Abstract
We report a case of an axillary abscess with Streptococcus pyogenes complicated by venous thrombosis. Bacterial etiology and typing were obtained by PCR and sequencing of the 16S rRNA and M-protein genes from abscess material. The bacterium was of serotype M41, and serology indicated that it had expressed procoagulant factors.
CASE REPORT
A 62-year-old woman presented at our department with a 7-day history of fever, chills, and nausea. She was previously healthy, apart from having atopic eczema, and she worked as a technician in a microbiology department handling bacterial specimens. For some months, she had experienced pain in the left part of her thoracic wall, which she related to repetitive movements. Two days prior to admission, she started to feel pain in her left axilla. On the day of admission, she had vomited and suffered from diarrhea. At admission, she had a temperature of 39.5°C. The routine physical examination was normal, except for a slight tenderness upon palpation of the left axilla. There were no signs of erysipelas, lymphangitis, or enlarged lymph nodes in the axilla. Laboratory investigation revealed a white blood cell count of 18 × 109/liter (the neutrophil count was 17 × 109/liter), a C-reactive protein (CRP) level of 53 mg/liter, and normal renal and liver function test results. Coagulation test results were within normal limits, with a PT(INR) [prothrombin time (international normalized ratio)] of 1.1, an aPTT (activated partial thromboplastin time) of 36 s, and a platelet count of 329 × 109/liter. After two aerobic and two anaerobic blood cultures (BacT/Alert; bioMérieux, Durham, NC) and a urinary culture were obtained, the patient was sent home and told to return if she got worse. No antibiotics were prescribed. Blood cultures turned out negative.
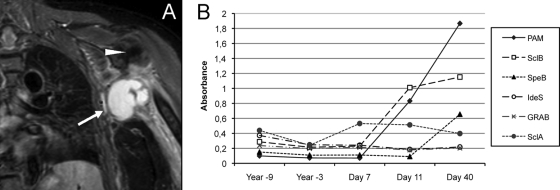
Seven days later, the patient returned with persistent axillary pain and intermittent chills and was hospitalized. Her body temperature fluctuated between 38.0°C and 39.9°C in the following days. Her white blood cell count was 21 × 109/liter (her neutrophil count was 19 × 109/liter), and her CRP level was 393 mg/liter. Upon examination of the axillary region, pain was provoked by palpation but no enlarged lymph nodes or suspected abscesses were felt and no signs of arthritis were noted. Treatment with cefuroxime and clindamycin was instituted due to suspicion of a soft-tissue infection in the axillary region. A plain X-ray of the shoulder showed degenerative changes in the acromio-clavicular joint, and ultrasonographic examination of the axilla was normal, with no signs of edema in the musculature or in the subcutaneous layer and no signs of abscess. A slight improvement occurred over the following days. Renewed blood cultures taken at the time of admission turned out negative. On the 6th day after admission, a swelling of the left arm developed and venous flebography confirmed the presence of a venous thrombosis in the axillary vein. Coagulation tests were done and showed a PT(INR) of 1.1, an aPTT of 40 s, and a platelet count of 430 × 109/liter. Low-molecular-weight heparin and warfarin treatment was initiated. A magnetic resonance imaging (MRI) scan revealed a multilobulated lesion of 7 by 4 by 7 cm in the left axilla approximately 1.5 cm from the skin enclosing the axillary vein with a contrast signal in the periphery and surrounding edema (Fig. 1A). A renewed ultrasonographic examination could visualize the abscess, which was punctured led by a computed tomography scan. Abscess material was added to an anaerobic blood culture bottle (BacT/Alert). Direct cultures were negative, and no growth in the bottle was detected.
FIG. 1.
S. pyogenes causing an axillary abscess. (A) MRI picture, T2 weighted with short inversion time inversion recovery sequencing, showing the abscess in the left axilla. The arrow indicates the abscess, and the arrowhead indicates the caput humeri. (B) Time course of titers of antibodies against various streptococcal surface antigens, where day 1 is the first day of illness.
Abscess material was also subjected to PCR amplification of the 16S rRNA gene and subsequently of the emm gene. DNA was extracted from 200 μl of abscess material using Bio Robot EZ-1 with a DNA Tissue kit (Qiagen, Hilden, Germany) after treatment with proteinase K according to the instructions of the manufacturer. Amplification was carried out in a 50-μl reaction mixture containing 1× PCR buffer (Qiagen), 3 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 1.0 U of HotStarTaq DNA polymerase (Qiagen), 10 pmol of each primer, and 5 μl of template. P515f (5′-TGCCAGCMGCCGCGGTWAT-3′ [12]) and P1067r (5′-AACATYTCACRACACGAGCT-3′[this study]) were used as PCR and sequencing primers. A pre-PCR step of 15 min at 95°C was followed by 40 cycles of 93°C for 50 s, 52°C for 50 s, and 72°C for 50 s. A final step of 5 min at 72°C terminated the amplification. Tubes with no target DNA and Escherichia coli DNA were included as negative and positive controls, respectively. Both strands of the approximately 520-bp PCR product were sequenced using the BigDye Terminator Cycle Sequencing kit (Applied Biosystems Inc., Foster City, CA) and analyzed on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems Inc.) by BMLabbet (Furulund, Sweden). The sequence was identical (523/523 bp) to the 16S rRNA gene of Streptococcus pyogenes available at the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov).
