Abstract
Enterotoxigenic Escherichia coli (ETEC) is a major cause of childhood diarrhea. The present study sought to determine the prevalence and distribution of toxin types, colonization factors (CFs), and antimicrobial susceptibility of ETEC strains isolated from Peruvian children. We analyzed ETEC strains isolated from Peruvian children between 2 and 24 months of age in a passive surveillance study. Five E. coli colonies per patient were studied by multiplex real-time PCR to identify ETEC virulence factors. ETEC-associated toxins were confirmed using a GM1-based enzyme-linked immunosorbent assay. Confirmed strains were tested for CFs by dot blot assay using 21 monoclonal antibodies. We analyzed 1,129 samples from children with diarrhea and 744 control children and found ETEC in 5.3% and 4.3%, respectively. ETEC was more frequently isolated from children >12 months of age than from children <12 months of age (P < 0.001). Fifty-two percent of ETEC isolates from children with diarrhea and 72% of isolates from controls were heat-labile enterotoxin (LT) positive and heat-stable enterotoxin (ST) negative; 25% and 19%, respectively, were LT negative and ST positive; and 23% and 9%, respectively, were LT positive and ST positive. CFs were identified in 64% of diarrheal samples and 37% of control samples (P < 0.05). The most common CFs were CS6 (14% and 7%, respectively), CS12 (12% and 4%, respectively), and CS1 (9% and 4%, respectively). ST-producing ETEC strains caused more severe diarrhea than non-ST-producing ETEC strains. The strains were most frequently resistant to ampicillin (71%) and co-trimoxazole (61%). ETEC was thus found to be more prevalent in older infants. LT was the most common toxin type; 64% of strains had an identified CF. These data are relevant in estimating the burden of disease due to ETEC and the potential coverage of children in Peru by investigational vaccines.
Enterotoxigenic Escherichia coli (ETEC) is one of the main causes of diarrhea in children from developing countries and in adult travelers from industrialized countries to the developing world (16, 21). According to the World Health Organization (WHO), ETEC is the second most common cause of diarrhea after rotavirus in children less than 5 years of age and is therefore an important target for vaccine development (11). Diarrhea due to ETEC develops between 8 and 72 h after initial infection, usually due to the ingestion of contaminated food and water (21). The disease varies from a mild illness to one of great severity, usually without leukocytes or fecal blood but often with vomiting and, potentially, dehydration (10).
The ability of ETEC to adhere to and colonize the human intestinal mucosa has been correlated with the presence of specific antigenic fimbriae called colonization factors (CFs), which have been designated colonization factor antigens (CFAs), coli surface antigens (CSs), or putative colonization factors (PCFs), followed by a numeric designation. The CFs are mainly fimbrial or fibrillar proteins, although some are not fimbrial in structure (21). To date, over 25 human ETEC CFs have been described. In turn, these CFs have been divided into different families: (i) a CFA/I-like group including CFA/I, CS1, CS2, CS4, CS14, and CS17; (ii) a CS5-like group including CS5, CS7, CS18, and CS20; and (iii) a unique group including CS3, CS6, and CS10 to CS12 (8, 21, 33).
Following CF-mediated mucosal adhesion, ETEC elaborates one or both of two enterotoxins: heat-labile toxin (LT), a protein multimer which shares many features with cholera toxin and which binds to intracellular adenylylcyclase, leading to increased cyclic AMP levels, and/or heat-stable toxin (ST), a small-peptide molecule that similarly activates guanylylcyclase and which produces increased intracellular cyclic GMP. For both toxins, the increased chloride secretion resulting from these toxins produces a watery diarrhea (10, 16). Both of these virulence factors are plasmid encoded. ST is encoded by two different genes: estA and st1, which produce STh (originally isolated from ETEC in humans) and STp (originally from a pig isolate), respectively. LT toxin is encoded by the eltA and eltB genes (12). The diagnosis of ETEC infection relies upon the detection of either the genes themselves or their gene products in clinical specimens.
Currently, derivatives of LT and the CFs are targets for the development of vaccines against ETEC. However, the great variability of ETEC CFs requires determination of the CF types prevalent in different geographic locations (21, 33). The aims of this study were (i) to determine the clinical and epidemiological characteristics of ETEC diarrhea in Peruvian children, (ii) to determine the presence of ST and LT, (iii) to determine the presence and distribution of colonization factors in these strains, and (iv) to determine the antibiotic susceptibilities of these strains.
MATERIALS AND METHODS
Study design.
