Abstract
We developed a multiprobe real-time PCR assay targeting hsp65 (HMPRT-PCR) to detect and identify mycobacterial isolates and isolates directly from sputum specimens. Primers and probes for HMPRT-PCR were designed on the basis of the hsp65 gene sequence, enabling the recognition of seven pathogenic mycobacteria, including Mycobacterium tuberculosis, M. avium, M. intracellulare, M. kansasii, M. abscessus, M. massiliense, and M. fortuitum. This technique was applied to 24 reference and 133 clinical isolates and differentiated between all strains with 100% sensitivity and specificity. Furthermore, this method was applied to sputum specimens from 117 consecutive smear-positive patients with smear results of from a trace to 3+. These results were then compared to those obtained using the rpoB PCR-restriction analysis method with samples from cultures of the same sputum specimens. The HMPRT-PCR method correctly identified the mycobacteria in 89 samples (76.0%, 89/117), and moreover, the sensitivity level was increased to 94.3% (50/53) for sputa with an acid-fast bacillus score equal to or greater than 2+. Our data suggest that this novel HMPRT-PCR method could be a promising approach for detecting pathogenic mycobacterial species from sputum samples and culture isolates routinely in a clinical setting.
Of the known species in the genus Mycobacterium, Mycobacterium tuberculosis is the most common and most important pathogen, causing 2 million deaths and over 8 million cases of tuberculosis worldwide annually (2, 3, 4, 7). In addition to M. tuberculosis, infections with nontuberculosis mycobacteria (NTM) can also cause clinical problems. Because of the different pathogenic potentials and susceptibilities of different mycobacterial species, the treatments of mycobacterial infections are different (13, 30, 33, 34). Thus, it is very important to differentiate between mycobacteria at the species level during early-stage diagnostics.
Instead of a culture-based identification scheme, which may take 4 to 6 weeks or longer to identify slowly growing mycobacteria, PCR-based protocols (sequencing or PCR-restriction analysis [PRA]) targeting chronometer molecules, such as 16S rRNA (5, 6, 28), hsp65 (17, 19, 25), and rpoB (1, 16, 21), have been widely used to identify mycobacteria. However, in spite of the successful application of these conventional PCR-based methods to culture isolates, there are some drawbacks in their direct application to clinical specimens. This is especially true for sputum samples, which also contain numbers of commensal bacteria from the respiratory tract, producing confusing results by the simultaneous amplification of both commensals and mycobacterial strains. We have recently developed several methods for mycobacterial species identification based on amplification of hsp65 gene sequences directly from sputum samples (15, 27). Limitations due to the intrinsic features of conventional PCR prevented feasible identification of mycobacterial species from sputum samples using this method.
The use of the real-time PCR assay in the diagnosis of many infectious diseases has been increasing, as it represents an appealing alternative to conventional PCR. It is an improvement over conventional methods because of its increased sensitivity and specificity, low contamination risk, and ease of performance and speed (8). In particular, fluorescence resonance energy transfer (FRET)-based real-time PCR permits not only the simultaneous identification of multiple target species but also the direct identification of target species from primary specimens such as sputum specimens through melting curve analysis of the amplification product (8). These characteristics of FRET-based real-time PCR provide a useful advantage for the identification of mycobacteria from sputum samples. Recently, several real-time PCR-based methods for mycobacterial detection and identification have been developed and evaluated (9, 22, 23, 26, 29). However, direct application of the real-time PCR-based method to primary specimens was generally limited to M. tuberculosis alone (11, 26). So far, a method which can simultaneously identify several pathogenic NTM as well as M. tuberculosis from primary sputum samples in a single reaction has not been developed.
In the present study, we sought to develop a multiprobe real-time PCR targeting the hsp65 gene (HMPRT-PCR) based on melting curve analysis (HybProbes). This enabled the simultaneous identification of several pathogenic mycobacteria, including M. tuberculosis, in a single PCR performed on cultures and sputum samples. The usefulness of these methods was evaluated by blindly applying them to cultured and sputum samples.
MATERIALS AND METHODS
Mycobacterial strains and sputa.
Twenty-four mycobacterial reference strains (Table 1), 133 clinical isolates (Table 2), and 117 sputum specimens suspected of harboring mycobacteria were used in this study. Twenty-two of the 24 mycobacterial reference strains were provided by the Korean Institute of Tuberculosis (KIT). M. intracellulare 05-1390 and M. avium ATCC 4006 were provided by the Seoul National University College of Medicine (SNUMC) and the Samsung Medical Center (SMC), respectively. One hundred thirty-three clinical isolates which had already been identified by molecular biology-based assays, such as rpoB PRA (21) and hsp65-based sequencing analysis (17, 19), were provided by SMC (51 strains), SNUMC (49 strains), the Kangbuk Samsung Medical Center (KBSMC) (24 strains), the Asan Medical Center (AMC) (5 strains), and the Chung-Ang University Medical Center (CAUMC) (4 strains). One hundred seventeen sputum specimens from different patients with positive acid-fast bacillus (AFB) smears detected between 1 April 2008 and 31 July 2008 at AMC, Seoul, Republic of Korea, were included in this study. The sputa were digested, decontaminated, and concentrated as recommended by the WHO (14). The processed sediment was stained using the Ziehl-Neelsen method (14). The results of the AFB smears were graded according to the recommendations of the American Thoracic Society and the Centers for Disease Control and Prevention (32). Sputa with trace AFB smear results (one to two bacilli in 300 fields) were also included in this study. The protocol for this study was approved by the Institutional Review Board of AMC.
