Abstract
Chlamydophila psittaci causes respiratory disease in poultry and can be transmitted to humans. We conducted a C. psittaci zoonotic risk assessment study of a chicken and turkey slaughterhouse. Eighty-five percent of the slaughtered chicken flocks tested positive by PCR and culture. Genotype D was discovered. Fifty-seven percent of the slaughtered turkey flocks tested positive by PCR and culture. Genotype D was present. For the chicken slaughterhouse employees, 7.5% and 6% tested positive for C. psittaci by PCR and culture, respectively. In the turkey slaughterhouse, 87% and 61% of the employees tested positive by PCR and culture, respectively. All genotyped human samples contained genotype D. Using stationary bioaerosol monitoring by means of an MAS-100 ecosampler and ChlamyTrap collection medium, chlamydial DNA, and viable organisms were detected in both the chicken and turkey slaughterhouses. Positive air samples were most frequently found in the animal reception area and evisceration room. Zoonotic transmissions were very common, especially from processed turkeys. Accurate diagnostic monitoring and reporting of C. psittaci infections should be promoted in poultry workers.
Chlamydophila psittaci, an obligate intracellular bacterium, causes psittacosis or parrot fever in parrots, parakeets, lories, and cockatoos (Psittaciformes) and is well-known as a zoonotic agent. In nonpsittacine birds, including poultry, the disease is often called ornithosis or more generally chlamydiosis. C. psittaci infections occur in at least 465 bird species spanning 30 different bird orders. The symptoms in birds are conjunctivitis, rhinitis, dyspnea, nasal discharge, diarrhea, polyuria, anorexia, and dullness (the birds are fluffed and inactive) (26). The transmission of C. psittaci to humans occurs through inhalation of contaminated aerosols from respiratory and eye secretions or dried feces from a diseased animal or asymptomatic carrier. Handling the plumage and tissues of infected birds and, in rare cases, mouth-to-beak contact or biting also present zoonotic risks. C. psittaci can cause a respiratory infection in humans with highly variable clinical symptoms. The disease may vary from being unapparent, as also recognized by the Centers for Disease Control and Prevention (CDC) (9), to fatal (7) in untreated patients. Symptoms frequently include high fever (up to 40.5°C) accompanied by a relatively low pulse, chills, headache, myalgia, nonproductive coughing, and difficult breathing. The incubation period is usually 5 to 14 days, although periods of up to 1 month have been reported. The disease is rarely fatal in properly treated patients. Therefore, early diagnosis and awareness are important.
During the 1980s, the 1990s, and the last decade, outbreaks of parrot fever were reported in the North American (6, 10) and European (8, 13, 14, 17, 21, 24) poultry industries as well as in China (11), India (3), and Australia (18). Evidence of human infections associated with outbreaks in commercially raised turkeys and ducks does exist (2, 8). However, reports on C. psittaci outbreaks on chicken farms or reports on zoonotic transmissions linked to contact with C. psittaci-infected chickens are extremely rare (5). It could be the case that chickens rarely become infected and/or that the strains infecting chickens are less virulent, presenting a minor risk for humans. Recently, we investigated the occurrence of C. psittaci by performing a retrospective study with 300 serum samples collected in 2005 from 10 randomly selected chicken breeder, broiler, and layer farms in Belgium. We examined 10 serum samples from each farm using a recombinant enzyme-linked immunosorbent assay (ELISA) (30) for the detection of antibodies against the C. psittaci major outer membrane protein (MOMP); and we obtained 98, 95, and 95% seropositive results for layers, broilers, and breeders, respectively (unpublished results, 2009). Seropositive birds were found on all farms. Therefore, the statement that C. psittaci infections occur less frequently in chickens is not true, at least for Belgium. To study whether the strains circulating in chickens are less easily transmitted to humans, we examined zoonotic transmissions of C. psittaci in a chicken slaughterhouse and a turkey slaughterhouse. Incoming flocks as well as employees were sampled. Additionally, C. psittaci bioaerosol monitoring was performed using a recently developed personal and stationary (19) bioaerosol monitoring technique allowing the detection of chlamydial DNA as well as live organisms. The aim was to determine the zoonotic risk at different times and locations in the slaughterhouses.
MATERIALS AND METHODS
Study concept.
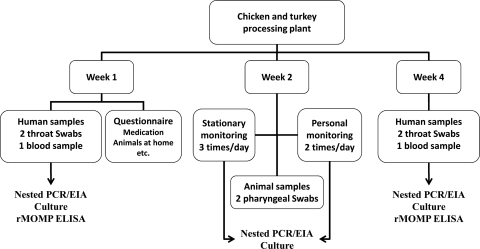
In October 2007, a C. psittaci zoonotic risk assessment study was conducted in a West Flanders, Belgium, chicken slaughterhouse. One month later the study was repeated in a West Flanders turkey slaughterhouse. The concept of each study was the following. During week 1, poultry workers on all working stations as well as members of the administrative personnel, all of whom provided informed consent, were medically examined (Fig. 1).
FIG. 1.
Study design.
They were asked to fill out a medical questionnaire designed to assess information on professional and nonprofessional activities, general health status, smoking habits, use of medication, allergies, and clinical signs specifically related to psittacosis. Participants provided a blood sample and two pharyngeal swab samples. Four weeks later, the medical examination and the sampling were repeated. Four scientists conducting this study were also medically examined and sampled at the same time points. Scientists wore a full-face P3 mask (3M 6800, EN 136 CL1; 3M, Diegem, Belgium) while having contact with poultry or poultry products.
