-11818, 2009). In the present study, the immunogenicity of recombinant RABV expressing MIP-1α (rHEP-MIP1α) was determined. It was found that intramuscular immunization of BALB/c mice with rHEP-MIP1α resulted in a higher level of expression of MIP-1α at the site of inoculation, increased recruitment of dendritic cells (DCs) and mature B cells into the draining lymph nodes and the peripheral blood, and higher virus-neutralizing antibody titers than immunization with the parent rHEP and recombinant RABVs expressing RANTES (CCL5) or IP-10 (CXCL10). Our data thus demonstrate that expression of MIP-1α not only reduces viral pathogenicity but also enhances immunogenicity by recruiting DCs and B cells to the site of immunization, the lymph nodes, and the blood.
Rabies continues to present public health problems worldwide and causes more than 55,000 human deaths each year, most of which occur in the developing nations of Asia and Africa, where dog rabies remains the main source of human exposure (8, 31). Current rabies vaccines are made with inactivated rabies virus (RABV) grown in cultured cells. Although these vaccines are safe and efficacious, multiple doses (at least 4) must be administered over an extended period of time (14 days) to stimulate optimal immune responses (24). Furthermore, the high cost of cell culture-based vaccines makes it difficult to utilize them effectively in developing countries where they are needed most (28). A live attenuated RABV vaccine (SAG-2) and a recombinant vaccinia virus expressing RABV glycoprotein (VRG) have been licensed particularly for use in the oral immunization of wild animals (10, 29). These vaccines are effective; however, VRG may cause intense skin inflammation and systemic vaccinia infection (3, 25), and SAG-2 induces a low level of virus-neutralizing antibody (VNA) responses in wild animals (11).
Recent studies demonstrated that the activation of innate immune responses is one of the mechanisms by which RABV is attenuated (16, 30). Induced innate-response genes include inflammatory chemokines and cytokines, interferons (IFNs) and IFN-related genes, and Toll-like receptors (14, 22-23). Furthermore, it was found that overexpression of the chemokine MIP-1α (CCL3) in mouse brain further decreased RABV pathogenicity, while overexpression of RANTES (CCL5) or IP-10 (CXCL10) increased RABV pathogenicity (35). In this study, the immunogenicity of the recombinant high egg passage (rHEP) Flury strain of RABV that contains the MIP-1α gene (rHEP-MIP1α) was investigated.
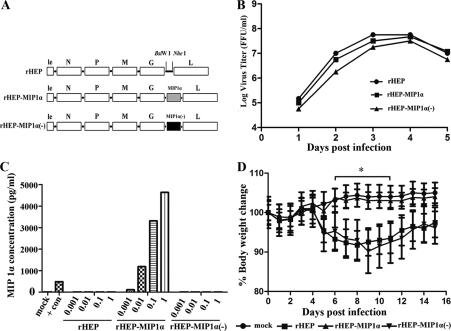
To ensure that the decreased pathogenicity of rHEP-MIP1α, as shown previously (35), is due to the overexpression of MIP-1α, another rHEP virus was constructed with a MIP-1α gene cloned into the rHEP genome that does not express MIP-1α protein because two stop codons were introduced near the N terminus of the MIP-1α gene, one stop codon (TAG) replacing TCA (residues 68 to 70) and the other replacing the codon ATG (residues 78 to 80). The recombinant RABV was rescued, using the procedures described by Inoue et al. (13), and was designated rHEP-MIP-1α(−) (Fig. 1A). Characterization of rHEP-MIP1α(−) in vitro revealed that its growth was similar to that of the parent rHEP virus and rHEP-MIP1α (Fig. 1B), and it failed to produce MIP-1α in infected cells (Fig. 1C). The pathogenicity of rHEP-MIP1α(−) in mice was determined by intracranial (i.c.) inoculation. Neither obvious clinical signs nor weight loss was observed in sham-infected mice or mice infected with rHEP-MIP1α (Fig. 1D). Since rHEP-Flury is one of the most attenuated RABVs (13), only one mouse in each group infected with rHEP or rHEP-MIP-1α(−) developed mild clinical signs, including rough fur and slow movement, at days 5 to 8 p.i., and they recovered very quickly. Compared with sham- or rHEP-MIP1α-infected mice, rHEP- or rHEP-MIP-1α(−)-infected mice lost about 7% of their body weight (P ≤ 0.05) between day 6 and 11 postinfection (p.i.). Together, these data indicate that the decreased pathogenicity of recombinant rHEP-MIP1α is indeed due to the expression of MIP-1α.
