Abstract
Eighteen Corynebacterium xerosis strains isolated from different animal clinical specimens were subjected to phenotypic and molecular genetic studies. On the basis of the results of the biochemical characterization, the strains were tentatively identified as C. xerosis. Phylogenetic analysis based on comparative analysis of the sequences of 16S rRNA and rpoB genes revealed that the 18 strains were highly related to C. xerosis, C. amycolatum, C. freneyi, and C. hansenii. There was a good concordance between 16S rRNA and partial rpoB gene sequencing results, although partial rpoB gene sequencing allowed better differentiation of C. xerosis. Alternatively, C. xerosis was also differentiated from C. freneyi and C. amycolatum by restriction fragment length polymorphism analysis of the 16S-23S rRNA gene intergenic spacer region. Phenotypic characterization indicated that besides acid production from d-turanose and 5-ketogluconate, 90% of the strains were able to reduce nitrate. The absence of the fatty acids C14:0, C15:0, C16:1ϖ7c, and C17:1ϖ8c can also facilitate the differentiation of C. xerosis from closely related species. The results of the present investigation demonstrated that for reliable identification of C. xerosis strains from clinical samples, a combination of phenotypic and molecular-biology-based identification techniques is necessary.
During the last decade, the genus Corynebacterium has undergone a significant expansion in the number of species described due to an increased concern about its potential pathogenic significance. About a quarter of the species currently recognized as belonging to the genus Corynebacterium (http://www.bacterio.net) have been isolated from animal sources, although only some of them can be considered well-established animal pathogens (5, 18). Corynebacterium xerosis is an unusual human pathogen (9) which has been recently isolated for the first time from animal clinical specimens (25). C. xerosis is phylogenetically closely related to Corynebacterium freneyi, Corynebacterium amycolatum, and Corynebacterium hansenii (19, 20, 26), but DNA-DNA hybridization experiments have demonstrated that they represent different species (19, 20). The unambiguous discrimination of these species based on phenotypic characteristics and comparative 16S rRNA gene analysis is problematic (7, 9, 20). Molecular-genetic methods, such as sequencing of the RNA polymerase beta subunit-encoding gene (rpoB), have been proposed as alternative or complementary molecular methods to 16S rRNA gene analysis for discerning phylogenetically closely related Corynebacterium species (13, 14). Also, restriction fragment length polymorphism (RFLP) analysis of the 16S-23S rRNA gene intergenic spacer region (IGS) has been proposed for the accurate differentiation of some of these species (7, 19, 20). However, these studies included a very limited number of C. xerosis strains because most of the strains previously classified as C. xerosis had been misidentified (9). Since the first description of C. xerosis from animals, additional strains have been recovered from clinical specimens of different animal species. In this work, we present the results of an extensive analysis of a significant number of C. xerosis strains from animals which could facilitate its identification and differentiation from other genotypically and phenotypically closely related species.
MATERIALS AND METHODS
Bacterial strains.
Eighteen bacterial strains suspected to be C. xerosis were isolated from different veterinary clinical specimens. Two human C. xerosis strains, CCUG 45245 and CCUG 39723, five C. freneyi and eight C. amycolatum human strains, as well as the C. xerosis CCUG 56051T, C. freneyi CCUG 45704T, C. hansenii CCUG 53252T, and C. amycolatum CCUG 35685T type strains, obtained from the Culture Collection of the University of Göteborg (CCUG), were included for comparison. Additional information regarding the strains included in this study is listed in Table 1. All of the strains were cultured on Columbia agar plates (bioMérieux) and incubated at 37°C for 48 h.
TABLE 1.
