Abstract
Phylogenetic analysis of respiratory syncytial virus (RSV) group B genotype BA strains from the 2002-2003 to 2009-2010 seasons collected in Niigata, Japan, revealed four distinct clusters, designated new BA genotypes BA7, BA8, BA9, and BA10. These new genotypes were not associated with large outbreaks in the community.
Respiratory syncytial virus (RSV) is a major cause of acute lower respiratory tract infections in infants and young children. RSV is classified into two groups, A and B (2, 6, 9, 14, 15), based on the variability of the attachment G glycoprotein (G protein) (7). The second variable region of this protein, located at the C-terminal end, is widely used to study the genetic diversity of RSV (4, 8). To date, several genotypes of each RSV group have been described: for RSV-A, GA1 to GA7 (10, 11), SAA1 (South Africa A1) (19), and most recently reported, NA1 and NA2 (13); for RSV-B, GB1 to GB4 (11), SAB1 to SAB3 (19), and BA1 to BA6 (Buenos Aires) (18).
We previously reported the emergence of two new RSV-A genotypes, designated NA1 and NA2, in Japan in the 2004-2005 and 2005-2006 seasons, respectively (13). Upon their emergence, large outbreaks of RSV infection in the community were observed. We also reported the emergence of thenew RSV-B genotype with a 60-nucleotide duplication in the G-protein gene (G gene), genotype BA, in the 2002-2003 season, but no big outbreaks or serious clinical manifestations were associated with its emergence (12). The RSV-B BA genotype BA5 was the predominant genotype when it emerged in the 2002-2003 season. Since then, BA viruses have been circulating in the community in increasing numbers and other genotypes within BA have been identified: BA2, BA4, and BA6. In this study, more detailed genetic analysis of RSV-B BA from the 2002-2003 season to the 2009-2010 season revealed four new clusters. These new clusters were assigned to additional BA genotypes BA7, BA8, BA9, and BA10.
Clinical samples were collected from children who visited an outpatient pediatric clinic in Niigata City, Niigata, Japan, from 2001 to 2010. Total viral RNA was extracted from 100-μl nasopharyngeal samples using the Extragen II kit (Kainos, Tokyo, Japan) and used for cDNA synthesis using random primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA). RSV groups and genotypes were determined by PCR and sequencing, respectively, according to a previously published method (12). Sequences were assembled using Lasergene SeqMan Pro version 7.2.1 (DNASTAR, Madison, WI) (3) and aligned by ClustalW. Subsequent phylogenetic analyses using the neighbor-joining method, the maximum composite likelihood model, and bootstrap analysis (1,000 replicates) were performed using the MEGA software version 4.0 (16). Only major nodes with bootstrap values of >50% were considered a cluster. Reference nucleotide sequences of RSV-B strains from other countries were obtained from GenBank.
There were 1,525 cases screened from November 2001 to February 2010, of which 707 (46.4%) were positive by reverse transcription-PCR (Table 1). Among these, 252 (35.6%) belonged to RSV-B. RSV surveillance in our laboratory since the 2001-2002 season showed that RSV-A was dominant in six of the nine seasons, while RSV-B was dominant in only three seasons: in the 2002-2003 season, when RSV-B genotype BA first appeared, in the 2007-2008 season, and in the 2009-2010 season (Table 1). No RSV-B strains were detected in the 2008-2009 season.
TABLE 1.
Prevalence of RSV-B from the 2001-2002 season to the 2009-2010 season
| Group | No. (%) of cases in season |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2001-2002 | 2002-2003 | 2003-2004 | 2004-2005 | 2005-2006 | 2006-2007 | 2007-2008 | 2008-2009 | 2009-2010 | |
| RSV+ | 33 | 60 | 75 | 45 | 121 | 154 | 80 | 79 | 60 |
| RSV-A | 31 | 13 | 67 | 36 | 85 | 106 | 14 | 79 | 24 |
| RSV-B | 2/2a | 44/47a | 6/8a | 7/9a | 34/36a | 36/48a | 66/66a | 0a | 36/36a |
| GB3 | 4 | 1 | |||||||
| SAB2 | 2 | ||||||||
| BA2 | 1 | 2 | |||||||
| BA4 | 4 | 5 | 5 | ||||||
| BA5 | 39 (88.6)b | 1 | |||||||
| BA6 | 1 | ||||||||
| BA7 | 1 | 28 (82.3)b | 8 | ||||||
| BA8 | 1 | 31 (86.1)b | 10 | ||||||
| BA 9 | 5 | 8 | 32 (88.9)b | ||||||
| BA10 | 40 (60.6)b | 2 | |||||||
The number of RSV-B samples sequenced over the total number of samples positive for PCR.