The specimens analyzed in this study were obtained as part of a prospective, passive surveillance cohort diarrhea study of children 2 to 24 months of age. Parents were asked to bring their children to the study clinic every time the children developed diarrhea that needed medical attention; there was no active surveillance at home for all diarrheal episodes. The study was conducted in periurban communities of Lima, Peru, between September 2006 and December 2007 (1,034 children) (18) and from January to July 2008 (529 children from the initial cohort were followed during this period).
Clinical data.
Clinical information on the diarrheal episodes was obtained from the cohort studies. We used a modified Vesikari score (23) to determine the severity of an ETEC-associated diarrhea episode. Elements of the score include the duration of diarrhea (in days; score, 0 to 3 points), the maximum number of stools per day during the episode (score, 1 to 3 points), the presence of vomiting (score, 0 or 1 point), the maximum number of emeses per day during the episode (score, 0 to 3 points), the presence of fever (score, 0 or 1 point), the presence of dehydration (score, 0 or 1 point), and treatment (score, 0 to 2 points). The maximum possible score was 14.
Strains.
A total of 1,102 samples from children with diarrhea (defined as three or more liquid or semiliquid stools in 24 h or a single bloody semiliquid stool in 24 h) and 742 samples from control children without diarrhea 1 week before and 1 week after the stool collection sample were analyzed. Samples were evaluated for common enteric pathogens (Shigella, Salmonella, Vibrio, Campylobacter, Giardia lamblia, Cryptosporidium, and rotavirus) by conventional methods. Five lactose-positive colonies were isolated from MacConkey plates and tested by multiplex PCR with specific DNA primers to detect virulence factors associated with enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), Shiga toxin-producing E. coli (STEC), enteroaggregative E. coli (EAEC), diffusely adherent E. coli (DAEC), and ETEC, as described previously (9), using a validated five-colony pool analysis method (1). Subsequently, individual colonies from positive ETEC samples were analyzed using separate PCR assays for the LT and ST genes. The primers used for detection of the ST genes were stIa-F (TTTCCCCTCTTTTAGTCAGTCAA), stIb-F (TGCTAAACCAGTAGAGTCTTCAAAA), and st-R (GCAGGATTACAACACAATTCACAGCAG); and those used for detection of the LT genes were lt-F (TCTCTATGTGCATACGGAGC) and lt-R (CCATACTGATTGCCGCAAT) (9). The number of positive ETEC colonies per sample varied from one to five. Two ST gene-positive and/or LT gene-positive colonies per sample (or one colony, if only a single positive colony was identified) were then selected for further analysis. The ETEC strains were stored at −70°C in Trypticase soy broth (TSB) containing 15% glycerol at the Naval Medical Research Center Detachment (NMRCD), Lima, Peru. Before the isolates were tested, one loop of bacteria from the frozen stock cultures was spread onto a MacConkey agar plate and was grown overnight at 37°C.
MAb dot blot assays.
Two colonies from MacConkey agar plates from each sample were inoculated onto a CFA agar plate with and without bile salts to test the phenotypic expression of CFs by a dot blot assay, as described previously (14, 26, 31). CFs were tested using 21 monoclonal antibodies (MAbs): MAbs to CFA/I, CS1 to CS7, CS12 (PCF0159), CS14 (PCF0166), CS17, CS18, CS20, CS8 (CFA/III), CS19, PCF039 (P19A6), PCF071 (P8C1), Ag150 (P1F2), Fim4264 (P3H5), Fim4089 (P5H10), and Fim7162 (P7H5). The MAbs were provided by A.-M. Svennerholm (University of Gothenburg, Gothenburg, Sweden) and S. Farid and H. Shaheen (NAMRU-3, Cairo, Egypt).
Toxin GM1-ELISA.
Two colonies from diarrheal samples and one colony from control samples were individually picked from each MacConkey plate using sterile toothpicks and inoculated onto GM1-coated enzyme-linked immunosorbent assay (ELISA) plates containing supplemented Luria-Bertani (LB) broth. A ganglioside GM1-specific ELISA (ganglioside GM1-ELISA) and an inhibition GM1-ELISA were performed to detect LT and ST, respectively, essentially as described previously (28, 29).
Antimicrobial susceptibility.
E. coli strains positive for ETEC genes were analyzed for their antimicrobial susceptibilities by disk diffusion, according to Clinical and Laboratory Standards Institute (CLSI) guidelines (5). The antibiotics analyzed were ampicillin (AMP; 10-μg disk), co-trimoxazole (SXT; 23.75/1.25-μg disk), tetracycline (TET; 30-μg disk), nitrofurantoin (NIT; 300-μg disk), nalidixic acid (NAL; 30-μg disk), chloramphenicol (CAF; 30-μg disk), ciprofloxacin (CIP; 5-μg disk), gentamicin (GTM; 10-μg disk), cefotaxime (CTX; 30-μg disk), amoxicillin-clavulanic acid (AMC; 30-μg disk), ceftazidime (CAZ; 30-μg disk), and azithromycin (AZD; 15-μg disk). Multidrug resistance (MDR) was defined as resistance to three or more classes of antimicrobial agents. Although azithromycin is commonly used as therapy for infections caused by some enteric pathogens, there are no approved resistance criteria for azithromycin for disk diffusion analysis of E. coli.