Details of the strains included in this study
| Strain | Source | Clinical history | Colony colorb | API Coryne numerical code |
|---|---|---|---|---|
| C. xerosis | ||||
| CCUG 56051T | Human ear discharge | Y | 3110325 | |
| St36404a | Goat liver | Suspected paratuberculosis | W-G | 2110325 |
| St33874a | Pig lung | Suspected erysipelas | Y | 3110325 |
| St34960a | Pig kidney | Respiratory problems | Y | 3110325 |
| St36130a | Pig skin | Subcutaneous abscess | Y | 3110325 |
| St38671a | Pig joint | Arthritis | W-G | 3110325 |
| St85640 | Cow milk | Mastitis | Y | 3110325 |
| St53041 | Pig fetus | Abortion | W-G | 3110325 |
| St51377/1 | Pig blood | Sudden death | Y | 3110325 |
| St53244 | Pig joint | Arthritic abscess | W-G | 3110325 |
| St51902 | Pig spleen | Septicemia | W-G | 3110325 |
| St51463 | Pig joint | Arthritis | W-G | 3110325 |
| St51377/2 | Sheep lungs | Respiratory problems | Y | 3110325 |
| St51377/3 | Pig. joint | Arthritis | Y | 3110325 |
| St46963 | Pig kidney | Septicemia | W-G | 3110325 |
| St49327a | Pig liver | Septicemia | Y | 3110325 |
| St47126a | Sheep uterus | Abortion | Y | 3110325 |
| St49485a | Pig joint | Subcutaneous abscess | W-G | 3110325 |
| CCUG 45245 | Not known | Not known | W-G | 2110325 |
| CCUG 39723 | Human bone | Osteomyelitis | W-G | 3110325 |
| C. freneyi | ||||
| CCUG 45704T | Human toe | W-G | 3110325 | |
| CCUG 54468 | Human duodenum | Duodenal biopsy specimen | W-G | 3110325 |
| CCUG 54466 | Human vagina | Not specified | W-G | 3100325 |
| CCUG 54465 | Human vagina | Not specified | W-G | 2110325 |
| CCUG 54469 | Human vagina | Not specified | W-G | 3110325 |
| CCUG 54467 | Human cervix | Not specified | W-G | 3110325 |
| M3 | Sheep eye | Conjunctivitis | Y | 2110325 |
| C. amycolatum | ||||
| CCUG 35685T | Human skin | Not specified | W-G | 0101324 |
| CCUG 58230 | Human femur | Wound | W-G | 2100324 |
| CCUG 57527 | Human blood | Not specified | W-G | 2100325 |
| CCUG 57358 | Human abdomen | Not specified | W-G | 3100325 |
| CCUG 56284 | Human skin | Wound | W-G | 3100325 |
| CCUG 46945 | Human | Not specified | W-G | 2100324 |
| CCUG 34699 | Human blood | Not specified | Y | 3100324 |
| CCUG 35623 | Human blood | Not specified | W-G | 2100325 |
| CCUG 38870 | Human skin | Not specified | W-G | 3100324 |
| C. hansenii CCUG 53252T | Human | Liposarcoma | Y | 2000325 |
Strain previously described by Vela et al. (24).
Y, yellowish; W-G, whitish-grayish.
Biochemical characterization.
All strains were biochemically characterized using the commercial API Coryne, API ZYM, and API 50 CH systems (bioMérieux). The API Coryne and API ZYM strips were used according to the manufacturer's instructions. The API 50 CH strips were inoculated with a 2 McFarland standard suspension of bacterial cells in CHB/E medium (bioMérieux) as recommended by the manufacturer and incubated at 37°C for up to 5 days. Glucose fermentation at 42°C and growth at 20°C were performed as described previously (26).
Cellular fatty acid analysis.
The cellular fatty acids of C. xerosis strains CCUG 56051T, CCUG 39723, St85640, St53041, St51377/1, and St53244 were determined by following the CCUG protocol (http://www.ccug.se). Briefly, isolates were grown aerobically on Columbia II agar base (BBL 4397596) with 5% horse blood for 16 to 48 h at 37°C. Cells were removed from the plate using a plastic inoculating loop, carefully scraped to avoid including medium in the sample. Fifty to 100 mg of cells was then transferred to glass tubes. Cells were saponified, and released fatty acids were methylated. Finally, fatty acid methyl esters were extracted. Analysis was carried out with a Hewlett Packard HP 5890 gas chromatograph.
DNA extraction.
For DNA extraction, three colonies of each strain grown on Columbia agar were suspended in 100 μl of distilled water and boiled for 10 min at 100°C. After boiling, bacterial suspensions were centrifuged (7,600 × g, 2 min) and the supernatant was used as a source of template DNA for PCRs.
16S-23S rRNA gene PCR-RFLP analysis.
The amplification of the 16S-23S rRNA gene IGS was carried out according to Aubel et al. (2) using primers G1 and L1 (12) with the following modifications. In vitro amplification was carried out with a reaction mixture of 100 μl containing template DNA (5 μl), 1 μM each primer (G1/L1), 200 μM each deoxynucleoside triphosphate (Biotools), and 2.5 U of Ultratools DNA polymerase (Biotools) and its amplification buffer. The amplifications were carried out in a Mastercycler gradient thermal cycler (Eppendorf) with an initial denaturation step of 94°C for 5 min; 30 serial cycles of denaturation at 94°C for 1 min, annealing at 47°C for 7 min, and extension at 72°C for 2 min; and a final extension step of 72°C for 10 min. Negative controls (no template DNA) were included in each batch of PCRs. PCR-generated products were detected by electrophoresis of 5 μl of each amplification mixture in 2% agarose gels supplemented with 1× SYBR Safe (Invitrogen, Eugene, OR). Amplified DNA was digested using the restriction enzyme CfoI (Fermentas Inc., Glen Burnie, MD). Approximately 15 μl of the PCR product was digested with 15 U of enzyme for 4 h at 37°C in a water bath. The electrophoretic patterns of digested products were photographed with a Fluor-S MultiImager (Bio-Rad Laboratories, Inc., Hercules, CA), and the data were analyzed using the Quantity One software package (Bio-Rad Laboratories, Inc.).