TABLE 1.
Mycobacterial reference strains used in the present study and specificity of hsp65 multiprobe real-time PCR for identification of target Mycobacterium species
| Species | Strain | Sourcea | Measured Tm (°C)b |
Real-time PCR identification | |||
|---|---|---|---|---|---|---|---|
| CH. 610 | CH. 640 | CH. 670 | CH. 705 | ||||
| M. abscessus | 19977T | ATCC | — | — | — | 68.2 ± 0.51 | M. abscessus |
| M. avium | 25291T | ATCC | 60.9 ± 0.00 | — | 74.0 ± 0.06 | 60.3 ± 0.03 | M. avium |
| M. celatum | 51131T | ATCC | — | — | — | — | |
| M. chelonae | 35749T | ATCC | — | — | 64.9 ± 0.01 | 62.6 ± 0.57 | |
| M. flavescens | 14474T | ATCC | — | — | 67.2 ± 0.05 | 57.9 ± 0.69 | |
| M. fortuitum | 6841T | ATCC | — | 60.5 ± 0.01 | — | — | M. fortuitum-M. peregrinum complex |
| M. gastri | 15754T | ATCC | 61.2 ± 0.03 | 57.7 ± 0.08 | — | 57.9 ± 0.18 | |
| M. genavense | 51233T | ATCC | — | — | 69.1 ± 0.23 | — | |
| M. gordonae | 14470T | ATCC | 61.6 ± 0.02 | — | 66.6 ± 0.13 | — | |
| M. haemophilum | 29548T | ATCC | 61.4 ± 0.11 | — | — | 58.3 ± 1.15 | |
| M. intracellulare | 13950T | ATCC | 61.4 ± 0.05 | 73.0 ± 0.02 | — | 58.1 ± 0.12 | M. intracellulare |
| M. kansasii | 12478T | ATCC | — | 67.0 ± 0.01 | — | 57.4 ± 0.23 | M. kansasii |
| M. malmoense | 29571T | ATCC | 61.6 ± 0.04 | — | — | 61.9 ± 0.20 | |
| M. massiliense | 19086 | KCTC | — | — | — | 70.8 ± 0.18 | M. massiliense |
| M. marinum | 29571T | ATCC | 61.6 ± 0.04 | — | 63.1 ± 0.14 | 58.1 ± 0.02 | |
| M. peregrinum | 27294T | ATCC | — | 60.0 ± 0.07 | — | — | M. fortuitum-M. peregrinum complex |
| M. phlei | 35784 | ATCC | — | — | 67.5 ± 0.05 | — | |
| M. scrofulaceum | 19981T | ATCC | 61.5 ± 0.15 | 61.5 ± 0.17 | 61.6 ± 0.04 | — | |
| M. smegmatis | 607 | ATCC | 61.6 ± 0.09 | — | — | 61.5 ± 0.33 | |
| M. szulgai | 35799T | ATCC | — | — | 58.8 ± 0.21 | — | |
| M. terrae | 15755T | ATCC | — | — | 60.3 ± 0.01 | 60.1 ± 0.20 | |
| M. tuberculosis | 27294T | ATCC | 66.9 ± 0.02 | — | — | 57.8 ± 0.13 | M. tuberculosis |
| M. ulcerans | 19423T | ATCC | 61.7 ± 0.12 | — | 63.2 ± 0.05 | 57.9 ± 0.18 | |
| M. xenopi | 19250T | ATCC | — | — | — | — | |
ATCC, American Type Culture Collection; KCTC, Korean Collection for Type Cultures.
Tms were obtained by duplicate real-time PCR and melting curve analyses, and the data represent the means ± standard deviations. Boldface, species-specific Tm; —, no significant Tm.
TABLE 2.
Identification of mycobacterial clinical isolates by HMPRT-PCR
| Species | No. of isolates detected by: |
|
|---|---|---|
| rpoB PRA | HMPRT-PCR | |
| M. abscessus | 33 | 16a |
| M. massiliense | 17a | |
| M. avium | 18 | 18 |
| M. fortuitum | 7 | 7 |
| M. intracellulare | 26 | 26 |
| M. kansasii | 25 | 25 |
| M. tuberculosis | 24 | 24 |
| Total | 133 | 133 |
Differentiation between M. abscessus and M. massiliense was confirmed by hsp65-based sequencing analysis.
DNA extraction.
Chromosomal DNA was extracted from the clinical isolates and sputum samples by the bead beater-phenol extraction method, as described previously (19).