Every day during week 2, all incoming flocks were tested for the presence of C. psittaci. For this purpose, we randomly selected 10 animals per flock and swabbed them pharyngeally. Stationary and personal bioaerosol monitoring was conducted daily at all workstations, including the administrative office. Stationary bioaerosol monitoring was performed at the start, in the middle, and at the end of the daily activity, while personal bioaerosol monitoring took place at the start and at the end of the daily activity. The study was approved by the Medical (approval EC UZG 2005/024) and Veterinary (approval EC 2005/20) Ethical Committees of Ghent University.
Sampling details and processing of samples prior to analyses.
Rayon-tipped aluminum-shafted swabs (Copan; Fiers, Kuurne, Belgium) were used to sample the pharynges of all participating humans during week 1 as well as during week 4. During week 2, the same type of swab was used for sampling the pharynges of arriving animals. Each time, two pharyngeal swab samples were taken. The first pharyngeal swab sample was deposed in 2 ml RNA/DNA stabilization reagent (Roche, Brussels, Belgium) and examined by a nested PCR-enzyme immunoassay (PCR-EIA). The second one, to be used for culture, was submersed in 2 ml chlamydia transport medium (25). The swabs were transported to the laboratory on ice and were stored at −80°C until they were processed.
During the medical examination at weeks 1 and 4, human blood samples were collected by venipuncture of the intermediate cubital vein using a Vacutainer system. Blood samples were stored overnight at room temperature. Serum for the detection of antichlamydial antibodies was obtained by centrifugation (325 × g, 10 min, 4°C) and was stored at −20°C until further testing.
During week 2, stationary bioaerosol monitoring was performed at different locations along the slaughter line: (i) the (live) poultry reception area, (ii) the plucking room, (iii) the evisceration line, (iv) the cutting room, (v) the packing line, and (vi) the administration area. Personal monitoring was performed at the same locations except the plucking room, as the plucking machine operated fully automatically. Stationary bioaerosol monitoring was performed using an MAS-100 ecosampler (Merck, Darmstadt, Germany), together with in-house-made semisolid collection medium, ChlamyTrap (19), at an airflow rate of 100 liters/min for 10 min. Personal bioaerosol monitoring was performed by use of an IOM personal inhalable dust sampler (SKC Inc., Eighty Four, PA) provided with a gelatin filter (3-μm pore size; SKC) at an airflow rate of 2 liters/min for 60 min.
Petri dishes with 20 ml semisolid collection medium were securely taped, stored on ice, and transported as such. Gelatin filters were gently removed from the personal sampling devices and transferred to sterile recipients before transport to the laboratory. The collection medium and filters were transported on ice. Each volume (20 ml) of collection medium was split in two. For the gelatin filter, 10 ml of sterile distilled water was added, followed by incubation at 37°C until the filter completely dissolved. Subsequently, the filter solution was split in two and added to either 5 ml chlamydia transport medium for culture or 5 ml RNA/DNA stabilization reagent (Roche) for nested PCR-EIA. All samples were ultracentrifuged (45,000 × g, 45 min) at 4°C. Pellets for culture were suspended in 500 μl chlamydia transport medium, while the ones for nested PCR-EIA were suspended in 198 μl standard (STD) buffer. The samples were stored at −80°C until further testing.
C. psittaci nested PCR-EIA.
DNA extraction from swabs and air samples was performed as described previously (22). Following DNA extraction, the samples from animals in each flock were pooled and tested as such. All human and air samples were tested individually. The presence of the C. psittaci outer membrane protein A (ompA) gene was examined using a nested PCR-EIA, as described by Van Loock et al. (22), by analyzing the results obtained by agarose gel electrophoresis (for swabs) and/or EIA (for air samples).
C. psittaci genotyping.
Human and animal samples positive by the nested PCR were genotyped using a microarray based on the ompA gene (15, 16).
C. psittaci culture.
The presence of viable C. psittaci cells in pharyngeal swab and air samples was examined by isolation of the cells in BGM cells and direct immunofluorescence staining at 6 days postinoculation (Imagen; Oxoid, Drongen, Belgium) (28). The numbers of C. psittaci-positive cells in randomly selected microscopic fields (magnification, ×400; Eclipse TE2000-E microscope; Nikon, Japan) were counted. A score from 0 to 4 was given (Table 1).
TABLE 1.
Isolation scores
| Score | Meaninga |
|---|---|
| 0 | No EBs or inclusions |
| 0.5 | 1-3 EBs |
| 1 | >3 EBs and/or 1-2 IPCs |
| 1.5 | 3-5 IPCs |
| 2 | >5 IPCs in less than one-quarter of the fields |
| 2.5 | IPCs in one-quarter of the fields |
| 3 | IPCs in one-half of the fields |
| 3.5 | Large IPCs in one-half of the fields |
| 4 | IPCs in all of the fields |
EBs, elementary bodies; IPCs, inclusion-positive cells.
Chlamydial antibody detection in human serum.
Human sera were examined with an adapted recombinant ELISA (rELISA) using the recombinant MOMP (rMOMP) antigen of a C. psittaci genotype D strain, as described previously (29, 30). rMOMP was produced in COS-7 cells (17). Sera were diluted 2-fold (starting at 1/100). Antibody titers were determined using rMOMP-coated ELISA plates and 1/500 dilutions of horseradish peroxidase-labeled goat anti-human IgG (H+L; Nordic Immunological Laboratories, Tilburg, Netherlands). The results were positive if the absorbance exceeded the cutoff value of the mean of three negative-control serum samples plus two times the standard deviation. Positive-control sera originated from three humans who were infected while visiting a C. psittaci-infected turkey farm (29).
Medical questionnaire.