Sequencing of the 16S rRNA and rpoB genes.
A nearly complete 16S rRNA gene fragment (>1,400 bp) was amplified as described previously (24). Amplification of the partial rpoB gene was performed as indicated by Khamis et al. (14), using previously described primers C2700F and C3130R (13). Multiple-sequence alignments and percent similarities of the rpoB and 16S rRNA genes of the various species were obtained with the CLUSTAL W program available from the EMBL-EBI web server (http://www.ebi.ac.uk/clustalw/). Phylogenetic trees were constructed according to three different algorithms: neighbor joining (21) using the programs SeqTools (http://www.seqtools.dk) and TREEVIEW (17), maximum likelihood using the PHYML software (11), and maximum parsimony using the software package MEGA (molecular evolutionary genetics analysis) version 3.1 (16). Bootstrap replicates were performed to estimate the stability (1,000 replicates) of the branching nodes of the phylogenetic tree.
Nucleotide sequence and culture collection accession numbers.
The 16S rRNA gene sequences of the strains isolated from veterinary clinical material included in this study have been deposited in the GenBank database under accession numbers FM213372 to FM213376, FN179318 to FN179334, and FN564566 to FN564568. The partial rpoB sequences of all of the strains characterized in this study have been deposited in the GenBank database under accessions numbers FN179293 to FN179317, FN564557 to FN564565, and FN552725. Strains St36130, St36404, and St47126 have been deposited in the Culture Collection of the University of Göteborg, Göteborg, Sweden, under collection numbers CCUG 53404 to CCUG 53406, and strains St85640, St53244, St53041, and St51377/1 have been deposited under collection numbers CCUG 57194 to CCUG 57197, respectively.
RESULTS
Genetic analysis.
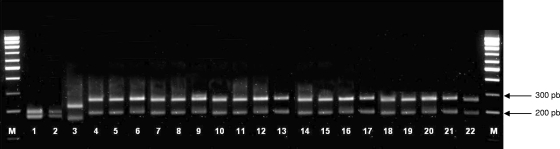
Three different patterns were observed after RFLP-IGS analysis. Most of the animal isolates (n = 17) suspected to be C. xerosis, as well as C. xerosis strains CCUG 56051T, CCUG 45245, and CCUG 39723 and C. hansenii CCUG 53252T, displayed identical fingerprinting patterns (Fig. 1, lanes 4 to 22), showing two bands of 280 and 200 bp. Animal strain M3 and the six C. freneyi strains presented the same fingerprinting pattern (Fig. 1, lanes 1 to 2), with bands of 200, 180, and 100 bp. Likewise, all of the C. amycolatum strains showed a pattern identical to that of C. amycolatum CCUG 35685T (Fig. 1, lane 3), displaying RFLP fragments of 245 and 172 bp. Therefore, C. xerosis, C. freneyi, and C. amycolatum displayed different RFLP patterns, whereas C. xerosis and C. hansenii showed indistinguishable patterns.
FIG. 1.
Digestion of the PCR-amplified 16S-23S rRNA gene IGS of Corynebacterium species using the restriction endonuclease CfoI. Lanes 1 to 22: C. freneyi CCUG 45704T, animal strain M3, C. amycolatum CCUG 35685T, C. hansenii CCUG 53252T, C. xerosis CCUG 56051T, and animal strains St36404, St33874, St34960, St36130, St38671, St85640, St53041, St51377/1, St53244, St51902, St51463, St51377/2, St51377/3, St46963, St49327, St47126, and St49485, respectively. Lanes M, molecular weight markers (100-bp ladder).