Primer and oligonucleotide probe design.
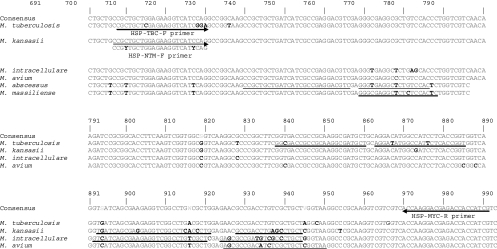
To obtain appropriate primer sequences for the amplification of mycobacterial hsp65 DNA and identify multiple probe sequences for the species-specific detection of mycobacteria, the hsp65 DNA sequences of a total of 140 mycobacterium-related strains were analyzed using SeqMan II software (DNAStar) (data not shown). An M. tuberculosis complex (TBC)-specific forward primer and an NTM-specific forward primer which are different from each other at 3 bases at the 3′ end and a reverse primer for all mycobacterial species were designed using the Oligo (version 6.5) program (Molecular Biology Insights). These primers produced 304-bp hsp65 amplicons (from the 698th to 991st nucleotides in the M. tuberculosis hsp65 gene) (Fig. 1; Table 3). We designed a set of 10 hybridization probes (an anchor probe and a sensor probe; HybProbe) for the specific simultaneous detection in a single reaction of seven representative mycobacterial species or complexes that are the most clinically important and most frequently isolated from the patients using LC PDS (version 2.0) software (Fig. 1; Table 3). We used four channels (CH.) for probes specific to seven mycobacterial species. We used CH. 610 for the detection of M. tuberculosis; CH. 640 for the detection of M. kansasii, M. intracellulare, and the M. fortiutum-M. peregrinum complex; CH. 670 for the detection of M. avium; and CH. 705 for the detection of M. abscessus and M. massiliense (Table 1). We determined species-specific regions of each target organism that appeared to be dissimilar from the sequences of the other organisms by more than 2 bases at the sensor probe-binding position or 4 bases at the anchor probe-binding position in the sequences of the other Mycobacterium species to ensure a highly specific signal. When designing the HybProbes, we ensured that the anchor and sensor probes were adjacently hybridized to the complementary target DNA for successful fluorescence emission by a mechanism of FRET. The potential presence of cross-complementarities among all the primers and probes was checked by using the LC PDS (version 2.0) software, and to obtain high sensitivity, we modified the primer or probe sequences while maintaining the primer and probe specificities. The diagnostic melting temperatures (Tms) of the designed probes and their specificities were investigated by calculation of the Tms of the sensor and anchor probes hybridizing to the target and nontarget mycobacterial DNAs by using the LC PDS software (Table 4). The probe specificity for each target Mycobacterium species was confirmed by comparing the sequences from 240 mycobacterial or related strains via BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The designed probes were purchased from Metabion.
FIG. 1.
Primer and probe positions developed for the identification of Mycobacterium species on the basis of the hsp65 DNA sequence alignment. Arrows indicate the primer positions. The primer sequence that is different from the one indicated by one of the arrows is shown under the arrow. Underlines indicate the probe positions and sequences designed for the specific identification of the respective species. The numbers indicate the nucleotide positions on the hsp65 DNA sequence of M. tuberculosis. Boldface bases denote the bases different from the ones in the consensus sequence. The strains used were as follows: M. tuberculosis, H37Rv; M. kansasii, ATCC 12478T; M. intracellulare, ATCC 13950T; M. avium, ATCC 25291T; M. abscessus, ATCC 19977T; M. massiliense, Icheon.
TABLE 3.
Primers and HybProbes developed for identification of Mycobacterium species on the basis of the hsp65 sequences in this study
| Primer or probe | Sequence (5′ → 3′)a | Tm (°C)b | Potential target organism(s) | Detection channel/Tm (°C)c |
|---|---|---|---|---|
| Primers | ||||
| HSP-NTM-F | CCGYTGCTGGAGAAGGTCATYCAG | 66.3-69.7 | NTM | |
| HSP-TBC-F | CGCTGCTCGAGAAGGTCATCGGA | 68.3 | MTCd | |
| HSP-MYC-R | CGATGATGGTGGTCTCGTCCTTGGT | 68.6 | Mycobacteria | |
| Probes | ||||
| MTC | GGCGACCGACGCAAGGCGATGCT-FL | 73.6 | M. tuberculosis complex | 610/67.2 |
| LC Red610-AGGATATGGCCATTCTCACCGGT-PH | 67.2 | |||
| KAN | TGATCAGCGAGGAGGTCGGCCTCACCCTG-FL | 75.3 | M. kansasii types I to VI | 640/65.1-66.7 |
| LC Red640-GCCGACCTGAGCCTGCT-PH | 65.2 | |||
| INT | GTCATCAGCGAAGAGGTCGGCCTGTCGCT-FL | 74.8 | M. intracellulare | 640/72.9 |
| LC Red640-GAGCGCCGATGTCGCCCTGCT-PH | 72.9 | M. fortuitum and M. peregrinum | 640/61.4 | |
| AVI | LC Red670-AGCGCCGACATCTCGCTGCTCGGTAA-PH | 73.7 | M. avium | 670/73.7 |
| M. aviumsubsp. hominissuis | 670/67.2 | |||
| ABS | CCGCTGCTGATCATCGCCGAGGACGTC-FL | 73.6 | M. abscessus | 705/63 |
| LC Red705h-GGGTGAGGCTCTGTCCAC-PH | 63 | |||
| MAS | LC Red705-GGGCGAGGCTCTCTCCACTC-PH | 67 | M. massiliense | 705/67 |
FL, fluorescein; LC Red610, LightCycler dye Red610; PH, phosphate; LC Red640, LightCycler dye Red640; LC Red670, LightCycler dye Red670; LC Red705, LightCycler dye Red705.