The emm gene encoding the S. pyogenes M protein was amplified from abscess material, as described above, using primers derived from conserved parts of the emm1 gene (5′-GCTTAGAAAATTAAAAACAGG-3′ [emm for] and 5′-GCGTTTTACAACTGCTGC-3′[emm rev]). A 1.2-kbp fragment was generated, and sequencing, as described above, with emmfor yielded a sequence which was highly similar (596/598 bp) to the hypervariable part of the emm41 gene. These results are strongly suggestive of S. pyogenes serotype M41 as the causative agent, and treatment with clindamycin was continued for a total of 3 weeks. The patient had an uncomplicated recovery.
Antibodies directed toward the variable part of the cell wall-attached M protein of S. pyogenes are believed to confer serotype-specific protection. Stored serum samples obtained from the patient several years before the present episode were available, and levels of immunoglobulin G (IgG) antibody against S. pyogenes PAM (plasminogen-binding group A streptococcal M-like protein), an M-like protein expressed by serotype M41 (19), and other virulence determinants in these samples were compared to IgG antibody levels in convalescence-phase sera. ELISA (enzyme-linked immunosorbent assay) was performed essentially as described previously (2). The following S. pyogenes antigens were used for coating: PAM at 0.5 μg/ml, GRAB (protein G-related α2M-binding protein) at 0.8 μg/ml, IdeS (IgG-degrading enzyme of S. pyogenes) at 1.1 μg/ml, SpeB (the secreted streptococcal cysteine proteinase) at 0.5 μg/ml, and SclA and SclB (streptococcal collagen-like proteins A and B, respectively, both from serotype M41) at 4 μg/ml. Antigens were purified as described previously (2, 13, 19). Serum samples were diluted 1:500 (PAM, GRAB, IdeS, and SpeB) or 1:50 (SclA and SclB). All antigens gave an absorbance of at least 0.5 when tested with Octagam (human IgG at 50 mg/ml; Octapharma) or a positive-control serum at the same dilutions as the patient serum samples. There was a marked increase in the levels of IgG antibodies against PAM, a collagen-like surface protein (SclB), and SpeB, whereas levels of IgG antibodies against other streptococcal surface proteins remained unchanged (Fig. 1B). Anti-streptolysin O and anti-DNase B antibody levels on day 21 of illness were elevated.
S. pyogenes, or group A Streptococcus, is an important human pathogen causing a variety of diseases ranging from mild skin infections like impetigo to life-threatening necrotizing fasciitis and toxic shock-like syndrome. Soft-tissue infections caused by S. pyogenes, such as erysipelas and cellulitis, are characterized by diffuse spreading of inflammation in the tissue. The bacterium also causes tonsillitis, and following this infection, abscess formation in the peritonsillar and pharyngeal tissues is relatively common. Abscess formation at other sites occurs rarely. Cases of abscesses with S. pyogenes in the brain (6, 7, 9, 17), in the epidural space (10, 16), in the mediastinum (5), in the lung (8), in the spleen (4), in the retroperitoneum (11), in the pericolic tissue (15), in muscular tissue (1, 3, 18), and in periprosthetic breast tissue (14) have been reported. Considering how common S. pyogenes infections are, abscess formation at sites other than those around the tonsils is distinctly uncommon. To our knowledge, this is the first reported case with an axillary abscess due to S. pyogenes. Though no signs of erysipelas or lymphangitis were present, we believe that the bacteria entered through the skin and spread to the axillary lymph nodes.
The complicating venous thrombosis, which drew attention to the abscess, was probably due to compression of the axillary vein by the abscess. A previous report (3) of an abscess with S. pyogenes causing venous thrombosis also indicated vein compression as the pathogenetic mechanism behind thrombosis formation. However, S. pyogenes binds many components of the coagulation system and the M41 serotype expresses the SclA and SclB proteins, which recruit thrombin-activatable fibrinolysis inhibitor to the bacterial surface (13). By serology, we could show that SclB was expressed during the infection, which could mediate a more procoagulatory state at the site of infection. This molecular mechanism may also have contributed to the thrombosis seen.
The use of 16S PCR and sequencing was invaluable for correct diagnosis in this case, as all cultures were negative. This diagnostic procedure should always be considered in cases where antibiotic treatment has already been commenced. Moreover, DNA extraction from the abscess material made molecular typing of the isolate possible, demonstrating that also the presence of, for example, resistance genes can be detected without culturable bacteria.
Acknowledgments
Ulrika Ringdahl and Lisa Påhlman generously provided the E. coli PAM-expressing clone and SclA and SclB, respectively. Anna Kahn helped with the MRI picture.
This work was supported in part by Swedish Government Funds for Clinical Research (ALF).
We have no conflicts of interest to report.
Footnotes
Published ahead of print on 30 June 2010.