FIG. 1.
Construction and characterization of recombinant RABV with the MIP-1α gene cloned but without the protein expressed. (A) rHEP-MIP1α(−) was constructed by introducing two stop codons near the N terminus of the MIP-1α gene as described previously. le, leader region; N, nucleoprotein; P, phosphoprotein; M, matrix protein; G, glycoprotein; L, RNA-dependent RNA polymerase. (B) Virus growth curves were determined by infecting mouse neuroblastoma (NA) cells with either the rHEP, rHEP-MIP1α, or rHEP-MIP1α(−) virus at a multiplicity of infection (MOI) of 0.01. At days 1, 2, 3, 4, and 5 after infection, the culture supernatants were harvested, and viral titers in NA cells were determined with fluorescein isothiocyanate (FITC)-conjugated antirabies antibodies (FujiRab, Melvin, PA). Antigen-positive foci were counted under a fluorescence microscope (Zeiss, Germany), and viral titers were calculated as FFU per milliliter. All titrations were carried out in quadruplicate. (C) Expression of MIP-1α was determined by infecting NA cells with either the rHEP, rHEP-MIP1α, or rHEP-MIP1α(−) virus at an MOI of 0.001, 0.01, 0.1, or 1. After a 24-h incubation at 34°C, the culture supernatants were harvested, and the amounts of MIP-1α were determined by a murine MIP-1α enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) according to the manufacture's protocol. The positive control (MIP-1α) was supplied with the ELISA kit. Checkered bar, rHEP; horizontally striped bar, rHEP-MIP1α; vertically striped bar, rHEP-MIP1α(−). (D) The pathogenicity of recombinant rHEP-MIP1α(−) was determined by inoculating BALB/c mice (6 to 8 weeks of age) i.c. with 105 FFU of either the rHEP, rHEP-MIP1α, or rHEP-MIP1α(−) virus or with medium alone (mock infection). Body weight was monitored daily. Data were obtained from 10 mice in each group and presented as mean values ± standard errors (SEs). The asterisk indicates a significant difference (P < 0.05) in results among the indicated experimental groups, as calculated by one-way analysis of variance (ANOVA) with the Holm-Sidak method.
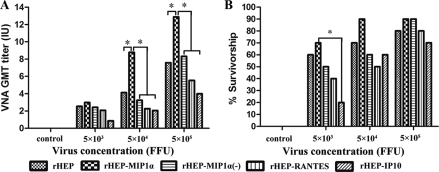
To determine the immunogenicity of recombinant RABV expressing chemokines, mice were immunized intramuscularly (i.m.) once with different doses of either rHEP or one of the recombinant RABVs [rHEP-MIP1α, rHEP-MIP1α(−), rHEP-RANTES, or rHEP-IP10]. Blood samples were collected 21 days p.i. for the determination of VNAs using the rapid fluorescent focus inhibition test (RFFIT) (27). Overall, the production of VNA was dose dependent for all the viruses (Fig. 2A). The VNA titers were not significantly different in mice infected with the different viruses at a low dose (5 × 103 focus-forming units [FFU]/mouse). Significantly higher VNA titers (P ≤ 0.05) were induced by RABV expressing MIP-1α (rHEP-MIP1α) than by the parent virus (rHEP) and other recombinant viruses [rHEP-MIP1α(−), rHEP-RANTES, or rHEP-IP10] when high doses (5 × 104 and 5 × 105 FFU/mouse) were used to immunize mice. Immunized mice were then challenged i.c. with 50% lethal doses (LD50) of challenge virus standard CVS-24 on day 21 after vaccination and observed for 2 weeks for the development of disease and death. As depicted in Fig. 2B, more mice survived challenge when immunized with rHEP-MIP1α than with the other viruses, particularly at lower doses, although no significant differences were observed among these groups except between the mice immunized with 5 × 103 FFU of rHEP-MIP1α and those immunized with rHEP-IP10. This could be due to multiple factors; for example, immune mechanisms other than VNA may be involved in protection (12). Nevertheless, these results indicate that RABV rHEP-MIP1α induces a higher level of adaptive immunity, presumably supported by a strong innate immune response, than the parent virus and viruses expressing other chemokines. Mice immunized with the recombinant rHEP-MIP1α(−) virus produced levels of antibodies similar to those immunized with the parent (rHEP) virus at different doses but significantly lower levels of antibodies than mice immunized with rHEP-MIP1α (Fig. 2B). Likewise, fewer mice immunized with rHEP-MIP1α(−) were protected after challenge with CVS-24 than were those immunized with rHEP-MIP1α. These data indicate that the increased immunogenicity of recombinant rHEP-MIP1α is indeed due to the expression of MIP-1α.