The number of cases with the predominant genotype and the percent distribution among RSV-B samples per season are in bold.
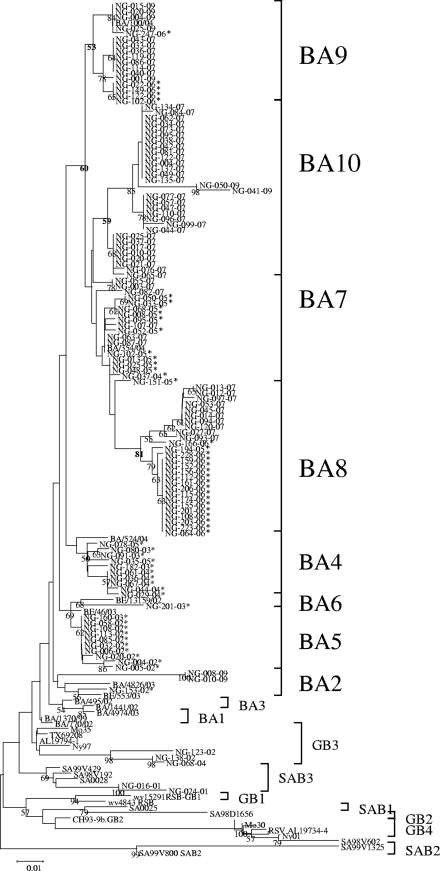
Genetic analysis of RSV-B strains showed that almost all (96.7%), except for seven strains, contain features characteristic of the BA genotype (1). BA viruses were previously classified into the BA2, BA4, BA5, and BA6 genotypes, with viruses from the 2003-2004 to 2006-2007 seasons collectively classified as BA4 (13). The addition of sequences from the 2007-2008 and 2009-2010 seasons led to further classification of the BA genotype viruses into four new distinct clusters (Fig. 1 ). Previously classified BA4 viruses were reclassified into four groups: BA4, BA7, BA8, and BA9 (Table 1; Fig. 1). Most of the strains from the 2003-2004 and 2004-2005 seasons clustered in the previously described BA4 genotype. Meanwhile, more than 80% of the strains from the 2005-2006, 2006-2007, and 2009-2010 seasons formed their own clusters, designated BA7, BA8, and BA9, respectively. A majority of the strains from the 2007-2008 season clustered together and were classified as BA10. Strains from this season also belong to the BA7, BA8, and BA9 genotypes.
FIG. 1.
Phylogenetic tree of RSV-B strains constructed by the neighbor-joining method. Partial nucleotide sequences of the second variable region of the G gene (270 to 330 bases) of strains from the 2001-2002 season to the 2009-2010 season were aligned. Reference sequences of group B genotypes from other studies were included in the analysis of the respective groups for comparison. Previously reported Niigata RSV-B BA sequences are marked with asterisks. The scale bar represents the number of nucleotide substitutions per site, and the values at the nodes are bootstrap values for 1,000 iterations. Only values of greater than 50% are shown. Genotypes are indicated at the right by brackets.
The bootstrap value at the major node between the four new genotypes and previously identified genotypes was 60%. This provided reasonable support for the distinction of the new genotypes from BA4. Genotypes BA8, BA9, and BA10 had bootstrap values of 81%, 53%, and 59% at their major nodes. BA7, however, does not have a significant bootstrap value. Most of the strains in this genotype were from the 2006-2007 season (Table 1), the earliest among the new genotypes. BA7 may be a progenitor of the more recent BA viruses.