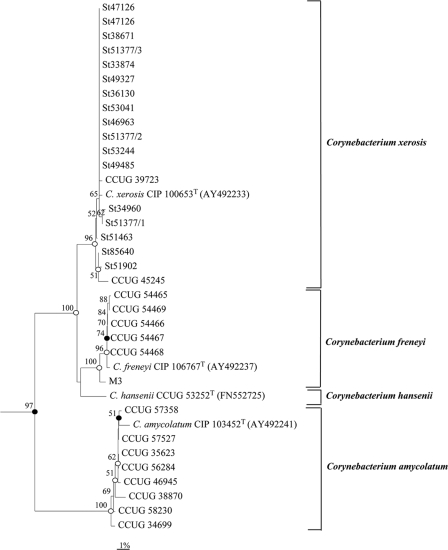
Phylogenetic analysis based on the rpoB gene sequence revealed that the strains of C. xerosis cluster together to form a distinct subline within the genus Corynebacterium that was separate from that formed by C. freneyi, C. amycolatum, or C. hansenii, which was supported by high bootstrap resampling values (Fig. 2). In agreement with the RFLP-IGS results, animal strain M3 was more closely related to C. freneyi strains (Fig. 2). A similar tree topology was obtained after phylogenetic analysis based on 16S rRNA gene sequences (Fig. 3).
FIG. 2.
Dendrogram based on rpoB gene sequence comparison obtained with the neighbor-joining algorithm showing the phylogenetic relationship between animal strains of C. xerosis and the close relatives C. freneyi, C. amycolatum, and C. hansenii. Bootstrap values (expressed as percentages of 1,000 replications) higher than 50% are given at the branching points. Filled circles indicate that the corresponding nodes (groupings) are also obtained on the parsimony tree. Open circles indicate that the corresponding nodes (groupings) are also obtained on the maximum-likelihood and parsimony trees. Corynebacterium bovis CIP 54.80T (AY492236) was used as an outgroup. Bar, 1% sequence divergence.
FIG. 3.
Dendrogram based on 16S rRNA gene sequence comparison obtained with the neighbor-joining algorithm showing the phylogenetic relationship between animal strains of C. xerosis and the close relatives C. freneyi, C. amycolatum, and C. hansenii. Bootstrap values (expressed as percentages of 1,000 replications) higher than 50% are given at the branching points. Filled circles indicate that the corresponding nodes (groupings) are also obtained on the parsimony tree. Open circles indicate that the corresponding nodes (groupings) are also obtained on the maximum-likelihood and parsimony trees. C. bovis NCTC 3324T (X82051) was used as an outgroup. Bar, 1% sequence divergence.
Comparative analysis of the partial rpoB sequences determined for the C. xerosis isolates revealed sequence similarities in the ranges of 100 to 99%, 97 to 93%, 86 to 84%, and 95 to 92% with the type strains C. xerosis CCUG 56051T, C. freneyi CCUG 45704T, C. amycolatum CCUG 35685T, and C. hansenii CCUG 53252T, respectively. Comparative analysis of the 16S rRNA gene sequences of the strains studied showed sequence similarities in the ranges of 100 to 99%, 99 to 98%, 98 to 96%, and 99 to 98% with the type strains C. xerosis CCUG 56051T, C. freneyi CCUG 45704T, C. amycolatum CCUG 35685T, and C. hansenii CCUG 53252T, respectively. These similarity values indicated that the strains studied are more close related to C. xerosis, C. freneyi, and C. hansenii than to C. amycolatum.
Phenotypic analysis.
All of the strains genetically confirmed as C. xerosis gave whitish-grayish or yellowish pigmented colonies with a dry, rough, wrinkled surface (Table 1). Every one of the C. freneyi strains examined gave whitish-grayish and wrinkled-surface colonies, and animal strain M3, genetically confirmed as C. freneyi, displayed yellowish wrinkled colonies (Table 1). The C. amycolatum strains analyzed in the present study displayed whitish-grayish colonies, except strain CCUG 34699, which formed yellowish wrinkled colonies. C. hansenii CCUG 53252T gave small, dry, rough, wrinkled-surface, yellowish pigmented colonies. Strains of the four Corynebacterium species were biochemically characterized using three commercial systems. With the API Coryne system, C. xerosis exhibited a quite homogeneous biochemical profile (Table 1) displaying the numerical profiles 3110325 (n = 18; 90.0%) and 2110325 (n = 2; 10.0%). These numerical profiles were also exhibited by four and two C. freneyi strains, respectively (Table 1). C. amycolatum exhibited great biochemical diversity, with five different profiles, none being displayed by C. xerosis. The numerical profile given by C. hansenii (2000325) was not shared by any C. xerosis strain either (Table 1). Biochemical characterization of the strains was extended by testing them for the abilities to ferment glucose at 42°C and grow at 20°C, as well as testing their enzymatic and carbohydrate metabolism, by using the API ZYM and API 50 CH systems (Table 2). Growth at 20°C was observed for C. xerosis, C. freneyi, and C. hansenii but not for C. amycolatum. On the other hand, C. xerosis and C. hansenii did not ferment glucose at 42°C, whereas C. freneyi and C. amycolatum were positive by this test (Table 2). C. xerosis, as well as the other three species included in this study, expressed esterase lipase (C8) activity and were able to ferment glucose, ribose, and maltose, but none fermented xylose, mannitol, fructose, or glycogen. None produced pyrrolidonyl arylamidase, β-galactosidase, α-galactosidase, β-glucuronidase, β-glucosidase, α-mannosidase, α-fucosidase, N-acetyl-β-glucosaminidase, valine arylamidase, cystine arylamidase, trypsin, or chymotrypsin, and none hydrolyzed esculin or urea. The biochemical characteristics with different results among the four species analyzed in this study are shown in Table 2.