Ethical aspects.
The study was approved by the Institutional Review Boards of the Universidad Peruana Cayetano Heredia, the Instituto de Investigación Nutricional, Instituto Nacional de Salud del Niño, and NMRCD, all in Lima, in accordance with relevant Peruvian and U.S. federal regulations.
Statistical analysis.
The results were analyzed using the EpiInfo program (version 3.4.3; Centers for Disease Control and Prevention, Atlanta, GA). The chi-square test or Fisher's exact test was used for comparisons between groups, as appropriate. Student's t test or nonparametric tests were used in case of continuous variables. Differences were considered statistically significant if the P value was less than 0.05.
RESULTS
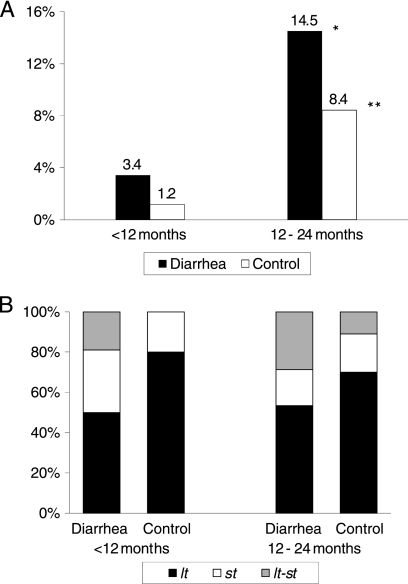
Between September 2006 and July 2008, we collected 1,129 samples from children with diarrhea and 744 samples from control children and found ETEC in 60 (5.3%) and 32 (4.3%) samples, respectively. ETEC was more commonly isolated from diarrheal samples than control samples, and it was isolated more frequently from children older than 12 months of age than younger children (14.5% and 3.4%, respectively; P < 0.001) (Fig. 1 A). Eighty-five strains out of 92 (92%) were available for further analyses.
FIG. 1.
(A) ETEC prevalence among children less than 12 months of age (n = 936 diarrheal samples and n = 424 control samples) and children 12 to 24 months of age (n = 193 diarrheal samples and n = 320 control samples). *, P < 0.001 for the comparison of ETEC prevalence between children <12 and 12 to 24 months of age for diarrheal samples (3.4% and 14.5%, respectively) and control samples (1.2% and 8.4%, respectively); **, P < 0.05 for the comparison of ETEC prevalence between diarrheal and control samples among children 12 to 24 months of age (14.5% and 8.4%, respectively). (B) Age distribution for toxin gene types of ETEC among diarrheal samples (n = 32 children <12 months of age and n = 28 children 12 to 24 months of age) and control samples (n = 5 children <12 months of age and n = 27 children 12 to 24 months of age).
LT-producing ETEC (ETEC-LT) strains were the most frequently detected strains in diarrheal and control samples (52% and 72%, respectively), followed by ST-producing ETEC (ETEC-ST) strains (25% and 19%, respectively) and ETEC-LT-ST strains (23% and 9%, respectively) (Fig. 1B).
We used a modified Vesikari score to determine the diarrhea severity and observed a tendency for severe diarrhea to be more frequently associated with ETEC-ST than ETEC-LT episodes (6.4 ± 2.3 and 5.2 ± 2.7, respectively); however, the difference was not significant (Table 1). Children with episodes due to ST-producing ETEC strains had a higher frequency of fever and vomiting and had a significantly greater rate of use of oral rehydration solutions (ORSs) (P < 0.05) (Table 1). The duration of diarrhea caused by ETEC-LT strains tended to be longer (up to 24 days).
TABLE 1.