Primer or probe Tm calculated by using LC PDS software (version 2.0).
Tm for species detection at the specified channel calculated by using LC PDS software (version 2.0).
MTC, M. tuberculosis complex.
TABLE 4.
Measurement of melting temperatures of target Mycobacterium species by hsp65 multiprobe real-time PCR
| Species | Strain | Sourcea | Tm (°C)b | Measured Tm (°C)c |
|||
|---|---|---|---|---|---|---|---|
| CH. 610 | CH. 640 | CH. 670 | CH. 705 | ||||
| M. tuberculosis | 27294T | ATCC | 67.2 | 66.9 ± 0.02 | — | — | 57.8 ± 0.10 |
| M. kansasii | 12478T | ATCC | 65.1-66.7 | — | 67.0 ± 0.01 | — | 57.4 ± 0.18 |
| M. intracellulare | 13950T | ATCC | 72.9 | 61.5 ± 0.02 | 73.0 ± 0.02 | — | 58.2 ± 0.31 |
| M. intracellulare | 05-1390 | SNUMC | 70.7 | 61.6 ± 0.02 | 70.0 ± 0.06 | — | 58.4 ± 0.18 |
| M. fortuitum | 6841T | ATCC | 61.4 | — | 60.6 ± 0.06 | — | — |
| M. avium | 25291T | ATCC | 73.7 | 60.9 ± 0.00 | — | 74.0 ± 0.05 | 60.4 ± 0.03 |
| M. avium | 4006 | SMC | 67.2 | 60.7 ± 0.05 | — | 70.5 ± 0.03 | 60.0 ± 0.07 |
| M. abscessus | 19977T | ATCC | 63.0 | — | — | — | 68.3 ± 0.37 |
| M. massiliense | 19086 | KCTC | 67.1 | — | — | — | 71.0 ± 0.07 |
ATCC, American Type Culture Collection; SNU, Seoul National University College of Medicine; KCTC, Korean Collection for Type Cultures.
Calculated by using LC PDS software (version 2.0).
Tms were obtained by triplicate real-time PCR and melting curve analyses, and the data represent the means ± standard deviations. Boldface, species-specific Tm; —, no significant Tm.
Real-time PCR.
A LightCycler (version 2.0) system was used for real-time PCR, and its four detection channels were calibrated for color compensation and activated for the experiment. The LightCycler Faststart DNA master HP kit (Roche Diagnostics) was used for the preparation of the master mixture, according to the protocol provided with the kit. A 10-μl reaction mixture was prepared for each sample as follows: 1 μl Taq buffer (which contains a deoxynucleoside triphosphate mixture and 10 mM MgCl2), additional 2 mM MgCl2, 0.4 μM NTM-specific forward primer (primer HSP-NTM-F), 0.3 μM TBC-specific forward primer (primer HSP-TBC-F), 1 μM mycobacterium-specific reverse primer (primer HSP-MYC-R), 0.2 μM HybProbes (which are presented in Table 3), 2 μl of culture-extracted DNA, or 4 μl of sputum-extracted DNA with 0.1 mg/ml bovine serum albumin (New England Biolabs) as templates, and sterile distilled water. The cycling conditions were 10 min at 95°C and 45 cycles of 10 s at 95°C, 20 s at 65°C (single acquisition of fluorescence signals), and 20 s at 72°C. Melting curve analysis followed by use of cycling for 10 s at 95°C and 30 s at 57°C, and the temperature was then increased from 57°C to 90°C at a temperature transition rate of 0.1°C/s, during which the fluorescence signal was continuously acquired. To determine the precise melting temperatures of the probes designed for the target species by real-time PCR, triplicate experiments were performed; and the average Tms for the Mycobacterium species were determined (Table 4; Fig. 2). The probe Tm specificity was verified from duplicate measurements of the Tms by real-time PCR with a panel of reference mycobacterial DNAs (Table 1). DNA from a total of 133 cultures (Table 2) and 117 sputum specimens (Table 5) was subsequently tested for species identification.