TABLE 2.
Distinctive biochemical characteristics of C. xerosis, C. freneyi, C. amycolatum, and C. hansenii
| Characteristica | % Positive reactions |
|||
|---|---|---|---|---|
| C. xerosis (n = 20) | C. freneyi (n = 7) | C. amycolatum (n = 9) | C. hansenii (n = 1) | |
| Fermentation of glucose at 42°C | 0 | 100 | 100 | 0 |
| Growth at 20°C | 100 | 100 | 0 | 100 |
| API Coryne | ||||
| Nitrate reduction | 90 | 71 | 44 | 0 |
| Fermentation of sucrose | 100 | 100 | 56 | 100 |
| Production of: | ||||
| Alkaline phosphatase | 100 | 100 | 100 | 0 |
| Pyrazinamidase | 100 | 100 | 89 | 100 |
| α-Glucosidase (pH 7.4) | 100 | 86 | 0 | 0 |
| API 50CH (fermentation of:) | ||||
| Glycerol | 17 | 0 | 100 | 100 |
| Erythritol | 5 | 0 | 0 | 0 |
| d-Arabinose | 5 | 0 | 0 | 0 |
| l-Arabinose | 5 | 0 | 11 | 0 |
| Adonitol | 5 | 0 | 0 | 0 |
| Galactose | 94 | 100 | 44 | 100 |
| d-Mannose | 84 | 83 | 100 | 100 |
| l-Sorbose | 0 | 16 | 0 | 0 |
| Rhamnose | 17 | 16 | 0 | 0 |
| Dulcitol | 0 | 16 | 0 | 0 |
| Sorbitol | 0 | 16 | 0 | 0 |
| α-Methyl-d-mannoside | 5 | 0 | 0 | 0 |
| α-Methyl-d-glucoside | 5 | 0 | 0 | 0 |
| N-Acetyl-glucosamine | 11 | 0 | 0 | 0 |
| Amygdaline | 27 | 16 | 11 | 0 |
| Arbutin | 17 | 33 | 0 | 0 |
| Salicin | 22 | 16 | 11 | 0 |
| Cellobiose | 11 | 33 | 33 | 0 |
| Melibiose | 5 | 0 | 22 | 0 |
| Trehalose | 99 | 100 | 56 | 100 |
| Inulin | 52 | 16 | 0 | 0 |
| d-Raffinose | 0 | 0 | 11 | 0 |
| Amidon | 22 | 33 | 11 | 0 |
| β-Gentiobiose | 27 | 16 | 0 | 0 |
| d-Turanose | 83 | 83 | 0 | 0 |
| d-Lyxose | 5 | 0 | 0 | 0 |
| d-Tagatose | 5 | 16 | 0 | 0 |
| d-Fucose | 11 | 0 | 0 | 0 |
| l-Fucose | 22 | 16 | 0 | 0 |
| d-Arabitol | 0 | 16 | 0 | 0 |
| l-Arabitol | 0 | 16 | 0 | 0 |
| Gluconate | 11 | 83 | 0 | 0 |
| 2-Ketogluconate | 5 | 16 | 0 | 0 |
| 5-Ketogluconate | 99 | 83 | 0 | 0 |
| API ZYM | ||||
| Esterase (C4) | 100 | 83 | 100 | 100 |
| Lipase (C14) | 61 | 66 | 89 | 0 |
| Leucine arylamidase | 99 | 100 | 89 | 100 |
| Acid phosphatase | 0 | 0 | 89 | 0 |
| Naphthol-AS-BI-phosphohydrolase | 61 | 16 | 67 | 0 |
Those tests that can be useful for the differentiation of C. xerosis strains from those of C. freneyi, C. amycolatum, and C. hansenii are in bold format.