Clinical characteristics of children with diarrhea episodes due to ETEC
| Characteristic | Result for the following source of infection: |
|||
|---|---|---|---|---|
| Single ETEC isolatesa (n = 38) | All ETEC isolates (n = 60) | ETEC-LTb (n = 31) | ETEC-STc (n = 15) | |
| Mean ± SD age (mo) | 12.4 ± 6.4 | 12.3 ± 6.2 | 12.9 ± 6.6 | 10.4 ± 5.8 |
| Gender (% male) | 61 | 58 | 61 | 60 |
| Median (range) duration of diarrhea (days) | 3.5 (1-24) | 4.0 (1-24) | 3.0 (1-24) | 3.0 (1-12) |
| % of children with: | ||||
| Persistent diarrhea | 11 | 8 | 6 | 0 |
| Blood in stools | 11 | 12 | 10 | 7 |
| Fever | 34 | 38 | 32 | 53 |
| Median (range) maximum no. of stools/day | 5.0 (3-11) | 5.0 (3-14) | 5.0 (3-10) | 4.0 (3-14) |
| % of children with vomiting | 29 | 35 | 32 | 40 |
| Median (range) maximum no. of vomiting occurrences/day | 2.0 (1-5) | 2.0 (1-7) | 2.0 (1-7) | 2.5 (1-5) |
| % of children who received ORSs | 39 | 40 | 23 | 60d |
| Mean ± SD severity scoree | 5.7 ± 2.5 | 6.0 ± 2.6 | 5.2 ± 2.7 | 6.4 ± 2.3 |
Excluding coinfections.
Including coinfections with EPEC (n = 2), DAEC (n = 1), EAEC (n = 1), Campylobacter (n = 7), and rotavirus (n = 2).
Including coinfections with EAEC (n = 3), Campylobacter (n = 3), and rotavirus (n = 2).
P < 0.05 for the comparison between ETEC-ST and ETEC-LT.
Modified Vesikari score.
Coinfections were identified in 37% (22/60) of ETEC diarrheal samples and 25% (8/32) of control samples, including coinfections with EPEC (8% and 9%, respectively), EAEC (7% and 13%, respectively), DAEC (2% and 3%, respectively), Campylobacter (20% and 6%, respectively), and Shigella (2% and 0%, respectively). Rotavirus was found in 10% (4/39) of diarrheal samples; control samples were not tested for rotavirus.
CFs were more frequently detected in ETEC strains from diarrheal samples than in strains from control samples (64% and 37%, respectively; P < 0.05). The most common CFs were CS6 (14% and 7%, respectively), CS12 (12% and 4%, respectively), and CS1 (9% and 4%, respectively) (Table 2). ETEC-ST strains showed a higher frequency of detectable CFs than ETEC-LT strains (81% and 42%, respectively; P < 0.01) (Table 2). Of interest, CS6 was detected only in ETEC-ST strains.
TABLE 2.
Age and toxin gene distributions of colonization factors of ETEC strains
| CF type | No. (%) of children |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <12 mo |
12-24 mo |
All ages |
Toxin gene type |
All strains (n = 85) | ||||||
| Diarrhea (n = 32) | Control (n = 4) | Diarrhea (n = 26) | Control (n = 23) | Diarrhea (n = 58) | Control (n = 27) | LT (n = 48) | ST (n = 21) | LT-ST (n = 16) | ||
| CFA/I | 2 (6) | 1 (4) | 2 (3) | 1 (4) | 3 (14) | 3 (4) | ||||
| CS1 | 1 (3) | 4 (15) | 1 (4) | 5 (9) | 1 (4) | 1 (5) | 5 (31) | 6 (7) | ||
| CS5 | 3 (12) | 3 (5) | 3 (14) | 3 (4) | ||||||
| CS6 | 6 (19) | 2 (8) | 2 (9) | 8 (14) | 2 (7) | 10 (48) | 10 (12) | |||
| CS7 | 2 (6) | 1 (4) | 1 (4) | 3 (5) | 1 (4) | 4 (8) | 4 (5) | |||
| CS8 | 1 (3) | 1 (2) | 1 (2) | 1 (1) | ||||||
| CS12 | 2 (6) | 5 (19) | 1 (4) | 7 (12) | 1 (4) | 3 (6) | 5 (31) | 8 (9) | ||
| CS17 | 1 (3) | 1 (4) | 2 (9) | 2 (3) | 2 (7) | 4 (8) | 4 (5) | |||
| CS18 | 1 (4) | 1 (2) | 1 (2) | 1 (1) | ||||||
| CS19 | 1 (4) | 1 (4) | 1 (2) | 1 (1) | ||||||
| CS20 | 1 (4) | 1 (2) | 1 (2) | 1 (1) | ||||||
| PCF071 | 1 (3) | 1 (2) | 1 (2) | 1 (1) | ||||||
| Ag150 | 1 (3) | 1 (4) | 1 (2) | 1 (4) | 2 (4) | 2 (2) | ||||
| Fim4089 | 1 (3) | 1 (4) | 2 (3) | 2 (4) | 2 (2) | |||||
| Mixed CFsa | 1 (3) | 0 (0) | 3 (12) | 1 (4) | 4 (7) | 1 (4) | 3 (6) | 1 (5) | 1 (6) | 5 (6) |
| CFs detectedb | 18 (56) | 0 (0) | 19 (73) | 10 (43) | 37 (64) | 10 (37)c | 20 (42) | 17 (81)d | 10 (63) | 47 (55) |
| CFs undetectede | 15 (47) | 4 (100) | 10 (38) | 14 (61) | 25 (43) | 18 (67) | 31 (65) | 5 (24) | 7 (44) | 43 (51) |
Five strains showed two CFs, including four from diarrheal samples: ETEC-LT with CS8 and Ag150, ETEC-LT with CS7 and CS18, ETEC-LT-ST with CS1 and CS12, and ETEC-ST with CS5 and CS6. Only one mixed-CF strain was isolated from a control sample: LT-ETEC with CS17 and CS19.