FIG. 2.
Immunogenicity of recombinant RABVs expressing chemokines. Groups of 10 ICR mice were immunized by the i.m. route with serial 10-fold dilutions of rHEP, rHEP-MIP1α, rHEP-MIP1α(−), rHEP-RANTES, or rHEP-IP10. (A) At day 20 after immunization, blood was obtained, and the sera were used to determine VNA titers, using the RFFIT as described previously (27). Titers were normalized to IU, using the WHO standard. GMT, geometric mean titer. (B) Mice were then challenged i.c. with 50 LD50 of CVS-24 and observed daily for 2 weeks. The numbers of survivors were recorded and compared. Data were analyzed with SigmaStat software (Systat Software, Inc., San Jose, CA). (A and B) Asterisks indicate significant differences (P < 0.05) in results among the experimental groups, as calculated by one-way ANOVA with the Holm-Sidak method.
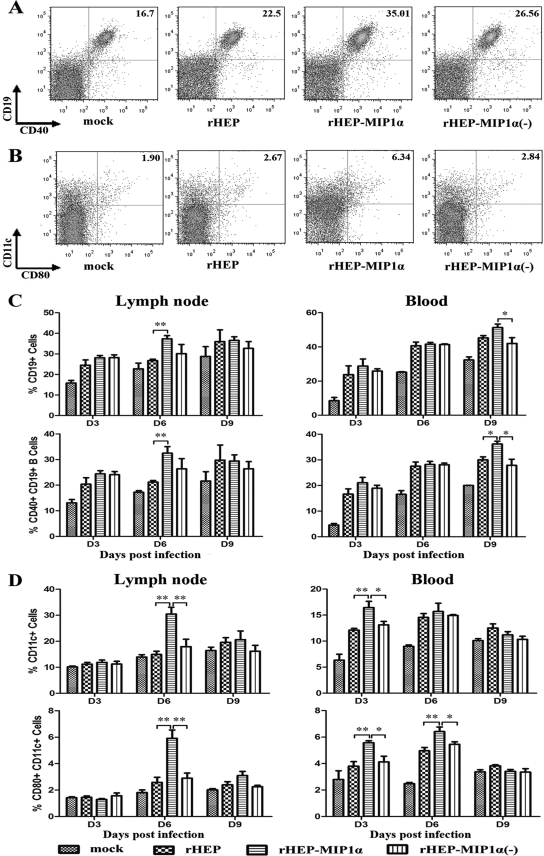
To determine if the increased production of VNA in mice immunized with rHEP-MIP1α correlates with the recruitment of B cells, CD19+/CD40+ B cells were analyzed by flow cytometry in the draining (inguinal) lymph nodes and peripheral blood. As shown in Fig. 3A and C, the numbers of CD19+ and CD19+/CD40+ cells increased as a function of time after immunization. Significantly more CD19+ and CD19+/CD40+ cells were found in the lymph nodes of mice infected with rHEP-MIP1α than in those of mice infected with the parent rHEP virus or rHEP-MIP1α(−) at day 6 p.i. Significantly more CD19+ and CD19+/CD40+ cells were found in the peripheral blood of mice infected with rHEP-MIP1α than in the peripheral blood of mice infected with the parent rHEP virus or rHEP-MIP1α(−) at day 9 p.i. The geometric mean fluorescences of CD40+ cells from the different groups were compared, but no significant difference could be found (data not shown). All these data indicate that expression of MIP-1α results in recruitment of B cells in the draining lymph nodes and the peripheral blood.
FIG. 3.