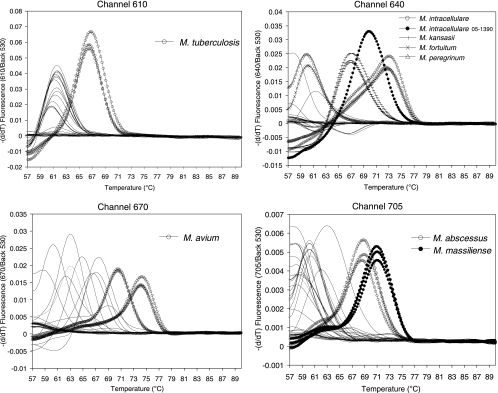
FIG. 2.
hsp real-time PCR melting curve analysis to identify the major target Mycobacterium species, M. tuberculosis, M. intracellulare, M. avium, M. kansasii, M. abscessus, and M. massiliense. All the target species were differentially identified by measurement of their specific melting temperatures. The strains tested are the same ones listed in Table 1 and were tested in duplicate or triplicate. Only the data for target strains are shown in triplicate to avoid complex plots. The y axis indicates the negative differential of fluorescence over temperature at the detection channel and normalized by the background fluorescence at channel 530.
TABLE 5.
Comparison of identification results between sputum HMPRT-PCR and same culture-based rpoB PRA for 117 sputum samples, according to mycobacterial species
| Species | Strain score by HMPRT-PCR for the following scores obtained by culture-based rpoB PRAa: |
Sensitivity (%)b | |||||
|---|---|---|---|---|---|---|---|
| Trace | 1+ | 2+ | 3+ | 4+ | Total | ||
| M. intracellulare | 12 (8/0/4) | 11 (10/0/1) | 4 (4/0/0) | 4 (4/0/0) | 1 (1/0/0) | 32 (27/0/5) | 84.3 |
| M. avium | 13 (6/0/7) | 3 (0/0/3) | 4 (4/0/0) | 7 (6/0/1) | 3 (3/0/0) | 30 (19/0/11) | 63.3 |
| M. abscessus | 8 (4/2/2) | 12 (7/0/5) | 4 (4/0/0) | 6 (5/0/1) | 0 | 30 (20/2/8)c | 66.7 |
| M. tuberculosis | 3 (3/0/0) | 1 (1/0/0) | 3 (3/0/0) | 7 (7/0/0) | 7 (7/0/0) | 21 (21/0/0) | 100 |
| M. fortuitum | 0 | 0 | 0 | 0 | 1 (1/0/0) | 1 (1/0/0) | 100 |
| M. kansasii | 0 | 0 | 1 (1/0/0) | 0 | 0 | 1 (1/0/0) | 100 |
| M. szulgai | 0 | 1 (0/0/1) | 0 | 0 | 0 | 1 (0/0/1) | 0 |
| M. celatum | 0 | 0 | 0 | 1 (0/0/1) | 0 | 1 (0/0/1) | 0 |
| Total | 36 (21/2/13) | 28 (18/0/10) | 16 (16/0/0) | 25 (22/0/3) | 12 (12/0/0) | 117 (89/2/26) | |
| Sensitivity (%) | 58.3 | 64.3 | 100 | 88 | 100 | 76.1 | |
Data in parentheses represent the number of isolates for which the results of the two methods are in agreement/number of isolates for which the results are in disagreement/number of isolates not identified by HMPRT-PCR.
Calculated from the concordant results between the two assays, rpoB PRA and HMPRT-PCR.
Differentiation between M. abscessus (12 samples) and M. massiliense (8 samples) was possible by HMPRT-PCR.
RESULTS
Determining the melting temperatures specific for target mycobacterial species by HMPRT-PCR.
To enable the species-specific identification of mycobacteria, we analyzed the melting temperatures specific for the 24 reference strains by melting curve analysis by HMPRT-PCR. All PCR products from the seven species (M. tuberculosis, M. avium, M. intracellulare, M. kansasii, M. abscessus, M. massiliense, and M. fortuitum) were clearly separated, showing species-specific melting temperatures similar to theoretical calculations (Table 1; Fig. 2). M. tuberculosis could be clearly separated in CH. 610, showing a melting temperature 5°C higher than the melting temperatures for the other reference strains. Differentiation between M. kansasii and M. gastri, which is not possible using the 16S rRNA gene-targeting method, was easily done, with an almost 10°C difference in the melting temperatures between two species being shown in CH. 640. M. avium could be clearly distinguished, as it had a distinct melting temperature 5°C higher than the melting temperatures for the other strains in CH. 670. Separation of two members of the M. abscessus complex (M. abscessus and M. massiliense) was also possible, with a more than 2°C difference in melting temperatures occurring in CH. 705. Even for M. avium (ATCC 25291T and ATCC 4006) and M. intracellulare (ATCC 13950T and SNUMC 05-1390), different melting temperatures between strains were observed (Table 1). Only separation of two M. fortuitum complex strains (M. fortuitum and M. peregrinum) was not possible, since both had similar melting temperatures in CH. 640.