REFERENCES
- 1.Abuelreish, M. A., and M. H. Rathore. 2005. Subpectoral abscess a rare group A beta-hemolytic Streptococcus infection. Pediatr. Infect. Dis. J. 24:1121-1122. [DOI] [PubMed] [Google Scholar]
- 2.Åkesson, P., M. Rasmussen, E. Mascini, U. von Pawel-Rammingen, R. Janulczyk, M. Collin, A. Olsen, E. Mattsson, M. L. Olsson, L. Björck, and B. Christensson. 2004. Low antibody levels against cell wall-attached proteins of Streptococcus pyogenes predispose for severe invasive disease. J. Infect. Dis. 189:797-804. [DOI] [PubMed] [Google Scholar]
- 3.Bertelsen, J., M. T. Severinsen, and H. Gregersen. 2006. Deep venous thrombosis caused by severe infection with group A streptococci. Ugeskr. Laeger 168:2260-2261. [PubMed] [Google Scholar]
- 4.Chang, K. W., C. H. Chiu, T. H. Jaing, and H. F. Wong. 2003. Splenic abscess caused by group A beta-haemolytic Streptococcus. Acta Paediatr. 92:510-511. [DOI] [PubMed] [Google Scholar]
- 5.Conway, J. H., A. C. Nyquist, and E. Goldson. 1996. Posterior mediastinal abscess caused by invasive group A Streptococcus infection. Pediatr. Infect. Dis. J. 15:547-549. [DOI] [PubMed] [Google Scholar]
- 6.Crum, N. F. 2004. Group A streptococcal brain abscess. Scand. J. Infect. Dis. 36:238-239. [DOI] [PubMed] [Google Scholar]
- 7.Dehority, W., S. Uchiyama, A. Khosravi, and V. Nizet. 2006. Brain abscess caused by Streptococcus pyogenes in a previously healthy child. J. Clin. Microbiol. 44:4613-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frieden, T. R., J. Biebuyck, and W. J. Hierholzer, Jr. 1991. Lung abscess with group A beta-hemolytic Streptococcus. Case report and review. Arch. Intern. Med. 151:1655-1657. [PubMed] [Google Scholar]
- 9.Khan, M. A., G. M. Viagappan, and J. Andrews. 2001. Group A streptococcal brain abscess. Scand. J. Infect. Dis. 33:159. [DOI] [PubMed] [Google Scholar]
- 10.LaQuinte, V., S. Gupta, and R. Kamat. 2009. Back abscess. Clin. Pediatr. (Phila.) 48:109-111. [DOI] [PubMed] [Google Scholar]
- 11.Llibre, J. M., P. Puig, A. Aloy, J. Roset, and J. Torne. 1992. Silent spontaneous retroperitoneal abscess caused by M-type 18 Streptococcus pyogenes. Eur. J. Clin. Microbiol. Infect. Dis. 11:205-207. [DOI] [PubMed] [Google Scholar]
- 12.Nikkari, S., F. A. Lopez, P. W. Lepp, P. R. Cieslak, S. Ladd-Wilson, D. Passaro, R. Danila, and D. A. Relman. 2002. Broad-range bacterial detection and the analysis of unexplained death and critical illness. Emerg. Infect. Dis. 8:188-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Påhlman, L. I., P. F. Marx, M. Mörgelin, S. Lukomski, J. C. Meijers, and H. Herwald. 2007. Thrombin-activatable fibrinolysis inhibitor binds to Streptococcus pyogenes by interacting with collagen-like proteins A and B. J. Biol. Chem. 282:24873-24881. [DOI] [PubMed] [Google Scholar]
- 14.Persichetti, P., M. Langella, G. F. Marangi, E. Vulcano, G. Gherardi, and G. Dicuonzo. 2008. Periprosthetic breast abscess caused by Streptococcus pyogenes after scarlet fever. Ann. Plast. Surg. 60:21-23. [DOI] [PubMed] [Google Scholar]
- 15.Procop, G. W., L. J. Harrell, M. K. Washington, C. H. Owen, and T. N. Pappas. 1996. Pericolic abscess due to Streptococcus pyogenes: report of a case that clinically mimicked acute cholecystitis. Clin. Infect. Dis. 23:182-183. [DOI] [PubMed] [Google Scholar]
- 16.Quach, C., B. Tapiero, and F. Noya. 2002. Group A Streptococcus spinal epidural abscess during varicella. Pediatrics 109:E14. [DOI] [PubMed] [Google Scholar]
- 17.Ralph, E., and G. Shoemaker. 1999. Group A streptococcal brain abscess. Scand. J. Infect. Dis. 31:206-207. [PubMed] [Google Scholar]
- 18.Viani, R. M., K. Bromberg, and J. S. Bradley. 1999. Obturator internus muscle abscess in children: report of seven cases and review. Clin. Infect. Dis. 28:117-122. [DOI] [PubMed] [Google Scholar]
- 19.Wistedt, A. C., U. Ringdahl, W. Müller-Esterl, and U. Sjöbring. 1995. Identification of a plasminogen-binding motif in PAM, a bacterial surface protein. Mol. Microbiol. 18:569-578. [DOI] [PubMed] [Google Scholar]