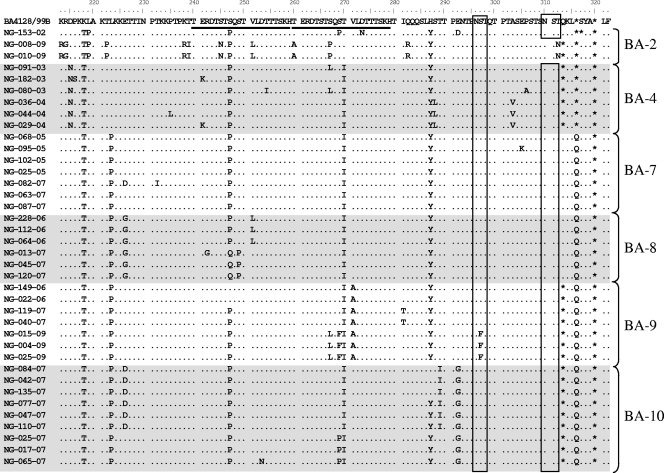
Genotype BA strains were compared with prototype strain BA4128/99B (Fig. 2 ), and characteristic amino acid changes for a particular genotype were identified. The BA7 genotype had an L223P substitution initially detected in 2005-2006 season strains and persisted until the 2009-2010 season. The BA8 genotype had an E226G substitution. The 2006-2007 BA8 strains had an additional V251L change, whereas those from the 2007-2008 season had S247Q and S249P changes. The BA9 genotype had a V271A substitution. The amino acid at position 247 of 2006-2007 BA9 strains reverted to that of the prototype. The 2007-2008 season strains had an additional I281T substitution. Furthermore, 2009-2010 season strains had S267L, S269F, and S297F changes. BA10 strains had an E292G change. A subset of the BA10 genotype had E226D and T289I changes. Another subset had an S269P change. Notably, two new viruses from the 2009-2010 season (NG-008-09 and NG-010-09), which are BA2-like, had 10 amino acid substitutions not seen in previous strains, one of which (T312N) resulted in the loss of a potential N-glycosylation site. This site, together with another N-glycosylation site at position 296, had been previously reported to be conserved in all BA viruses (1). This is the first time that a loss of an N-glycosylation site at position 310 has been reported.
FIG. 2.
Deduced amino acid alignments of the second variable region of the G genes of representative RSV-B BA genotype strains from Niigata. Alignments are shown relative to prototype BA strain BA4128/99B (GenBank accession number AY333364), with amino acid numbers corresponding to amino acid positions 213 to 315 of the prototype sequence. Identical residues are indicated by dots, and stop codons are indicated by asterisks. The duplicated 20-amino-acid regions are underlined, and potential N-glycosylation sites are indicated by rectangles.
RSV-B genotype BA was the predominant genotype when it emerged in the 2002-2003 season. It has been circulating in the community for 8 years, and new genotypes within BA have been identified each year. The BA genotype, however, predominated only in three seasons and did not contribute to large epidemics, as opposed to RSV-A genotypes, which caused large outbreaks in the 2005-2006 and 2006-2007 seasons. The high incidence of RSV-A infection in these years may have consequently led to the predominance of RSV-B in the 2007-2008 season.
We were able to distinguish four new clusters, BA7, BA8, BA9, and BA10, which contained strains from the 2005-2006 season to the 2009-2010 season, from the previously described BA4 genotype (Fig. 1). Cocirculation of several genotypes was observed in all of the seasons, with one genotype dominant in one season and replaced by another in the following season. The clustering of viruses that circulated in the same year observed in the BA7 to BA10 genotypes supports the previously described temporal nature of RSV (5, 20).
The BA genotype is of considerable interest because of its 60-nucleotide insertion in the G gene's second variable region, the largest modification in this gene described so far (17). It had replaced previously circulating RSV-B genotypes in the community, although no major outbreaks had been associated with any new BA genotype. This might suggest that the duplication did not result in a significant change in antigenicity, although the mutations within and outside the duplication regions contributed to the increased genetic diversity of BA strains.
The results of this study further stress the importance of continued surveillance of RSV infections in detecting and characterizing new genotypes that circulate not only in local communities but also worldwide.
Nucleotide sequence accession numbers.
Representative sequences used in this study were submitted to GenBank under accession numbers HM459856 to HM459892.
Footnotes
Published ahead of print on 7 July 2010.