Differentiation of clinical mycobacterial strains by HMPRT-PCR.
To evaluate the usefulness of the method developed for the identification of clinical isolates, we blind tested clinical isolates that had been identified by rpoB PRA and compared the results obtained by the two methods. In this experiment, a total of 133 clinical isolates were analyzed, including M. tuberculosis, M. avium, M. intracellulare, M. kansasii, M. abscessus, M. massiliense, and M. fortuitum isolates. All 133 isolates could be clearly differentiated by HMPRT-PCR, showing melting temperatures almost identical to those of the reference strains. Differentiation between M. abscessus and M. massiliense, which is not possible by rpoB PRA, could be achieved by HMPRT-PCR. A total of 33 strains identified as M. abscessus by rpoB PRA were further separated into 16 M. abscessus and 17 M. massiliense strains, and this result was completely concordant with the results obtained by hsp65 sequence analysis (Table 2).
Enrolled patients and specimens.
Sputa from 117 different patients were stained and found to be positive by AFB smear during the study period. Among the 117 patients, 58 patients (49.6%) were male and their median age was 59 years. A trace AFB smear was the most common (36 sputum specimens, 30.8%), followed by results of 1+ (28 sputum specimens, 23.9%), 3+ (25 sputum specimens, 21.4%), 2+ (16 sputum specimens, 13.7%), and 4+ (12 sputum specimens sputa, 10.3%) (Table 5).
Application of HMPRT-PCR to sputum samples.
HMPRT-PCR was directly applied to 117 sputum samples with diverse AFB staining scores. We blind tested the samples, and the results were compared with those obtained by the rpoB PRA method performed with the same cultures (18). Of the 117 samples, 81 (77.8%) were successfully analyzed by HMPRT-PCR. For 79 (97.5%) samples, complete agreement (71/81, 87.7%) or partial agreement (8/81, 9.9%) between the results of the two different methods were observed. In general, a higher sensitivity of this method was achieved with sputa showing higher AFB scores. While the sensitivity was only 60.9% (39/64) for sputa with a trace or 1+ score, the sensitivity was 94.3% (50/53) for sputa with AFB scores equal to or greater than 2+ (Table 5).
Comparing the sensitivity levels of HMPRT-PCR for detection of the four most common mycobacterial species (M. tuberculosis, M. avium, M. intracellulare, and M. abscessus), the highest levels of sensitivity were observed for the detection of M. tuberculosis (100%, 21/21) and M. intracellulare (84.3%, 27/32). The sensitivities for the M. abscessus-M. massiliense complex (66.7%, 20/30) and M. avium (63.3%, 19/30) followed (Table 5).
The two sputum specimens showing different results between two methods belonged to the group having trace AFB scores. These were identified as M. abscessus by culture-based rpoB PRA but as M. tuberculosis by HMPRT-PCR. Repeated assays showed the same results (data not shown). In the case of the eight sputum specimens whose results showed partial agreement between the two methods, all the sputum specimens were identified being infected with a single species by rpoB PRA but were proven to be coinfected with two Mycobacterium spp. by HMPRT-PCR. The most frequently encountered case of coinfection is simultaneous infection with M. intracellulare and M. kansasii (three cases). The species involved most frequently in coinfection was M. intracellulare (five cases) (Table 6).
TABLE 6.
Sputum samples from the same culture showing discrepancies between results by HNPRT-PCR and rpoB PRAa
| Patient no. | Age (yr) | Genderb | AFB score | rpoB PRA result | HMPRT-PCR result | Interpretation |
|---|---|---|---|---|---|---|
| 1 | 72 | M | 1+ | M. abscessus | M. abscessus, M. intracellulare | Coinfection |
| 2 | 80 | M | 3+ | M. abscessus | M. avium, M. massiliense | Coinfection |
| 3 | 44 | F | 1+ | M. abscessus | M. masiiliense, M. tuberculosis | Coinfection |
| 4 | 84 | F | Trace | M. intracellulare | M. avium, M. intracellulare | Coinfection |
| 5 | 52 | F | Trace | M. intracellulare | M. kansasii, M. intracellulare | Coinfection |
| 6 | 33 | M | Trace | M. intracellulare | M. kansasii, M. intracellulare | Coinfection |
| 7 | 71 | M | 2+ | M. intracellulare | M. kansasii, M. intracellulare | Coinfection |
| 8 | 33 | F | 4+ | M. tuberculosis | M. fortuitum, M. tuberculosis | Coinfection |
| 9 | 66 | M | Trace | M. abscessus | M. tuberculosis | Disagreement |
| 10 | 47 | F | Trace | M. abscessus | M. tuberculosis | Disagreement |
Most discrepancies may be caused by the overgrowth of a rapid grower.
M, male; F, female.