A medical questionnaire was filled out. The questionnaire included some general questions about the work environment, including the working station(s) of the employee. The working stations were grouped according to the location on the slaughter line: “unclean” (before evisceration) and “clean” (from the evisceration and further steps). A question about contact with birds outside the work environment, e.g., pets, was also asked. Scores from 0 to 3 were given. A score of 0 meant no contact at all, a score of 1 meant rare contact with birds, a score of 2 meant weekly contact, and a score of 3 meant daily contact. Respiratory complaints such as wheezing, dyspnea, shortness of breath, sore throat, chest pain, runny nose, coughing, sneezing, and coughing up mucus were scored. A score of 0 indicated no complaints; and scores of 1, 2, and 3 were given when the complaint occurred once, several times, and regularly, respectively. Scores for respiratory complaints were summed to obtain one overall score for each patient. Two groups of ocular complaints were scored as described above, and again, the scores were added. Group 1 consisted of dry, itchy, and irritated eyes; and group 2 consisted of tired or painful eyes.
Statistical analyses were performed using an independent-sample t test (>30 subjects) or the Mann-Whitney nonparametric test (<30 subjects) (SPSS software, version 17; SPSS Inc., Chicago, IL). The results were considered significantly different at the level of a P value of <0.05.
RESULTS
C. psittaci in chickens and turkeys.
During week 2, pharyngeal swab samples from incoming chickens and turkeys, pooled for each flock, were tested by nested PCR. Subsequently, swab samples from all nested PCR-positive flocks were examined individually by culture to determine the presence and amount of live chlamydial organisms.
Eleven of 13 (85%) slaughtered chicken flocks tested positive by the nested PCR (Table 2), and all of the chickens were excreting live organisms, as demonstrated by culture (Table 2). In 5 of the 11 positive chicken flocks, genotype D was discovered. Genotyping was unsuccessful for the remaining six positive flocks. On every day from days 1 to 5, C. psittaci-positive chicken flocks were processed. The proportions of culture-positive chicken swab samples tested varied from 30 to 100%. Three of 11 (27%) chicken flocks (flocks 2A, 4A, and 4C) had been treated with antibiotics active against C. psittaci, albeit only at the start of the brood. Flocks 2A and 4C received enrofloxacin from production days 4 to 8, even though no clinical symptoms were observed. They were excreting live C. psittaci organisms when they arrived at the slaughterhouse. Flock 4A, negative when it arrived at the abattoir, had experienced colibacillosis and was treated with doxycycline from production days 6 to 8.
TABLE 2.
Information on slaughtered chicken flocks and results of C. psittaci detection in pharyngeal swab specimens from 10 animals from each flock
| Day | Flock | Na | Treated with antibiotics | Nested PCR result | Genotype | Mean isolation score ± SD (% positive chickens) |
|---|---|---|---|---|---|---|
| 1 | 1A | 6,160 | No | + | /b | 0.30 ± 0.42 (40) |
| 1 | 1B | 21,330 | No | + | / | 1.3 ± 0.26 (100) |
| 1 | 1C | 7,750 | No | − | NDc | ND |
| 2 | 2A | 21,060 | Yes | + | / | 0.60 ± 0.52 (60) |
| 2 | 2B | 19,380 | No | + | / | 0.5 ± 0.60 (50) |
| 3 | 3A | 19,300 | No | + | / | 1.1 ± 0.76 (70) |
| 3 | 3B | 15,000 | No | + | D | 1.0 ± 0.58 (90) |
| 3 | 3C | 12,500 | No | + | D | 0.55 ± 0.55 (60) |
| 4 | 4A | 27,000 | Yes | − | ND | ND |
| 4 | 4B | 18,600 | No | + | D | 1.2 ± 0.44 (100) |
| 4 | 4C | 15,660 | Yes | + | / | 0.78 ± 0.44 (89) |
| 5 | 5A | 22,770 | No | + | D | 0.30 ± 0.48 (30) |
| 5 | 5B | 18,750 | No | + | D | 0.50 ± 0.43 (67) |
N, number of animals per flock.
/, genotyping was not possible.
ND, not determined.
At the turkey abattoir, four of seven (57%) flocks tested positive by PCR (Table 3). All DNA-positive flocks were excreting live organisms, as demonstrated by culture. In one of the four positive turkey flocks, genotype D was discovered. Genotyping was unsuccessful for the remaining three positive flocks. On every day from days 1 to 5, with the exception of day 4, when the abattoir was closed, C. psittaci-positive turkey flocks were processed. The proportions of culture-positive turkeys per flock varied from 38 to 100%. Two out of seven processed turkey flocks (flocks 2A and 5A) were treated with enrofloxacin (during production weeks 4 and 3, respectively) and doxycycline (during production weeks 12 and 6, respectively). We could speculate that the treatment was effective, as no PCR-positive swabs were found in the treated turkey flocks. Although flock 5B was treated with doxycycline during week 13, 38% of the turkeys were excreting live C. psittaci organisms.
TABLE 3.
Information on slaughtered turkey flocks and results of C. psittaci detection in pharyngeal swab specimens from 10 animals from each flock
| Day | Flock | Na | Treated with antibiotics | Nested PCR result | Genotype | Mean isolation score ± SD (% positive turkeys) |
|---|---|---|---|---|---|---|
| 1 | 1A | 4,997 | No | + | /b | 1.3 ± 0.79 (100) |
| 2 | 2A | 5,610 | Yes | − | NDc | ND |
| 2 | 2B | NAd | No | + | / | 1.1 ± 0.68 (89) |
| 3 | 3A | 4,997 | No | + | D | 1.1 ± 0.89 (89) |
| 3 | 3B | 4,000 | No | − | ND | ND |
| 5 | 5A | NA | Yes | − | ND | ND |
| 5 | 5B | 6,000 | Yes | + | / | 0.56 ± 0.43 (38) |
N, number of animals per flock.