Including strains with mixed CFs.
P < 0.05 for the comparison between diarrheal and control samples for all ages.
P < 0.05 for the comparison between ST- and LT-producing strains.
CFs were undetected with the panel of 21 monoclonal antibodies used.
PCR showed that 56% (48/85) of all strains were ETEC-LT, 25% were ETEC-ST (21/85), and 19% (16/85) were ETEC-LT-ST. However, only 63% (53/85) were positive for one or both toxin types by GM1-ELISA. Among PCR-positive ETEC strains, 60% (29/48) expressed LT, 90% (19/21) expressed ST, and 31% (5/16) expressed both toxins (LT and ST).
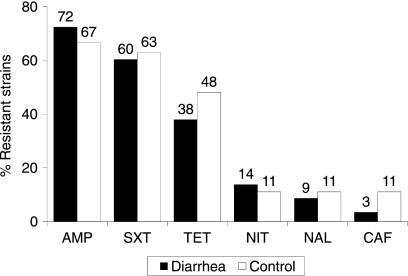
The ETEC strains isolated from diarrheal and control samples had high frequencies of antimicrobial drug resistance (Fig. 2). No resistance to ciprofloxacin, gentamicin, or cefotaxime was found among the diarrheal and control samples. The strains from 19% and 15% of diarrheal and control samples, respectively, were resistant to amoxicillin-clavulanate; however, 28% and 26% showed intermediate susceptibility to this drug. Although we identified no evidence of extended-spectrum beta-lactamase production, 19% and 3% of diarrheal samples were resistant to amoxicillin-clavulanate and ceftazidime, respectively. Multidrug resistance was common in diarrheal and control samples (34% and 48%, respectively). The most common multidrug resistance phenotypes were AMPr SXTr TETr (16 strains), AMPr SXTr TETr NITr (8 strains), AMPr SXTr NALr (2 strains), AMPr SXTr TETr (2 strains), AMPr TETr CAFr (2 strains), SXTr TETr NALr CAFr (2 strains), and AMPr SXTr TETr NALr (1 strain). The distribution of the growth-inhibitory zones for azithromycin by disc diffusion for 58 diarrheal strains and 27 control strains were as follows: 2% and 11%, respectively, had azithromycin inhibitory diameter zones of less than 10 mm; 57% and 19%, respectively, had zones of from 11 to 15 mm; 22% and 11%, respectively, had zones of from 16 to 20 mm, and 19% and 59%, respectively, had zones of more than 21 mm.
FIG. 2.
Antimicrobial resistance of ETEC strains from diarrheal (n = 58) and control (n = 27) samples by disk diffusion. There was no resistance noted to ciprofloxacin, gentamicin, or cefotaxime.
DISCUSSION
As in many other areas of the developing world, ETEC is one of the most common and severe etiologic agents associated with childhood diarrhea in Peru. We identified a prevalence of ETEC of 5.3% in children with diarrhea between 2 and 24 months of age. This prevalence rate is lower than the rates reported from other studies performed in developing countries: 38% in children <2 years of age in Nicaragua (19), 33% in children <1 year of age in Mexico (6), and 18% in children <5 years of age in Argentina (32). These differences may be related to the age of the population and the type of study (passive versus active surveillance and a community-based versus hospital-based study). The current study was a prospective, community-based passive surveillance of diarrhea cases in our outpatient facility.
We found the prevalence of ETEC to be similar in children without diarrhea (4%), showing that children are colonized with these pathogens early in life (18). The incidence of ETEC diarrhea in our sample was significantly more frequent in children >12 months of age than in younger children. In a similar study of children and adults in Bangladesh that included 37 children less than 9 years of age, the proportion of children infected with ETEC was higher in children under 2 years of age, with the prevalence decreasing as the age of the children increased, suggesting the development of immunity (3). In general, the prevalence of ETEC infection in developing countries decreases after 5 years of age (21), although there is a significantly higher ETEC isolation rate among older infants.