Effects of MIP-1α expression on the recruitment of DCs and B cells in the draining lymph nodes and the peripheral blood. Female BALB/c mice of 6 to 8 weeks of age were inoculated i.m. with 105 FFU of one of the recombinant RABVs [rHEP, rHEP-MIP-1α, or rHEP-MIP-1α(-)] or with medium alone (sham infection). At days 3, 6, and 9 p.i., single-cell suspensions were prepared from the draining (inguinal) lymph nodes or the peripheral blood and stained with antibodies to B cell (CD19 and CD40) or DC (CD11c and CD80) markers. Data collection and analysis were performed with a BD LSR II flow cytometer and BD FACSDiva software (BD Pharmingen). (A and B) Representative flow cytometric plots of the infiltration of mature B cells (CD19+/CD40+) (A) or DCs (CD11c+) or activated DCs (CD11c+/CD80+) (B) into the inguinal lymph nodes at day 6 p.i. are shown. (C and D) The percentages of B cells (C) or DCs (D) in the inguinal lymph nodes and peripheral blood at different time points were quantified from the results for 4 mice in each group and presented as mean values ± standard errors. Asterisks (*) indicate significant differences (*, P < 0.05; **, P < 0.01) in results between the indicated experimental groups, as calculated by one-way ANOVA with the Holm-Sidak method.
To determine if the recruitment of B cells is due to the recruitment of DCs, antibodies to CD11c and CD80 were used to determine the number of DCs in the inguinal lymph nodes and peripheral blood. As shown in Fig. 3B and D, there were significantly more CD11c+ and CD11c+/CD80+ cells in the draining lymph nodes of mice infected with rHEP-MIP1α than in those of mice infected with any other recombinant RABV at day 6 p.i. In the peripheral blood, there were significantly more CD11c+ cells (at day 3 p.i.) and CD11c+/CD80+ cells (at days 3 and 6 p.i.) in samples from mice infected with rHEP-MIP1α than in those from mice infected with the parent rHEP virus or rHEP-MIP1α(−). A similar trend was observed when another DC activation marker, CD86, was used for the flow cytometric studies. However, there was no significant difference in the geometric mean fluorescences of CD80+ or CD86+ cells from the different groups (data not shown). All these data indicate that expression of MIP-1α results in the recruitment of DCs in the draining lymph nodes and the peripheral blood.
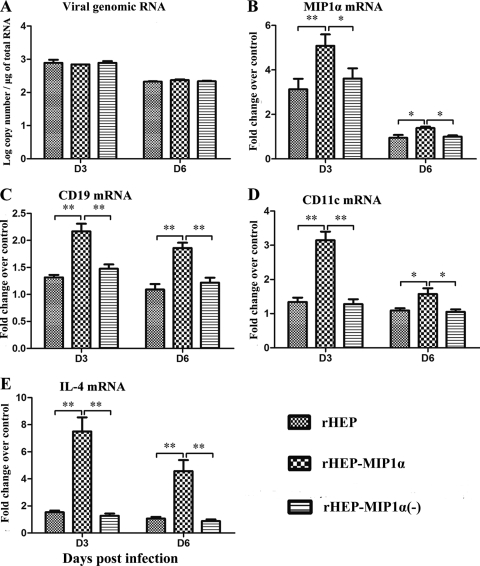
To determine if the enhancement of innate and adaptive immune responses in rHEP-MIP-1α-infected mice is due to virus replication, expression of chemokines, and/or recruitment of immune cells at the local sites, muscle tissue at the inoculation site was harvested at days 3 or 6 p.i., and total RNA was extracted. Quantitative reverse transcription-PCR (QRT-PCR) was performed to determine the levels of viral RNA replication and expression of MIP-1α, CD19, CD11c, and IL-4. As shown in Fig. 4A, there was no difference in virus replication in mice infected with the different viruses at either day 3 or day 6 p.i., indicating that the induction of innate and adaptive immune responses by rHEP-MIP1α is not due to the rate of virus replication at the local site. Increased levels of MIP-1α mRNA were detected in mice infected with the different RABVs at day 3 p.i., but levels were stable by day 6 p.i. However, significantly more MIP-1α, CD19, CD11c, and IL-4 (a major cytokine produced by Th2 cells to enhance the growth and differentiation of activated B cells [21]) mRNA was detected in the muscle tissues of mice infected with rHEP-MIP1α than in those of mice infected with the parent virus rHEP or rHEP-MIP1α(−) at days 3 and 6 p.i. (Fig. 4B to E). All of these data suggest that MIP-1α expression at the local site may be responsible for recruitment of the DCs and/or B cells observed at the site of immunization as well as in the lymph nodes and the peripheral blood.