/, genotyping was not possible.
ND, not determined.
NA, not available.
Bioaerosol monitoring for C. psittaci.
At the chicken slaughterhouse, two to three culture-positive flocks, with a mean of 17,319 animals per flock, were processed each day. However, using stationary bioaerosol monitoring, chlamydial DNA was detected only on days 2, 4, and 5 (Table 4). Not all DNA-positive air samples were culture positive, as the culture scores ranged from 0 to 2.5. At the turkey slaughterhouse (days 1, 2, 3, and 5), only one culture-positive flock, with a mean number of 5,267 turkeys per positive flock, was processed each day. Nevertheless, on each day except day 5, stationary bioaerosol monitoring showed the presence of chlamydial DNA in the air (Table 4).
TABLE 4.
Nested PCR-EIA results for air samples from the chicken and turkey abattoirsa
| Location | Time of day of sampling | PCR-EIA resultb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chicken slaughterhouse |
Turkey slaughterhouse |
|||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 1 | Day 2 | Day 3 | Day 5 | ||
| Input area | Morning | − | − | − | + | + | − | − | − | − |
| Midday | − | + | − | + | + | + | − | + | − | |
| Evening | − | − | − | + | + | − | − | − | − | |
| Plucking machine | Morning | − | − | − | + | + | − | − | − | − |
| Midday | − | − | − | + | + | − | − | − | − | |
| Evening | − | − | − | + | + | − | + | − | − | |
| Evisceration line | Morning | − | − | − | + | + | − | + | − | − |
| Midday | − | − | − | + | + | − | − | − | − | |
| Evening | − | − | − | + | + | − | − | − | − | |
| Cutting room | Morning | − | − | − | + | + | − | + | − | − |
| Midday | − | − | − | + | + | − | − | − | − | |
| Evening | − | − | − | + | + | − | − | − | − | |
| Processing area | Morning | − | − | − | + | + | − | − | + | − |
| Midday | − | − | − | + | + | − | − | − | − | |
| Evening | − | − | − | + | + | − | − | − | − | |
| Administration | Morning | − | − | − | + | + | − | − | + | − |
| Midday | − | − | − | + | − | − | − | − | − | |
| Evening | − | − | − | + | + | − | − | − | − | |
Air samples were collected by means of stationary bioaerosol monitoring at different locations and times along the turkey slaughter line. Chicken carcasses were air chilled for 4 h and turkey carcasses were air chilled for 24 h before they went to the cutting room.
+, positive result; −, negative result.
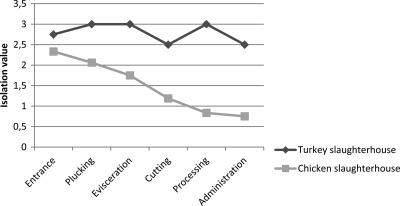
DNA-positive air samples were most frequently found at the chicken and turkey reception areas, and all were culture positive, with the isolation scores ranging from 2.5 to 3.0. All turkey abattoir workstations contained large amounts of viable C. psittaci organisms in the air (Fig. 2), indicating that live organisms were still present in the air sampled at the end of the slaughter line. Remarkably, even the administrative office tested positive for live organisms. In the chicken abattoir, live airborne organisms were mainly present at the point of input of living chickens, and the amount of live organisms in the air declined along the slaughter line (Fig. 2). Few viable organisms were present in the administrative office.
FIG. 2.
Total culture scores for air samples taken during week 2 of the study at different locations in the chicken or turkey abattoir.
All gelatin filters used for personal bioaerosol monitoring were negative.
C. psittaci zoonotic transmission.
Pharyngeal swab specimens from employees working in the chicken or turkey slaughterhouse were examined by both nested PCR and culture. Four of 53 (7.5%) chicken slaughterhouse employees tested positive for C. psittaci (Table 5). One of them was positive only by PCR, while the other three persons were positive by both PCR and culture. Genotyping of these four samples revealed no result. On the other hand, 33 of 38 (87%) turkey slaughterhouse employees tested positive by PCR (Table 5), and 23 (70%) of these employees were positive by both PCR and culture. Ten samples from week 1 and three samples from week 4 could be genotyped, and all were found to be genotype D. The isolate in the specimen from only one person could be genotyped at both time points. Thus, 12 out of 23 positive employees were infected with genotype D. Genotyping of the 11 remaining positive samples revealed no result. Interestingly, the rates of seropositivity were comparable for both groups of employees: 79% for chicken slaughterhouse employees versus 71% for turkey slaughterhouse employees (Table 6). Human antibody titers ranged from 1/100 to 1/800 in both slaughterhouses, with a slightly higher average occurring in the turkey abattoir (1/285 versus 1/197 for the chicken abattoir). However, seroconversion was never observed. Serum antibodies could not be detected in one PCR- and culture-positive chicken slaughterhouse employee. Some seropositive persons tested negative by either PCR or culture. One and five seropositive employees of the chicken and turkey slaughterhouses, respectively, tested negative by both PCR and culture.
TABLE 5.