Among the ETEC strains isolated from diarrheal samples, the distribution of enterotoxin types was similar to that in other studies of childhood diarrhea, such as those conducted in Argentina (32) and India (27), although ST-producing ETEC predominated in other studies conducted in Bangladesh (22) and Egypt (25). These studies detected enterotoxins by phenotypic and not molecular methods, and therefore, the real frequency of LT may have been underestimated.
CS6, CS12, and CS1 were among the most prevalent CFs detected in this cohort study (12%, 9%, and 7%, respectively). CFA/I was present in only 4% of the isolates, in contrast to the findings for other developing countries, where CFA/I is the most prevalent CF type present in up to 21% of samples (17, 21, 25). Thus, variations in the prevalence of CF antigens may be related to location. CS6 was detected only in ETEC-ST strains; the ST-producing CS6 phenotype was the most prevalent among all strains (12%, 10/85), including those from diarrhea and control samples.
The local and regional enterotoxin type distributions are relevant for estimation of vaccine coverage against ETEC strains, as vaccines are mainly based on LT and common colonization factors (15). The success of an ETEC vaccine targeting infants and children in developing countries will depend on the use of a combination of maximally antigenic vaccine preparations and regimens for their delivery which will produce optimal immune responses to these antigens (33). To provide broad protection, an ETEC vaccine should contain the most prevalent fimbrial antigens, that is, CFA/I and CS1 to CS6 and/or an LT toxoid (30). Svennerholm and Tobias developed an oral vaccine consisting of inactivated ETEC strains with the CFs CFA/I and CS1 to CS5 plus the B subunit of recombinant cholera toxin (which is antigenically related to LT toxin) (30). This vaccine had good efficacy in adult travelers, but the results of studies with children were disappointing (13, 20). Using a prototype vaccine (with LT toxoid, CFA/I, and CS1 to CS6) as a model and the data from the current study, the estimated vaccine coverage rate in children in Lima will be 95% (81/85) (48 LT-producing strains plus 11 LT-ST-producing strains [whose CFs are not included in the vaccine CFs] and 22 strains whose CFs are included in the vaccine CFs).
In this study, 51% of all ETEC strains did not express a known CF type (determined using 21 monoclonal antibodies). Blackburn et al. recently reported that 58% of ETEC strains were positive for an Escherichia coli common pilus (ECP), a percentage that is even higher than that for the rate of positivity for the most prevalent CFs and that is independent of the presence of CFs (4). This suggests an important role for ECP in the biology of ETEC, particularly in CF-negative strains and in human infections (4). Further studies are needed to determine the role of ECP in our sample.
We did not find a good correlation between the results of PCR and GM1-ELISA for the detection of ETEC toxins; 37% of PCR-positive strains were negative by GM1-ELISA. It is possible that either the eltB or the estA gene is present as a silent gene or, alternatively, that the levels of expression of the genes for these toxins are so low that the toxins are not detected by the ELISA method. The probability of phenotypic silencing may increase even more during storage and recultivation (26). Sjöling et al. have shown the multiplex PCR for toxins to be an efficient method for the detection of LT and ST, having better sensitivity and specificity than the ELISA method. However, the selection of either method for a given laboratory depends on the availability of equipment and resources (26).
Antibiotic resistance, mainly to common antibiotics such as ampicillin (72%) and co-trimoxazole (60%), was widespread among the ETEC strains in the diarrheal samples. This finding is similar to descriptions of the susceptibilities of strains from children in Argentina (75% and 64%, respectively) (2), Egypt (63% and 52%, respectively) (24), and Mexico (73% and 65%, respectively) (7). In children with acute gastroenteritis, use of specific antimicrobial drugs should be limited to well-defined bacterial agents. If ETEC is suspected or confirmed, especially for traveler's diarrhea in Peru, high levels of resistance should preclude the empirical use of ampicillin or co-trimoxazole. Ciprofloxacin remains appropriate as empirical therapy for adults, but the 10% of strains resistant to nalidixic acid remind us of the importance of ongoing surveillance.
There are some methodological limitations in our study. Some patients may have been ill with viral enterocolitis caused by a virus other than rotavirus (i.e., adenovirus, astrovirus, or sapovirus), leading to a potential underestimation of the number of mixed ETEC-virus infections. Because this was a passive surveillance study, we may lack information on milder illnesses not requiring medical attention and thus underestimate the total ETEC diarrhea prevalence in this population. Not all currently described CFs were evaluated in this study, although we have included the classical CFs evaluated in most vaccine coverage studies. Conversely, there are important advantages of this study design. The sample is representative of the overall study population, and the combined use of phenotypic and genotypic methods may provide a better estimate of the local ETEC prevalence.