FIG. 4.
Virus replication and chemokine expression at the inoculation site. BALB/c mice (6 to 8 weeks of age) were inoculated i.m. in the hind leg with 105 FFU of one of the recombinant viruses [rHEP, rHEP-MIP-1α, or rHEP-MIP-1α(−)] or with medium alone. The hind leg muscles of 4 mice from each group were removed at days 3 and 6 p.i. Total RNA was extracted from the muscle tissue, and viral genomic RNA (A), MIP-1α mRNA (B), CD19 mRNA (C), CD11c mRNA (D), or IL-4 mRNA (E) was analyzed by QRT-PCR. For absolute quantities of viral genomic RNA, a standard curve was generated from a serially diluted, in vitro-transcribed RNA, using a plasmid expressing RABV N, and the copy numbers of viral genomic RNA were normalized to that of 1 μg of total RNA. For MIP-1α, CD19, CD11c, and IL-4 expression, mRNA copy numbers were normalized to that of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Levels of gene expression in a test sample are presented as the fold increase over that detected in sham-infected controls. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) between the indicated experimental groups, as calculated by one-way ANOVA with the Holm-Sidak method.
Activation of the innate immune responses, particularly chemokines and IFNs, has been reported as one of the mechanisms by which RABV is attenuated (16, 30). To further investigate the role of chemokines in RABV attenuation, we compared rHEP viruses with MIP-1α, RANTES, and IP-10 individually cloned into the rHEP genome (35). Pathogenicity studies with these viruses indicated that overexpression of MIP-1α alone in mouse brain further attenuated the virus by inducing a transient innate immune response and mild inflammation, whereas overexpression of RANTES or IP-10 increased viral pathogenicity by inducing prolonged innate immune responses and extensive inflammation (35). In the present study, the immunogenicities of these recombinant viruses were compared in BALB/c mice. Our studies indicate that the rHEP expressing MIP-1α induced a stronger innate immune response at the local site of inoculation, resulting in recruitment of DCs and B cells in the draining lymph nodes and the peripheral blood, which led to the production of higher levels of VNA than were induced by the parent rHEP virus and the recombinant viruses expressing RANTES or IP-10.
MIP-1α is one of the major chemoattractants for monocytes, especially immature DCs and macrophages (1, 19-20). Recently, one in vitro study showed that infection with both attenuated and pathogenic RABV strains potently induced maturation of DCs (18). In our present study, more CD11c+ cells (DC markers) (9, 17) and CD11c+/CD80+ cells (markers for DC activation) (34) were detected in the draining lymph nodes and peripheral blood of mice infected with rHEP-MIP1α than in those of mice infected with other viruses. DCs are the most potent antigen-presenting cells (APCs) (4). They process antigen, migrate to the T cell zone, and stimulate antigen-specific naïve T cell activation. Activated T cells stimulate the proliferation and differentiation of antigen-specific naïve B cells into antibody-producing plasma cells (7). Interleukin-4 (IL-4) mRNA was shown to be significantly increased in the muscle tissues of mice infected with MIP-1α compared with that in the muscle tissues of mice infected with the other rHEP viruses, which may indicate activation of Th2 cells as well. DCs can also directly regulate B cell maturation and play an important role in B cell function (4). DCs can capture and retain unprocessed antigen and directly transfer this antigen to naïve B cells to initiate antigen-specific antibody responses (33). DCs can also induce proliferation of B cells independently of CD40, but proliferation is stronger in the presence of CD40 (32). Dubois et al. (7) reported that small numbers (250 to 1,000) of DCs can directly stimulate the proliferation and antibody production of activated B cells. In the present study, it was not surprising to observe that more CD19+ cells (B cell markers) (15) and CD19+/CD40+ cells (expressed on all mature B lymphocytes; they play a crucial role in B cell activation) (2) were detected in the inoculation sites, draining lymph nodes, and peripheral blood of mice infected with rHEP-MIP1α than in those of mice infected with the other viruses. The recruitment of B cells observed in this study could also be due to a direct function of MIP-1α, since it has been reported to attract B cells (5, 26). Recruitment of B cells could explain why the highest levels of VNA (the primary immune effector for RABV clearance [6]) were detected in mice immunized with rHEP-MIP1α.