Results of C. psittaci detection in pharyngeal swab specimens of employees of the chicken and turkey slaughterhouse
| Slaughterhouse and time | No. (%) of employees |
Mean isolation score ± SD | |||
|---|---|---|---|---|---|
| Total tested | Nested PCR positive | Culture positivea | Total positive | ||
| Chicken | |||||
| Wk 1 | 53 | 4 | 3 | 4 (7.5) | 0.75 ± 0.65 |
| Wk 4 | 53 | 0 | 0 | 0 (0) | 0 ± 0.0 |
| Total | 53 | 4 | 3 | 4 (7.5) | 0.75 ± 0.65 |
| Turkey | |||||
| Wk 1 | 38 | 20 | 13 | 20 (53) | 0.65 ± 0.59 |
| Wk 4 | 38 | 28b | 22c | 28 (74) | 1.1 ± 0.77 |
| Total | 38 | 20 + 13b = 33 | 13 + 10c = 23d | 33 (87) | 0.92 ± 0.73 |
All culture-positive employees tested positive by nested PCR.
Thirteen additional employees who were negative during week 1 were found to be positive by nested PCR during week 4.
Ten additional employees who were negative during week 1 were found to be positive by nested PCR during week 4.
All employees were nested PCR-EIA positive.
TABLE 6.
Results of chlamydial antibody detection (rMOMP-ELISA) in human sera
| Slaughterhouse and time | No. (%) of employees |
Mean antibody titer ± SD | |
|---|---|---|---|
| Total tested | Seropositive | ||
| Chicken | |||
| Wk 1 | 53 | 35 (66) | 1/206 ± 1/181 |
| Wk 4 | 53 | 32a (60) | 1/187 ± 1/143 |
| Total | 53 | 35 + 7a = 42 (79) | 1/197 ± 1/163 |
| Turkey | |||
| Wk 1 | 38 | 18 (47) | 1/361 ± 1/233 |
| Wk 4 | 38 | 22b (58) | 1/223 ± 1/92 |
| Total | 38 | 18 + 9b = 27 (71) | 1/285 ± 1/182 |
Seven additional employees who were negative during week 1 were found to be seropositive during week 4.
Nine additional persons who were negative during week 1 were found to be found seropositive during week 4.
The four scientists tested were PCR and culture negative in October 2007, at the start of the study. However, two of them (50%) were seropositive and at that time had titers of 1/100 and 1/200, respectively. None of the scientists became PCR or culture positive during their work in the chicken slaughterhouse. The ELISA results confirmed this, as all scientists became seronegative by the end of the chicken slaughterhouse study. Scientists were still PCR, culture, and ELISA negative in November 2007, when the study of the turkey slaughterhouse began. However, at the end of that study, they all tested positive by PCR and ELISA, with titers of 1/100 for persons 1 and 2, 1/200 for person 3, and 1/400 for person 4. Two persons (with titers of 1/400 and 1/100) were also positive by culture. None of the scientists realized that they had been infected, as they felt healthy during and after the study. Only one scientist caught a cold during the study of the turkey abattoir, but it was considered normal given the time of year (October-November). The symptoms were not directly linked to C. psittaci, and differential diagnostics were not performed.
Statistics.
Fifty-three of 87 (61%) and 30 of 329 (9%) of the chicken and turkey slaughterhouse employees, respectively, participated in the study. They all filled out a medical questionnaire. For the chicken abattoir, the study population consisted of 23 women and 30 men, while for the turkey abattoir, the study population consisted of 8 women and 30 men. The ages, body lengths, weights, and blood pressure (systolic and diastolic) of the two populations did not differ significantly (data not shown). However, the body mass indexes (BMIs) were significantly different, with the means being 25.60 ± 0.56 kg/m2 for the chicken slaughterhouse employees and 27.39 ± 0.84 kg/m2 for the turkey slaughterhouse employees, probably because significantly more women worked in the chicken slaughterhouse.
The numbers of PCR-EIA-positive workers, namely, 4 in the chicken slaughterhouse and 33 in the turkey slaughterhouse, were significantly different, with the sigma value being <0.001. The percentages of seropositive individuals (sigma value, 0.374) and the mean culture scores (sigma value, 0.748) were not significantly different. There was a significant difference between the number of C. psittaci PCR-positive men and women, as men were more often infected than women (sigma value, 0.006). People who were C. psittaci PCR positive had more contact with birds outside the workplace than people who were C. psittaci PCR negative, but the difference was not significant (sigma value, 0.710). Employees of the unclean side of the slaughter line were significantly more often PCR-EIA positive than people from the clean side (sigma value, 0.04).
The mean scores for respiratory complaints reported by employees of the chicken abattoir (mean score, 4.72) or turkey abattoir (mean score, 3.63) were not significantly different (sigma value, 0.113). The mean complaint scores for dry, itchy, or irritated eyes reported by employees of the chicken and turkey abattoirs were also not significantly different (sigma value, 0.308). However, the mean complaint scores for tired or painful eyes reported by chicken and turkey abattoir employees were significantly different: 0.53 and 0.16, respectively (sigma value, 0.034).
DISCUSSION
The present study examines the occurrence of C. psittaci zoonotic transmission in two different risk environments, namely, a turkey slaughterhouse and a chicken slaughterhouse. All over the world, C. psittaci is known to be highly prevalent in turkey broilers, being an important player in the so-called turkey respiratory disease complex (1, 21, 24, 29). Chickens, on the other hand, are believed to be less sensitive to C. psittaci infection (1). Thus, handling and processing of turkeys would present a higher risk for public health.
However, using sera from chicken broilers raised in Belgium during 2005, we demonstrated that the seroprevalence of C. psittaci in chickens was as high as that in turkey broilers (more than 90%; unpublished results). Thus, we were actually not surprised to find C. psittaci in 85% of the processed chicken flocks examined during the present study. The percentage of infected chicken flocks was even higher than the percentage of infected turkeys flocks (57%). However, we sampled only 10 randomly selected animals per slaughtered flock, examining 13 chicken flocks and 7 turkey flocks, so we cannot really draw conclusions on the differences in the percentages of positive chicken and turkey flocks.