DISCUSSION
The most important advantage of our HMPRT-PCR method is that it enables the simultaneous identification of seven mycobacterial species, the most commonly encountered mycobacteria in clinical settings, in a single reaction. Particularly in the Republic of Korea, M. tuberculosis infections account for more than 93% of mycobacterial infections; and almost 98% of clinically significant NTM infections are known to be caused by M. avium, M. intracellulare, the M. abscessus complex, the M. fortuitum complex, and M. kansasii (20). So, at least in the Republic of Korea, this assay can detect nearly all infections caused by mycobacteria. To apply our method to species differentiation, species- or strain-specific characteristics should be evaluated. If there were different profiles between strains of a given species, our method would be too complicated for mycobacterial species identification. A substantial number of clinical isolates (133 isolates) have now been identified using our new HMPRT-PCR method in parallel with the rpoB PRA method. All the isolates were clearly identified at the species level using our method, as they showed species- or strain-specific melting temperature profiles. One characteristic feature of the HMPRT-PCR method is that it can differentiate between two members of M. abscessus complex strains, M. abscessus and M. massiliense. Recently, their separation has been gaining importance since it was reported that there are differences in the antibiotic susceptibility patterns, epidemiologic features, and clinical outcomes between the two strains (18, 31). However, separation of these two strains is difficult due to the high degree of sequence conservation between them. Therefore, sequencing of hsp65 (18) or rpoB (1) has generally been performed to separate these strains. To our knowledge, the HMPRT-PCR method described here is the only real-time PCR-based identification method that can differentiate between the two strains. These results suggest that our HMPRT-PCR method can effectively be used for the identification of mycobacterial culture isolates in the clinical laboratory.
Another advantage of our HMPRT-PCR method is that it can directly identify mycobacterial infections from sputum samples. In particular, this assay showed a sensitivity level of 94.3% (50/53 sputum samples) for the identification of sputum samples with an AFB score equal to or greater than 2+, which seems to be acceptable for the identification of mycobacterial organisms from sputum in a clinical laboratory. However, a relatively low level of sensitivity in identification was observed for sputum samples with 1+ and trace AFB scores. To improve the sensitivity of this assay, use of a nested PCR strategy should be considered for samples with AFB scores lower than 1+.
Moreover, our HMPRT-PCR method can identify mycobacterial coinfections directly from sputa. Recently, reports regarding mycobacterial coinfections have increased along with the increase in the incidence of AIDS and immune-compromised patients (10, 12, 24). Thus, the detection of coinfection in clinical specimens has gained much attention in the mycobacterial diagnostic field. Culture-based methods can underestimate the incidence of genuine coinfections in specimens, probably due to physiological differences, particularly differences in the growth rates between Mycobacterium spp. or host immune status. Our method could detect coinfections in eight sputum samples identified as having a single infection by a culture-based protocol (Table 6). Identical results were found in repeated experiments (data not shown).
Interestingly, the two misidentified cases observed in sputum samples with trace AFB scores were similar. In both cases, the organisms were identified as M. abscessus by culture-based rpoB PRA but were identified as M. tuberculosis by the HMPRT-PCR method (Table 6). Although we had no clinical evidence proving M. tuberculosis infections in these cases, the possibility that the difference in the results obtained by the two methods may be due to differences in the sensitivity of the HMPRT-PCR method in the detection of these two strains cannot be excluded. Our data showed that the sensitivity level of this method was lower for the detection of M. abscessus (66.7%, 20/30) than for the detection of M. tuberculosis (100%, 21/21) (Table 5). The other possible explanation is that the difference in the results obtained by the two methods may be due to the difference in the growth rates between the slowly growing species, M. tuberculosis, and the rapidly growing species, M. abscessus. Because the two mismatched cases were found exclusively for sputum samples with trace AFB scores, it is possible that increasing the sensitivity of the HMPRT-PCR method using a nested PCR strategy may solve this problem.
The mycobacteria in 2 of 26 sputum specimens whose identification could not be achieved by HMPRT-PCR were confirmed to be M. celatum and M. szulgai by culture-based hsp65 sequencing analysis (Table 5). Identification of the two strains is not possible through our HMPRT-PCR method. Although both strains are known to be rarely encountered in the Republic of Korea, the addition of probes to detect these two strains deserves to be considered for the next version of this assay.
In conclusion, our data suggest that a novel HMPRT-PCR method to detect pathogenic mycobacterial species from sputum samples, as well as culture isolates, could be effective as a routine method in the clinical setting.