In summary, our studies demonstrate that overexpression of MIP-1α results not only in reduced RABV pathogenicity (attenuation) by inducing transient innate immunity but also in enhanced RABV immunogenicity by recruiting DCs and B cells in the periphery. The recombinant RABV expressing MIP-1α thus has the potential to be developed as an avirulent RABV vaccine.
Acknowledgments
This work was partially supported by Public Health Service grant AI-051560 from the National Institute of Allergy and Infectious Diseases.
The authors thank Robert J. Hogan for helpful discussions and Guoqing Zhang and Yongjun Wen for technical help.
Footnotes
Published ahead of print on 30 June 2010.
REFERENCES
- 1.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castigli, E., F. Young, A. M. Carossino, F. W. Alt, and R. S. Geha. 1996. CD40 expression and function in murine B cell ontogeny. Int. Immunol. 8:405-411. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2009. Human vaccinia infection after contact with a raccoon rabies vaccine bait—Pennsylvania, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:1204-1207. [PubMed] [Google Scholar]
- 4.Clark, E. A. 1997. Regulation of B lymphocytes by dendritic cells. J. Exp. Med. 185:801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcione, A., G. Tortolina, R. Bonecchi, N. Battilana, G. Taborelli, F. Malavasi, S. Sozzani, L. Ottonello, F. Dallegri, and V. Pistoia. 2002. Chemotaxis of human tonsil B lymphocytes to CC chemokine receptor (CCR) 1, CCR2 and CCR4 ligands is restricted to non-germinal center cells. Int. Immunol. 14:883-892. [DOI] [PubMed] [Google Scholar]
- 6.Dietzschold, B., M. Kao, Y. M. Zheng, Z. Y. Chen, G. Maul, Z. F. Fu, C. E. Rupprecht, and H. Koprowski. 1992. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc. Natl. Acad. Sci. U. S. A. 89:7252-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois, B., J. M. Bridon, J. Fayette, C. Barthelemy, J. Banchereau, C. Caux, and F. Briere. 1999. Dendritic cells directly modulate B cell growth and differentiation. J. Leukoc. Biol. 66:224-230. [DOI] [PubMed] [Google Scholar]
- 8.Fu, Z. F. 1997. Rabies and rabies research: past, present and future. Vaccine 15(Suppl.):S20-S24. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann, F., S. Jung, and D. R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71-82. [DOI] [PubMed] [Google Scholar]
- 10.Hanlon, C. A., M. Niezgoda, A. N. Hamir, C. Schumacher, H. Koprowski, and C. E. Rupprecht. 1998. First North American field release of a vaccinia-rabies glycoprotein recombinant virus. J. Wildl. Dis. 34:228-239. [DOI] [PubMed] [Google Scholar]
- 11.Hanlon, C. A., M. Niezgoda, P. Morrill, and C. E. Rupprecht. 2002. Oral efficacy of an attenuated rabies virus vaccine in skunks and raccoons. J. Wildl. Dis. 38:420-427. [DOI] [PubMed] [Google Scholar]
- 12.Hooper, D. C., K. Morimoto, M. Bette, E. Weihe, H. Koprowski, and B. Dietzschold. 1998. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J. Virol. 72:3711-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue, K., Y. Shoji, I. Kurane, T. Iijima, T. Sakai, and K. Morimoto. 2003. An improved method for recovering rabies virus from cloned cDNA. J. Virol. Methods 107:229-236. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, N., C. S. McKimmie, K. L. Mansfield, P. R. Wakeley, S. M. Brookes, J. K. Fazakerley, and A. R. Fooks. 2006. Lyssavirus infection activates interferon gene expression in the brain. J. Gen. Virol. 87:2663-2667. [DOI] [PubMed] [Google Scholar]
- 15.Kozmik, Z., S. Wang, P. Dorfler, B. Adams, and M. Busslinger. 1992. The promoter of the Cd19 gene is a target for the B-cell-specific transcription factor BSAP. Mol. Cell. Biol. 12:2662-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuang, Y., S. N. Lackay, L. Zhao, and Z. F. Fu. 2009. Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection. Virus Res. 144:18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurts, C. 2008. CD11c: not merely a murine DC marker, but also a useful vaccination target. Eur. J. Immunol. 38:2072-2075. [DOI] [PubMed] [Google Scholar]