Interestingly, we noticed that turkey flocks had been treated more frequently with antibiotics active against C. psittaci than the incoming chicken flocks (57% and 23%, respectively). The treatments were apparently performed during weeks 3 to 4 and 6 to 12, which is not unusual. Van Loock et al. showed the occurrence of two waves of C. psittaci infection on Belgian turkey broiler farms: the first at the age of 3 to 6 weeks, soon after maternal antibodies disappeared, and the second at the age of 8 to 12 weeks (21). Broiler chickens are slaughtered at only 6 weeks of age, while turkey broilers are raised until the age of 15 to 17 weeks. Thus, the chance that turkeys will become infected with C. psittaci is much higher. In addition, since the life span of turkeys is much longer than that of chickens, C. psittaci can multiply for a longer time in turkeys, which could result in higher bacterial loads. This may explain why the infection rates in the turkey flocks examined were indeed higher than the ones found in the broiler chicken flocks and why individual turkeys revealed higher mean culture scores than individual chickens. Powell et al. found that the innate immunity in turkeys was less potent than that in chickens, resulting in a higher disease burden for Histomonas meleagridis (12). However, the higher bacterial loads in turkeys could also be explained by the occurrence of more virulent ompA genotypes and/or by the presence of mixed infections in turkeys. Mixed infections do occur in turkeys. In the past we even found three different genotypes (genotypes D, F, and E/B) simultaneously in turkey broilers (20). In the present study, ompA genotyping was performed but revealed only genotype D in both turkeys and chickens. Of course, we cannot exclude the possibility of the presence of additional genotypes. Genotype D is highly virulent, and it is excreted extensively and is therefore more easily discovered (27). Less is known about the C. psittaci genotypes in chickens. Until now, only genotypes B, E/B, and C have been detected in chickens (5, 23, 32). This is the first time that genotype D has been identified in chickens.
To examine the C. psittaci zoonotic risk in the slaughterhouses, employees provided pharyngeal swab specimens for both PCR and culture and sera for a recombinant MOMP-based ELISA. As far as we know, we are the first to conduct such an examination in chicken and turkey slaughterhouses in the absence of a psittacosis outbreak. In the turkey slaughterhouse, 61% and 87% of the employees examined tested positive by culture and PCR, respectively. On the other hand, for the chicken slaughterhouse, only 6% and 7.5% of the employees was positive by culture and PCR, respectively. Genotyping was successful only for turkey slaughterhouse employees and revealed the presence of genotype D. Samples from the chicken slaughterhouse employees could not be genotyped, probably because they contained less DNA. Differences in human infection status could reflect the results of bioaerosol monitoring, as more live chlamydial organisms were discovered in air samples originating from the turkey slaughterhouse. However, results on the human infection status must be handled with care, as the (voluntary) participation rate differed significantly for chicken (61%) and turkey (9%) slaughterhouse employees. While these results could indicate the self-selection of employees feeling less healthy, the analysis of the answers on the questionnaires indicated that there were no significant differences in respiratory complaints between the facilities. Surprisingly, chicken slaughterhouse employees did complain significantly more about tired or painful eyes. This could be due to C. psittaci infections (4) but could, of course, also be due to infrastructural (ventilation, quality of light) issues rather than a chlamydial infection.
Nevertheless, infected turkeys seem to present a higher zoonotic risk, as the four scientists, who were PCR negative before starting this study, stayed negative after visiting the chicken slaughterhouse. However, at the end of the subsequent study of the turkey slaughterhouse, they all tested positive by PCR and two of them were also culture positive. Our results are consistent with those in the literature in suggesting that contact with C. psittaci-infected turkeys presents a substantial zoonotic risk (29). On the other hand, zoonotic transmission from chickens to humans seems to occur less frequently.
In general, employees at the poultry reception area and the ones performing the evisceration were significantly more frequently infected than others. Living animals are actively excreting C. psittaci cells (due to crowding and stress), and during evisceration, infected air sacs and lungs are exposed to the environment. This has also been observed by Tiong et al. (18), examining an outbreak of ornithosis in a poultry abattoir.
Although the human infection rate was significantly higher in the turkey slaughterhouse, the rates of respiratory complaints did not differ between the two slaughterhouses. Poultry workers are almost continuously exposed to C. psittaci and therefore could have natural immunity against disease.
During the present study we also evaluated the practical application of a personal and stationary bioaerosol monitoring technique especially designed for entrapping C. psittaci DNA as well as live organisms (19). The stationary bioaerosol sampling method proved to be of high value for quantifying the chlamydial organisms in the air and allowed us to determine the zoonotic risk in both slaughterhouses. Most human infections were indeed detected at workstations where large amounts of live chlamydial organisms were measured in the air. The personal bioaerosol sampling method, however, was not suitable for use in poultry slaughterhouses. The technique is about 10 times less sensitive than the stationary bioaerosol monitoring method, as less air is collected (120 liters and 1,000 liters, respectively). Moreover, clogging of the gelatin filter occurred in dusty (feather dust) rooms, and the tube connecting the IOM sampler with the air aspiration pump often disconnected through movements of the employee. Stationary bioaerosol monitoring using the MAS-100 ecosampler and ChlamyTrap collection medium is therefore more suited for C. psittaci zoonotic risk assessment in the field.