REFERENCES
- 1.Agrawal, A., M. Sarkar, S. Ghosh, M. Chawla-Sarkar, N. Chakraborty, M. Basak, and T. Naik. 2009. Prevalence of respiratory syncytial virus group B genotype BA-IV strains among children with acute respiratory tract infection in Kolkata, eastern India. J. Clin. Virol. 45:358-361. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, L., J. Hierholzer, C. Tsou, R. Hendry, B. Fernie, Y. Stone, and K. McIntosh. 1985. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 151:626-633. [DOI] [PubMed] [Google Scholar]
- 3.Burland, T. 2000. DNASTAR's Lasergene sequence analysis software. Methods Mol. Biol. 132:71-91. [DOI] [PubMed] [Google Scholar]
- 4.Coggins, W., E. Lefkowitz, and W. Sullender. 1998. Genetic variability among group A and group B respiratory syncytial viruses in a children's hospital. J. Clin. Microbiol. 36:3552-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Silva, L., F. Spilki, A. Riccetto, R. de Almeida, E. Baracat, and C. Arns. 2008. Genetic variability in the G protein gene of human respiratory syncytial virus isolated from the Campinas metropolitan region, Brazil. J. Med. Virol. 80:1653-1660. [DOI] [PubMed] [Google Scholar]
- 6.Hendry, R., A. Talis, E. Godfrey, L. Anderson, B. Fernie, and K. McIntosh. 1986. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. J. Infect. Dis. 153:291-297. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, P., M. Spriggs, R. Olmsted, and P. Collins. 1987. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. U. S. A. 84:5625-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melero, J., B. García-Barreno, I. Martínez, C. Pringle, and P. Cane. 1997. Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J. Gen. Virol. 78 (Pt. 10):2411-2418. [DOI] [PubMed] [Google Scholar]
- 9.Mufson, M., C. Orvell, B. Rafnar, and E. Norrby. 1985. Two distinct subtypes of human respiratory syncytial virus. J. Gen. Virol. 66(Pt. 10):2111-2124. [DOI] [PubMed] [Google Scholar]
- 10.Peret, T., C. Hall, G. Hammond, P. Piedra, G. Storch, W. Sullender, C. Tsou, and L. Anderson. 2000. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J. Infect. Dis. 181:1891-1896. [DOI] [PubMed] [Google Scholar]
- 11.Peret, T., C. Hall, K. Schnabel, J. Golub, and L. Anderson. 1998. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J. Gen. Virol. 79(Pt. 9):2221-2229. [DOI] [PubMed] [Google Scholar]
- 12.Sato, M., R. Saito, T. Sakai, Y. Sano, M. Nishikawa, A. Sasaki, Y. Shobugawa, F. Gejyo, and H. Suzuki. 2005. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J. Clin. Microbiol. 43:36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shobugawa, Y., R. Saito, Y. Sano, H. Zaraket, Y. Suzuki, A. Kumaki, I. Dapat, T. Oguma, M. Yamaguchi, and H. Suzuki. 2009. Emerging genotypes of human respiratory syncytial virus subgroup A in Japan. J. Clin. Microbiol. 47:2475-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullender, W., L. Anderson, K. Anderson, and G. Wertz. 1990. Differentiation of respiratory syncytial virus subgroups with cDNA probes in a nucleic acid hybridization assay. J. Clin. Microbiol. 28:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullender, W., L. Sun, and L. Anderson. 1993. Analysis of respiratory syncytial virus genetic variability with amplified cDNAs. J. Clin. Microbiol. 31:1224-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 17.Trento, A., M. Galiano, C. Videla, G. Carballal, B. García-Barreno, J. Melero, and C. Palomo. 2003. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J. Gen. Virol. 84:3115-3120. [DOI] [PubMed] [Google Scholar]
- 18.Trento, A., M. Viegas, M. Galiano, C. Videla, G. Carballal, A. Mistchenko, and J. Melero. 2006. Natural history of human respiratory syncytial virus inferred from phylogenetic analysis of the attachment (G) glycoprotein with a 60-nucleotide duplication. J. Virol. 80:975-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venter, M., S. Madhi, C. Tiemessen, and B. Schoub. 2001. Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. J. Gen. Virol. 82:2117-2124. [DOI] [PubMed] [Google Scholar]
- 20.Viegas, M., and A. Mistchenko. 2005. Molecular epidemiology of human respiratory syncytial virus subgroup A over a six-year period (1999-2004) in Argentina. J. Med. Virol. 77:302-310. [DOI] [PubMed] [Google Scholar]