- 18.Li, J. W., J. P. McGettigan, M. Faber, M. J. Schnell, and B. Dietzschold. 2008. Infection of monocytes or immature dendritic cells (DCs) with an attenuated rabies virus results in DC maturation and a strong activation of the NF kappa B signaling pathway. Vaccine 26:419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurer, M., and E. von Stebut. 2004. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 36:1882-1886. [DOI] [PubMed] [Google Scholar]
- 20.McKay, P. F., D. H. Barouch, S. Santra, S. M. Sumida, S. S. Jackson, D. A. Gorgone, M. A. Lifton, and N. L. Letvin. 2004. Recruitment of different subsets of antigen-presenting cells selectively modulates DNA vaccine-elicited CD4(+) and CD8(+) T lymphocyte responses. Eur. J. Immunol. 34:1011-1020. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 22.Nakamichi, K., S. Inoue, T. Takasaki, K. Morimoto, and I. Kurane. 2004. Rabies virus stimulates nitric oxide production and CXC chemokine ligand 10 expression in macrophages through activation of extracellular signal-regulated kinases 1 and 2. J. Virol. 78:9376-9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Préhaud, C., F. Megret, M. Lafage, and M. Lafon. 2005. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J. Virol. 79:12893-12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rupprecht, C. E., D. Briggs, C. M. Brown, R. Franka, S. L. Katz, H. D. Kerr, S. M. Lett, R. Levis, M. I. Meltzer, W. Schaffner, and P. R. Cieslak. 2010. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recommend. Rep. 59(RR-2):1-9. [PubMed] [Google Scholar]
- 25.Rupprecht, C. E., L. Blass, K. Smith, L. A. Orciari, M. Niezgoda, S. G. Whitfield, R. V. Gibbons, M. Guerra, and C. A. Hanlon. 2001. Human infection due to recombinant vaccinia-rabies glycoprotein virus. N. Engl. J. Med. 345:582-586. [DOI] [PubMed] [Google Scholar]
- 26.Schall, T. J., K. Bacon, R. D. Camp, J. W. Kaspari, and D. V. Goeddel. 1993. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J. Exp. Med. 177:1821-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, J. S., P. A. Yager, and G. M. Baer. 1996. A rapid fluorescence focus inhibition test (RFFIT) for determining rabies virus-neutralisng antibody, p. 181-192. In F.-X. Meslin, M. M. Kaplan, and H. Koprowski (ed.), Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland.
- 28.Trabelsi, K., S. Rourou, H. Loukil, S. Majoul, and H. Kallel. 2006. Optimization of virus yield as a strategy to improve rabies vaccine production by Vero cells in a bioreactor. J. Biotechnol. 121:261-271. [DOI] [PubMed] [Google Scholar]
- 29.Wandeler, A. I., S. Capt, A. Kappeler, and R. Hauser. 1988. Oral immunization of wildlife against rabies: concept and first field experiments. Rev. Infect. Dis. 10(Suppl. 4):S649—S653. [DOI] [PubMed] [Google Scholar]
- 30.Wang, Z. W., L. Sarmento, Y. Wang, X. Q. Li, V. Dhingra, T. Tseggai, B. Jiang, and Z. F. Fu. 2005. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J. Virol. 79:12554-12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. 2005. WHO expert consultation on rabies: first report, p. 1-121. WHO technical report series; 931. World Health Organization, Geneva, Switzerland. [PubMed]
- 32.Wykes, M., and G. MacPherson. 2000. Dendritic cell-B-cell interaction: dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology 100:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wykes, M., A. Pombo, C. Jenkins, and G. G. MacPherson. 1998. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161:1313-1319. [PubMed] [Google Scholar]
- 34.Yong, M., D. Mitchell, A. Caudron, I. Toth, and C. Olive. 2009. Expression of maturation markers on murine dendritic cells in response to group A streptococcal lipopeptide vaccines. Vaccine 27:3313-3318. [DOI] [PubMed] [Google Scholar]
- 35.Zhao, L., H. Toriumi, Y. Kuang, H. Chen, and Z. F. Fu. 2009. The roles of chemokines in rabies virus infection: overexpression may not always be beneficial. J. Virol. 83:11808-11818. [DOI] [PMC free article] [PubMed] [Google Scholar]