This study indicated that zoonotic transmissions of C. psittaci are very common, especially in a turkey slaughterhouse, urging the need for higher awareness. Even though it seems that many infections were asymptomatic, there is always a possibility of a virulent psittacosis outbreak in slaughterhouses (10, 18, 31). Accurate diagnostic monitoring and reporting of infections in both poultry and poultry workers should be promoted. Additionally, an efficient veterinary vaccine, preventive measures, and information campaigns could be beneficial to public health.
Acknowledgments
This study was funded by the Federal Public Service of Health, Food Chain Safety and Environment (convention RF-6177). D. S. A. Beeckman is a postdoctoral fellow of the Research Foundation Flanders (FWO-Vlaanderen), and this institution is acknowledged for providing a grant.
The directors and employees of the poultry slaughterhouses are acknowledged for their participation. Thanks go to the Hogeschool UGent (I. Scholtis and N. Deschuyffeleer) for allowing us to use their IOM personal bioaerosol samplers and the extra MAS-100 ecosampler needed for this study. DGZ Vlaanderen (M. Verlinden) is acknowledged for providing the chicken sera from 2005 used for the retrospective C. psittaci study.
Footnotes
Published ahead of print on 30 June 2010.
REFERENCES
- 1.Andersen, A. A., and D. Vanrompay. 2003. Avian chlamydiosis (psittacosis, ornithosis), p. 863-879. In Y. M. Saif, H. J. Barnes, A. M. Fadly, J. R. Glisson, L. R. McDougald, and D. E. Swayne (ed.), Diseases of poultry. Iowa State University Press, Ames, IA.
- 2.Beeckman, D. S., and D. C. Vanrompay. 2009. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin. Microbiol. Infect. 15:11-17. [DOI] [PubMed] [Google Scholar]
- 3.Chahota, R., R. C. Katoch, S. P. Singh, S. Verma, and A. Mahajan. 2000. Concurrent outbreak of chlamydiosis and aflatoxicosis among chickens in Himachal Pradesh, India. Vet. Arch. 70:207-213. [Google Scholar]
- 4.Dean, D., R. P. Kandel, H. K. Adhikari, and T. Hessel. 2008. Multiple Chlamydiaceae species in trachoma: implications for disease pathogenesis and control. PLoS Med. 5:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaede, W., K. F. Reckling, B. Dresenkamp, S. Kenklies, E. Schubert, U. Noack, H. M. Irmscher, C. Ludwig, H. Hotzel, and K. Sachse. 2008. Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health 55:184-188. [DOI] [PubMed] [Google Scholar]
- 6.Grimes, J. E., and P. B. Wyrick. 1991. Chlamydiosis (ornithosis), p. 311-325. In B. V. V. Clenk, H. J. Barnes, C. W. Beard, W. M. Reid, and H. W. Yoder (ed.), Diseases of poultry. Iowa State University Press, Ames, IA.
- 7.Kovacova, E., J. Majtan, R. Botek, T. Bokor, H. Blaskovicova, M. Solavova, M. Ondicova, and J. Kazar. 2007. A fatal case of psittacosis in Slovakia, January 2006. Euro. Surveill. 12:E070802. [DOI] [PubMed] [Google Scholar]
- 8.Laroucau, K., F. Vorimore, R. Aaziz, A. Berndt, E. Schubert, and K. Sachse. 2009. Isolation of a new chlamydial agent from infected domestic poultry coincided with cases of atypical pneumonia among slaughterhouse workers in France. Infect. Genet. Evol. 9:1240-1247. [DOI] [PubMed] [Google Scholar]
- 9.Moroney, J. F., R. Guevara, C. Iverson, F. M. Chen, S. K. Skelton, T. O. Messmer, B. Plikaytis, P. O. Williams, P. Blake, and J. C. Butler. 1998. Detection of chlamydiosis in a shipment of pet birds, leading to recognition of an outbreak of clinically mild psittacosis in humans. Clin. Infect. Dis. 26:1425-1429. [DOI] [PubMed] [Google Scholar]
- 10.Newman, C. P., S. R. Palmer, F. D. Kirby, and E. O. Caul. 1992. A prolonged outbreak of ornithosis in duck processors. Epidemiol. Infect. 108:203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni, A. P., G. Y. Lin, L. Yang, H. Y. He, C. W. Huang, Z. J. Liu, R. S. Wang, J. S. Zhang, J. Y. Yu, N. Li, J. B. Wang, and H. Y. Yang. 1996. A seroepidemiologic study of Chlamydia pneumoniae, Chlamydia trachomatis and Chlamydia psittaci in different populations on the mainland of China. Scand. J. Infect. Dis. 28:553-557. [DOI] [PubMed] [Google Scholar]
- 12.Powell, F. L., L. Rothwell, M. J. Clarkson, and P. Kaiser. 2009. The turkey, compared to the chicken, fails to mount an effective early immune response to Histomonas meleagridis in the gut. Parasite Immunol. 31:312-327. [DOI] [PubMed] [Google Scholar]
- 13.Prukner-Radovcic, E., D. Horvatek, I. C. Grozdanic, I. Majetic, and Z. Bidin. 2005. Chlamydophila psittaci in turkey in Croatia, p. 161-166. In R. Cevenini and V. Sambri (ed.), Proceedings of the 3rd Workshop, Diagnosis and Pathogenesis of Animal Chlamydioses, Sienna, Italy. Bononia University Press, Bologna, Italy.