In summary, ETEC was isolated more frequently from older infants; LT was the most common toxin type, and two-thirds of the strains had an identified CF. These data are relevant in estimating the current burden of disease due to ETEC and the potential coverage of children in Peru with investigational vaccines. Further intervention studies are needed to implement strategies to minimize the inappropriate use of antibiotics and the development of antimicrobial resistance.
Acknowledgments
T. J. Ochoa is supported by grant 1K01TW007405. This work has been partially funded by institutional research funds (Fondo Concursable) from the Universidad Peruana Cayetano Heredia and from the Instituto Nacional de Salud del Niño, Lima, Peru; Institutional Research Funds to C. F. Lanata; and work unit number 60000.000.0.B0017 from the United States Military Infectious Disease Research Program (MIDRP).
We thank Ann-Mari Svennerholm (University of Gothenburg) and Sami Farid and Hind Shaheen (NAMRU-3) for providing the monoclonal antibodies and David Cepeda for his technical assistance at NMRCD.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of the Navy, the U.S. Department of Defense, or the U.S. Government or that of the National Institutes of Health and the other funding institutions.
M. Bernal, R. Meza, E. R. Hall, and R. C. Maves are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
There is no conflict of interest for any of us.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Barletta, F., T. J. Ochoa, L. Ecker, A. I. Gil, C. F. Lanata, and T. G. Cleary. 2009. Validation of a five-colony pool analysis using multiplex real-time PCR for detection of diarrheagenic Escherichia coli. J. Clin. Microbiol. 47:1915-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binsztein, N., A. M. Picandet, R. Notario, E. Patrito, M. E. De Lesa, A. De Petris, D. Maurel, O. Nader, M. Rivas, M. Szefner, and M. Vergara. 1999. Antimicrobial resistance among species of Salmonella, Shigella, Escherichia, and Aeromonas isolated from children with diarrhea in 7 Argentinian centers. Rev. Latinoam. Microbiol. 41:121-126. [PubMed] [Google Scholar]
- 3.Black, R. E., M. H. Merson, B. Rowe, P. R. Taylor, A. R. Abdul Alim, R. J. Gross, and D. A. Sack. 1981. Enterotoxigenic Escherichia coli diarrhoea: acquired immunity and transmission in an endemic area. Bull. World Health Organ. 59:263-268. [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn, D., A. Husband, Z. Saldaña, R. A. Nada, J. Klena, F. Qadri, and J. A. Girón. 2009. Distribution of the Escherichia coli common pilus among diverse strains of human enterotoxigenic E. coli. J. Clin. Microbiol. 47:1781-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S18. Vol. 28, no. 1, January 2008. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Cravioto, A., R. E. Reyes, F. Trujillo, F. Uribe, A. Navarro, J. M. De La Roca, J. M. Hernández, G. Pérez, and V. Vázquez. 1990. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am. J. Epidemiol. 131:886-904. [DOI] [PubMed] [Google Scholar]
- 7.Estrada-García, T., J. F. Cerna, L. Paheco-Gil, R. F. Velázquez, T. J. Ochoa, J. Torres, and H. L. DuPont. 2005. Drug-resistant diarrheogenic Escherichia coli, Mexico. Emerg. Infect. Dis. 11:1306-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaastra, W., and A.-M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 9.Guion, C. E., T. J. Ochoa, C. M. Walker, F. Barletta, and T. G. Cleary. 2008. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J. Clin. Microbiol. 46:1752-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 11.Lanata, C. F., W. Mendoza, and R. E. Black. 2002. Improving diarrhoea estimates. WHO, Geneva, Switzerland. http://www.who.int/child_adolescent_health/documents/pdfs/improving_diarrhoea_estimates.pdf.