C. xerosis exhibited large to moderate amounts of the fatty acids C16:0 (10.6 to 16.2%), C18:0 (15.8 to 9.2%), C18:1ϖ9c (58.8 to 49.7%), and C18:2ϖ6,9c/anteiso-C18:0 (7.4 to 19.5%). These fatty acids were also detected in similar relative percentages as predominant fatty acids in C. freneyi, C. amycolatum, and C. hansenii. The fatty acid C17 present in small amounts in C. xerosis (0.0 to 5.5%) was also detected in C. freneyi (6.7 to 13.3%), C. amycolatum (2.4 to 18.5%), and C. hansenii (0.4%). The fatty acids C14:0, C15:0, C16:1ϖ7c, and C17:1ϖ8c were not detected in C. xerosis, but they were present in C. freneyi and C. amycolatum. In addition, the fatty acid C16:1ϖ9c detected in C. xerosis (0.7 to 2.0%) was not present in C. freneyi and the fatty acid C17:1ϖ8c was present in C. hansenii.
DISCUSSION
C. xerosis is rarely isolated from clinical samples, and most of the previously identified isolates were subsequently recognized as C. amycolatum (9). Thus, most of the studies that have analyzed the phenotypic or genetic characteristics of C. xerosis have included a very limited number of strains (7, 20). In the present work, most (n = 17; Table 1) of the animal clinical strains suspected to be C. xerosis belonged to this species. To our knowledge, this represents the largest phenotypic and genetic study of C. xerosis strains. Moreover, we describe the first isolation of C. freneyi from animals (strain M3; Table 1). Previously, C. freneyi had only been isolated from human clinical samples (3, 7, 19). Macroscopically, C. xerosis gave dry, rough, and yellowish pigmented colonies that are considered typical for this microorganism (7); however, colonies of C. xerosis also had a wrinkled surface (100% of the strains; Table 1) and were whitish-grayish (50% of the strains; Table 1). These last characteristics had not been reported for this species before. The macroscopic characteristics of the colonies obtained with C. freneyi, C. amycolatum, and C. hansenii strains were in agreement with those previously described for these species (7, 20, 26). Therefore, the colonies formed by C. xerosis were very similar to those formed by C. freneyi, C. amycolatum, and C. hansenii and the macroscopic characteristics of colonies do not seem to be very useful for the presumptive differentiation of clinical strains of C. xerosis.
Most of the biochemical characteristics exhibited by C. xerosis were also in agreement with the current description of this species, which is based on two strains (ATCC 373T and ATCC 7711) (6, 9, 19). The most significant discrepancy was that most of the C. xerosis strains reduced nitrate (90%; Table 2), and in our opinion, this trait should now be considered positive and not variable, as it has been considered so far (8). This result is relevant because reduction of nitrate is one of the key characteristics commonly used for the differentiation of Corynebacterium species (6, 8). The biochemical characteristics of the C. freneyi, C. amycolatum, and C. hansenii strains in the present study matched those previously described for these species (9, 19, 21, 26). Using the API Coryne strips, which are widely used for the identification of coryneform microorganisms from clinical samples (1, 8, 10, 22), all of the strains of C. xerosis analyzed gave the numerical profile 3110325 or 2110325 (Table 1). Both of these numerical profiles were also exhibited by most of the C. freneyi strains (Table 1), which is in agreement with previous results (19). C. xerosis could be differentiated from C. amycolatum by the failure of this species to produce α-glucosidase and from C. hansenii because this species does not reduce nitrate and does not produce the enzymes alkaline phosphatase and α-glucosidase (Tables 1 and 2). However, atypical strains of C. xerosis cannot express α-glucosidase activity (26) and some strains of C. amycolatum can be α-glucosidase positive (9, 19, 26), which would limit the utility of this test. Likewise, 10% of the C. xerosis strains did not reduce nitrate (numerical code 2110325; Table 1). Thus, according to these data, the API Coryne system may not be sufficient for the accurate identification of C. xerosis. No characteristics distinguishing between C. xerosis and C. freneyi were found by using either the API 50 CH or the API ZYM system, and only glucose fermentation at 42°C would be a reliable test to differentiate these two species (Table 2). C. xerosis could still be distinguished from C. amycolatum and C. hansenii because these species do not produce acid from d-turanose and 5-ketogluconate when tested with API 50 CH strips (Table 2). The growth of C. xerosis at 20°C and its inability to ferment glucose at 42°C or produce acid phosphatase (API ZYM) could also help in its differentiation from C. amycolatum (Table 2). Overall, after the extensive biochemical characterization, several tests (underlined in Table 2) could be useful to improve the identification of C. xerosis in clinical microbiology laboratories. Phenotypic identification of those C. xerosis strains with atypical results for these characteristics (26) (Table 2) could be obtained by whole-cell fatty acid analysis, which is a useful tool for the identification of corynebacteria (23). C. xerosis can easily be differentiated from C. freneyi and C. amycolatum by the lack of the fatty acids C14:0, C15:0, C16:1ϖ7c, and C17:1ϖ8c. C. xerosis and C. hansenii can be differentiated by the presence of the fatty acid C17:1ϖ8c in the latter species.