- 14.Ryll, M., K. H. Hinz, U. Neumann, and K. P. Behr. 1994. Pilot study of the occurrence of Chlamydia psittaci infections in commercial turkey flocks in Niedersachsen. Dtsch. Tierarztl. Wochenschr. 101:163-165. (In German.) [PubMed] [Google Scholar]
- 15.Sachse, K., K. Laroucau, H. Hotzel, E. Schubert, R. Ehricht, and P. Slickers. 2008. Genotyping of Chlamydophila psittaci using a new DNA microarray assay based on sequence analysis of ompA genes. BMC Microbiol. 8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachse, K., K. Laroucau, F. Vorimore, S. Magnino, J. Feige, W. Muller, S. Kube, H. Hotzel, E. Schubert, P. Slickers, and R. Ehricht. 2009. DNA microarray-based genotyping of Chlamydophila psittaci strains from culture and clinical samples. Vet. Microbiol. 135:22-30. [DOI] [PubMed] [Google Scholar]
- 17.Sting, R., E. Lerke, H. Hotzel, S. Jodas, C. Popp, and H. M. Hafez. 2006. Comparative studies on detection of Chlamydophila psittaci and Chlamydophila abortus in meat turkey flocks using cell culture, ELISA, and PCR. Dtsch. Tierarztl. Wochenschr. 113:50-54. (In German.) [PubMed] [Google Scholar]
- 18.Tiong, A., T. Vu, M. Counahan, J. Leydon, G. Tallis, and S. Lambert. 2007. Multiple sites of exposure in an outbreak of ornithosis in workers at a poultry abattoir and farm. Epidemiol. Infect. 135:1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Droogenbroeck, C., Van Risseghem, M., L. Braeckman, and D. Vanrompay. 2009. Evaluation of bioaerosol sampling techniques for the detection of Chlamydophila psittaci in contaminated air. Vet. Microbiol. 135:31-37. [DOI] [PubMed] [Google Scholar]
- 20.Van Droogenbroeck, C. M., D. S. Beeckman, K. Verminnen, M. Marien, H. Nauwynck, L. D. Boesinghe, and D. Vanrompay. 2009. Simultaneous zoonotic transmission of Chlamydophila psittaci genotypes D, F and E/B to a veterinary scientist. Vet. Microbiol. 135:78-81. [DOI] [PubMed] [Google Scholar]
- 21.Van Loock, M., T. Geens, L. De Smit, H. Nauwynck, E. P. Van, C. Naylor, H. M. Hafez, B. M. Goddeeris, and D. Vanrompay. 2005. Key role of Chlamydophila psittaci on Belgian turkey farms in association with other respiratory pathogens. Vet. Microbiol. 107:91-101. [DOI] [PubMed] [Google Scholar]
- 22.Van Loock, M., K. Verminnen, T. O. Messmer, G. Volckaert, B. M. Goddeeris, and D. Vanrompay. 2005. Use of a nested PCR-enzyme immunoassay with an internal control to detect Chlamydophila psittaci in turkeys. BMC Infect. Dis. 5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanrompay, D., P. Butaye, C. Sayada, R. Ducatelle, and F. Haesebrouck. 1997. Characterization of avian Chlamydia psittaci strains using omp1 restriction mapping and serovar-specific monoclonal antibodies. Res. Microbiol. 148:327-333. [DOI] [PubMed] [Google Scholar]
- 24.Vanrompay, D., P. Butaye, A. Van Nerom, R. Ducatelle, and F. Haesebrouck. 1997. The prevalence of Chlamydia psittaci infections in Belgian commercial turkey poults. Vet. Microbiol. 54:85-93. [DOI] [PubMed] [Google Scholar]
- 25.Vanrompay, D., R. Ducatelle, and F. Haesebrouck. 1992. Diagnosis of avian chlamydiosis: specificity of the modified Gimenez staining on smears and comparison of the sensitivity of isolation in eggs and three different cell cultures. Zentralbl. Veterinarmed. B 39:105-112. [DOI] [PubMed] [Google Scholar]
- 26.Vanrompay, D., R. Ducatelle, and F. Haesebrouck. 1995. Chlamydia psittaci infections: a review with emphasis on avian chlamydiosis. Vet. Microbiol. 45:93-119. [DOI] [PubMed] [Google Scholar]
- 27.Vanrompay, D., J. Mast, R. Ducatelle, F. Haesebrouck, and B. Goddeeris. 1995. Chlamydia psittaci in turkeys: pathogenesis of infections in avian serovars A, B and D. Vet. Microbiol. 47:245-256. [DOI] [PubMed] [Google Scholar]
- 28.Vanrompay, D., A. Van Nerom, R. Ducatelle, and F. Haesebrouck. 1994. Evaluation of five immunoassays for detection of Chlamydia psittaci in cloacal and conjunctival specimens from turkeys. J. Clin. Microbiol. 32:1470-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verminnen, K., B. Duquenne, D. De Keuleleire, B. Duim, Y. Pannekoek, L. Braeckman, and D. Vanrompay. 2008. Evaluation of a Chlamydophila psittaci infection diagnostic platform for zoonotic risk assessment. J. Clin. Microbiol. 46:281-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verminnen, K., M. Van Loock, H. M. Hafez, R. Ducatelle, F. Haesebrouck, and D. Vanrompay. 2006. Evaluation of a recombinant enzyme-linked immunosorbent assay for detecting Chlamydophila psittaci antibodies in turkey sera. Vet. Res. 37:623-632. [DOI] [PubMed] [Google Scholar]
- 31.Yung, A. P., and M. L. Grayson. 1988. Psittacosis—a review of 135 cases. Med. J. Aust. 148:228-233. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, F., S. Li, J. Yang, W. Pang, L. Yang, and C. He. 2008. Isolation and characterization of Chlamydophila psittaci isolated from laying hens with cystic oviducts. Avian Dis. 52:74-78. [DOI] [PubMed] [Google Scholar]