- 12.Lasaro, M. A., J. F. Rodrigues, C. Mathias-Santos, B. E. Guth, A. Balan, M. E. Sbrogio-Almeida, and L. C. Ferreira. 2008. Genetic diversity of heat-labile toxin expressed by enterotoxigenic Escherichia coli strains isolated from humans. J. Bacteriol. 190:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine, M. M. 2006. Enteric infections and the vaccines to counter them: future directions. Vaccine 24:3865-3873. [DOI] [PubMed] [Google Scholar]
- 14.López-Vidal, Y., J. J. Calva, A. Trujillo, A. Ponce de León, A. Ramos, A.-M. Svennerholm, and G. M. Ruiz-Palacios. 1990. Enterotoxins and adhesins of enterotoxigenic Escherichia coli: are they risk factors for acute diarrhea in the community? J. Infect. Dis. 162:442-447. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie, R., A. L. Bourgeois, S. A. Frech, D. C. Flyer, A. Bloom, K. Kazempour, and G. M. Glenn. 2007. Transcutaneous immunization with the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine 25:3684-3691. [DOI] [PubMed] [Google Scholar]
- 16.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nirdnoy, W., O. Serichantalergs, A. Cravioto, C. LeBron, M. Wolf, C. W. Hoge, A.-M. Svennerholm, D. N. Taylor, and P. Echeverria. 1997. Distribution of colonization factor antigens among enterotoxigenic Escherichia coli strains isolated from patients with diarrhea in Nepal, Indonesia, Peru, and Thailand. J. Clin. Microbiol. 35:527-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochoa, T. J., L. Ecker, F. Barletta, M. L. Mispireta, A. I. Gil, C. Contreras, M. Molina, I. Amemiya, H. Verastegui, E. R. Hall, T. G. Cleary, and C. F. Lanata. 2009. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from periurban areas in Lima, Peru. Clin. Infect. Dis. 49:1694-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paniagua, M., F. Espinoza, M. Ringman, E. Reizenstein, A.-M. Svennerholm, and H. Hallander. 1997. Analysis of incidence of infection with enterotoxigenic Escherichia coli in a prospective cohort study of infant diarrhea in Nicaragua. J. Clin. Microbiol. 35:1404-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadri, F., T. Ahmed, F. Ahmed, R. Sack, D. A. Sack, A.-M. Svennerholm, and the PTE Study Group. 2003. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi children 18-36 months of age. Vaccine 21:2394-2403. [DOI] [PubMed] [Google Scholar]
- 21.Qadri, F., A.-M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qadri, F., A. Saha, T. Ahmed, A. Al Tarique, Y. A. Begum, and A.-M. Svennerholm. 2007. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect. Immun. 75:3961-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruuska, T., and T. Vesikari. 1990. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand. J. Infect. Dis. 22:259-267. [DOI] [PubMed] [Google Scholar]
- 24.Shaheen, H. I., S. B. Khalil, M. R. Rao, R. Abu Elyazeed, T. F. Wierzba, L. F. Peruski, S. Putnam, A. Navarro, B. Z. Morsy, A. Cravioto, J. D. Clemens, A.-M. Svennerholm, and S. J. Savarino. 2004. Phenotypic profiles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J. Clin. Microbiol. 42:5588-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaheen, H. I., I. A. Abdel Messih, J. D. Klena, A. Mansour, Z. El-Wakkeel, T. F. Wierzba, J. W. Sanders, S. B. Khalil, D. M. Rockabrand, M. R. Monteville, P. J. Rozmajzl, A.-M. Svennerholm, and R. W. Frenck. 2009. Phenotypic and genotypic analysis of enterotoxigenic Escherichia coli in samples obtained from Egyptian children presenting to referral hospitals. J. Clin. Microbiol. 47:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöling, A., G. Wiklund, S. J. Savarino, D. I. Cohen, and A.-M. Svennerholm. 2007. Comparative analyses of phenotypic and genotypic methods for detection of enterotoxigenic Escherichia coli toxins and colonization factors. J. Clin. Microbiol. 45:3295-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommerfelt, H., H. Steinsland, H. M. Grewal, G. I. Viboud, N. Bhandari, W. Gaastra, A.-M. Svennerholm, and M. K. Bhan. 1996. Colonization factors of enterotoxigenic Escherichia coli isolated from children in north India. J. Infect. Dis. 174:768-776. [DOI] [PubMed] [Google Scholar]
- 28.Svennerholm, A.-M., and G. Wiklund. 1983. Rapid GM1-enzyme-linked immunosorbent assay with visual reading for identification of Escherichia coli heat-labile enterotoxin. J. Clin. Microbiol. 17:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svennerholm, A.-M., M. Wikström, M. Lindblad, and J. Holmgren. 1986. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svennerholm, A.-M., and J. Tobias. 2008. Vaccines against enterotoxigenic Escherichia coli. Expert Rev. Vaccines 7:795-804. [DOI] [PubMed] [Google Scholar]
- 31.Viboud, G. I., N. Binsztein, and A.-M. Svennerholm. 1993. Characterization of monoclonal antibodies against putative colonization factors of enterotoxigenic Escherichia coli and their use in an epidemiological study. J. Clin. Microbiol. 31:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viboud, G. I., M. J. Jouve, N. Binsztein, M. Vergara, M. Rivas, M. Quiroga, and A.-M. Svennerholm. 1999. Prospective cohort study of enterotoxigenic Escherichia coli infections in Argentinean children. J. Clin. Microbiol. 37:2829-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker, R. I., D. Steele, T. Aguado, and Ad Hoc ETEC Technical Expert Committee. 2007. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 25:2545-2566. [DOI] [PubMed] [Google Scholar]