Unlike cellular fatty acid analysis, which is not available for routine diagnostics in most clinical laboratories, sequencing of the 16S rRNA gene is probably the most used molecular technique for identifying unusual bacteria in clinical microbiology (4, 15). Alternatively, partial rpoB gene sequencing is a successful and straightforward approach for discerning closely related Corynebacterium species (13, 14). Data obtained by 16S rRNA gene and partial rpoB gene analyses were congruent, and the phylogenetic trees constructed with both genes using the neighbor-joining method placed all of the C. xerosis strains in a separate branch clearly different from those formed by C. freneyi, C. amycolatum, and C. hansenii (Fig. 2 and 3). However, deeper branches were obtained using partial rpoB gene sequences as a result of the higher degree of polymorphism of the rpoB sequences than the 16S rRNA gene sequences (13, 14). Thus, the average similarities of partial rpoB gene sequences of C. xerosis strains compared to the partial rpoB gene sequences of the C. freneyi, C. amycolatum, and C. hansenii type strains were 94.6%, 85.7%, and 94.7%, respectively, whereas the average similarities for the almost complete 16S rRNA gene sequences were 99.1%, 97.1%, and 99.0%, respectively. A similarity of ≥95% based on the rpoB sequences has been proposed as a cutoff value for differentiating Corynebacterium species (14). These results support the utility of partial rpoB gene sequencing as an alternative to the 16S rRNA gene sequencing, allowing better discrimination of C. xerosis from closely phylogenetically related Corynebacterium species. Alternatively, C. xerosis can also be differentiated from C. freneyi and C. amycolatum by RFLP analysis of the 16S-23S rRNA gene IGS. All of the C. xerosis strains displayed the same pattern (Fig. 1, lanes 5 to 22), which could be distinguished from those obtained for C. freneyi and C. amycolatum (Fig. 1, lanes 1 to 3). In contrast, the digestion profiles of the C. xerosis strains and C. hansenii CCUG 53252T were identical (Fig. 1, lanes 4 to 22), which agrees with previous results (20).
The initial description of C. xerosis from animals included strains from pigs and one goat isolated from different clinical specimens (25). In the present study, nine additional C. xerosis strains (Table 1) were recovered from different clinical samples from pigs, one cow, and one sheep, expanding the range of animals and specimens from which this microorganism has been isolated to date. These data, together with the difficulty of its correct phenotype-based identification in routine clinical microbiology laboratories, may imply that the clinical significance of C. xerosis in veterinary medicine could be higher than currently considered. Therefore, the results of the present study can contribute to the better recognition and identification of this microorganism in clinical samples in veterinary laboratories, thereby allowing better knowledge of its distribution and clinical relevance as an animal pathogen.
Acknowledgments
We thank Juncal Fernández-Garayzábal for her assistance with the English reviewing of the manuscript.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Almuzara, M. N., C. De Mier, C. R. Rodríguez, A. M. Famiglietti, and C. A. Vay. 2006. Evaluation of API Coryne System, version 2.0, for diphteroid [sic] gram-positive rods identification with clinical relevance. Rev. Argent. Microbiol. 38:197-201. [PubMed] [Google Scholar]
- 2.Aubel, D., F. N. Renaud, and J. Freney. 1997. Genomic diversity of several Corynebacterium species identified by amplification of the 16S-23S rDNA gene spacer region. Int. J. Syst. Bacteriol. 47:767-772. [Google Scholar]
- 3.Auzias, A., C. Bollet, R. Ayari, M. Drancourt, and D. Raoult. 2003. Corynebacterium freneyi bacteremia. J. Clin. Microbiol. 41:2777-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarridge, J. E. 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17:840-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Garayzábal, J. F., M. D. Collins, R. A. Hutson, F. Fernández, R. Monasterio, R. Marco, and L. Domínguez. 1997. Corynebacterium mastitidis sp. nov., isolated from milk of sheep with subclinical mastitis. Int. J. Syst. Bacteriol. 47:1082-1085. [DOI] [PubMed] [Google Scholar]
- 6.Funke, G., and K. A. Bernard. 2003. Coryneform gram-positive rods, p. 472-501. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 7.Funke, G., and R. Frodl. 2008. Comprehensive study of Corynebacterium freneyi strains and extended and emended description of Corynebacterium freneyi Renaud, Aubel, Riegel, Meugnier, and Bollet 2001. J. Clin. Microbiol. 46:638-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funke, G., A. von Graevenitz, J. E. Clarridge, and K. A. Bernard. 1997. Clinical microbiology of coryneform bacteria. Clin. Microbiol. Rev. 10:125-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funke, G., P. A. Lawson, K. A. Bernard, and M. D. Collins. 1996. Most Corynebacterium xerosis strains identified in the routine clinical laboratory correspond to Corynebacterium amycolatum. J. Clin. Microbiol. 34:1124-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funke, G., F. N. Renaud, J. Freney, and P. Riegel. 1997. Multicenter evaluation of the updated and extended API (RAPID) Coryne database 2.0. J. Clin. Microbiol. 35:3122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guindon, S., and O. Gascuel. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696-704. [DOI] [PubMed] [Google Scholar]
- 12.Jensen, M. A., J. A. Webster, and N. Straus. 1993. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl. Environ. Microbiol. 59:945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khamis, A., D. Raoult, and B. La Scola. 2004. rpoB gene sequencing for identification of Corynebacterium species. J. Clin. Microbiol. 42:3925-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khamis, A., D. Raoult, and B. La Scola. 2005. Comparison between rpoB and 16S rRNA gene sequencing for molecular identification of 168 clinical isolates of Corynebacterium. J. Clin. Microbiol. 43:1934-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiratisin, P., L. Li, P. R. Murray, and S. H. Fischer. 2003. Identification of bacteria recovered from clinical specimens by 16S rRNA gene sequencing. Eur. J. Clin. Microbiol. Infect. Dis. 22:628-631. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 17.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 18.Quinn, P. J., M. E. Carter, B. Markey, and G. R. Carter. 1999. Corynebacterium species and Rhodococcus equi, p. 137-143. In P. J. Quinn, M. E. Carter, B. Markey, and G. R. Carter (ed.), Clinical veterinary microbiology. Mosby, Edinburgh, United Kingdom.
- 19.Renaud, F. N. R., D. Aubel, P. Riegel, H. Meugnier, and C. Bollet. 2001. Corynebacterium freneyi sp. nov., α-glucosidase-positive strains related to Corynebacterium xerosis. Int. J. Syst. Evol. Microbiol. 51:1723-1728. [DOI] [PubMed] [Google Scholar]
- 20.Renaud, F. N. R., A. Le Coustumier, N. Wilhem, D. Aubel, P. Riegel, C. Bollet, and J. Freney. 2007. Corynebacterium hansenii sp. nov., an α-glucosidase-negative bacterium related to Corynebacterium xerosis. Int. J. Syst. Evol. Microbiol. 57:1113-1116. [DOI] [PubMed] [Google Scholar]
- 21.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 22.Soto, A., J. Zapardiel, and F. Soriano. 1994. Evaluation of the API Coryne for identifying coryneform bacteria. J. Clin. Pathol. 47:756-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Velde, S., K. Lagrou, K. Desmet, G. Wauters, and J. Verhaegen. 2006. Species identification of corynebacteria by cellular fatty acid analysis. Diagn. Microbiol. Infect. Dis. 54:99-104. [DOI] [PubMed] [Google Scholar]
- 24.Vela, A. I., A. Mateos, M. D. Collins, V. Briones, R. Hutson, L. Domínguez, and J. F. Fernández-Garayzábal. 2003. Corynebacterium suicordis sp. nov., from pigs. Int. J. Syst. Evol. Microbiol. 53:2027-2031. [DOI] [PubMed] [Google Scholar]
- 25.Vela, A. I., E. Gracia, A. Fernández, L. Domínguez, and J. F. Fernández-Garayzábal. 2006. Isolation of Corynebacterium xerosis from animal clinical specimens. J. Clin. Microbiol. 44:2242-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wauters, G., B. Van Bosterhaut, M. Janssens, and J. Verhaegen. 1998. Identification of Corynebacterium amycolatum and other nonlipophilic fermentative corynebacteria of human origin. J. Clin. Microbiol. 36:1430-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]