Acknowledgments
This study was supported by grant A101205 from the Korean Healthcare Technology R&D project, Ministry for Health, Welfare & Family Affairs, Republic of Korea, and in part supported by grant 04-2008-0860 from the SNUH Research Fund.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Adékambi, T., P. Colson, and M. Drancourt. 2003. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J. Clin. Microbiol. 41:5699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, P. F., A. B. Bloch, P. T. Davison, and D. E. Sneider, Jr. 1991. Tuberculosis in patients with human immunodeficiency virus infection. N. Engl. J. Med. 324:1644-1649. [DOI] [PubMed] [Google Scholar]
- 3.Bloom, B. R. 1992. Back to a frightening future. Nature 358:538-539. [DOI] [PubMed] [Google Scholar]
- 4.Bloom, B. R., and C. J. L. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 5.Cloud, J. L., H. Neal, R. Rosenberry, C. Y. Turenne, M. JAMA, D. R. Hillyard, and K. C. Carroll. 2002. Identification of Mycobacterium spp. by using a commercial 16S ribosomal DNA sequencing kit and additional sequencing libraries. J. Clin. Microbiol. 40:400-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coll, P., M. Garrigo, C. Moreno, and N. Marti. 2003. Routine use of Gen-Probe Amplified Mycobacterium Tuberculosis Direct (MTD) test for detection of Mycobacterium tuberculosis with smear-positive and smear-negative specimens. Int. J. Tuber. Lung Dis. 7:886-891. [PubMed] [Google Scholar]
- 7.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 8.Espy, M. J., J. R. Uhl, L. M. Sloan, S. P. Buckwalter, M. F. Jones, E. A. Vetter, J. D. Yao, N. L. Wengenack, J. E. Rosenblatt, F. R. Cockerill, III, and T. F. Smith. 2006. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin. Microbiol. Rev. 19:165-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foongladda, S., S. Pholwat, B. Eampokalap, P. Kiratisin, and R. Sutthent. 2009. Multi-probe real-time PCR identification of common Mycobacterium species in blood culture broth. J. Mol. Diagn. 11:42-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopinath, K., and S. Singh. 2009. Multiplex PCR assay for simultaneous detection and differentiation of Mycobacterium tuberculosis, Mycobacterium avium complexes and other mycobacterial species directly from clinical specimens. J. Appl. Microbiol. 107:425-435. [DOI] [PubMed] [Google Scholar]
- 11.Halse, T. A., J. Edwards, P. L. Cunningham, W. J. Wolfgang, N. B. Dumas, V. E. Escuyer, and K. A. Musser. 2010. Combined real-time PCR and rpoB gene pyrosequencing for rapid identification of Mycobacterium tuberculosis and determination of rifampin resistance directly in clinical specimens. J. Clin. Microbiol. 48:1182-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huminer, D., S. Dux, Z. Samra, L. Kaufman, A. Lavy, C. S. Block, and S. D. Pitlik. 1993. Mycobacterium simiae infection in Israeli patients with AIDS. Clin. Infect. Dis. 17:508-509. [DOI] [PubMed] [Google Scholar]
- 13.Joh, J. S., C. H. Lee, J. E. Lee, Y. K. Park, G. H. Bai, E. C. Kim, S. K. Han, Y. S. Shim, and J. J. Yim. 2007. The interval between initiation of anti-tuberculosis treatment in patients with culture-positive pulmonary tuberculosis and receipt of drug-susceptibility test results. J. Korean Med. Sci. 22:26-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent, P., and G. Kubica. 1985. Public health mycobacteriology—a guide for the level III laboratory. Centers for Disease Control and Prevention, Atlanta, GA.
- 15.Kim, B. J., J. H. Park, S. A. Lee, H. Kim, C. Y. Cha, Y. H. Kook, E. C. Kim, S. I. Joo, J. S. Lee, and J. J. Yim. 2008. Differentiation of mycobacteria in sputa by duplex polymerase chain reaction for mycobacterial hsp65 gene. Diagn. Microbiol. Infect. Dis. 62:193-198. [DOI] [PubMed] [Google Scholar]
- 16.Kim, B. J., S. H. Lee, M. A. Lyu, S. J. Kim, G. H. Bai, G. T. Chae, E. C. Kim, C. Y. Cha, and Y. H. Kook. 1999. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 37:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, H., S. H. Kim, T. S. Shim, M. N. Kim, G. H. Bai, Y. G. Park, S. H. Lee, G. T. Chae, C. Y. Cha, Y. H. Kook, and B. J. Kim. 2005. Differentiation of Mycobacterium species by analysis of the heat-shock protein 65 gene (hsp65). Int. J. Syst. Evol. Microbiol. 55:1649-1656. [DOI] [PubMed] [Google Scholar]
- 18.Kim, H. Y., Y. Kook, Y. J. Yun, C. G. Park, N. Y. Lee, T. S. Shim, B. J. Kim, and Y. H. Kook. 2008. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J. Clin. Microbiol. 46:3384-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, H. J., H. S. Mun, H. Kim, E. J. Oh, Y. Ha, G. H. Bai, Y. G. Park, C. Y. Cha, Y. H. Kook, and B. J. Kim. 2006. Differentiation of mycobacterial species by hsp65 duplex PCR followed by duplex-PCR-based restriction analysis and direct sequencing. J. Clin. Microbiol. 44:3855-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh, W. J., O. J. Kwon, and K. S. Lee. 2005. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J. Korean Med. Sci. 20:913-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, H., H. J. Park, S. N. Cho, G. H. Bai, and S. J. Kim. 2000. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J. Clin. Microbiol. 38:2966-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung, K. L., C. W. Yip, W. F. Cheung, A. C. Lo, W. M. Ko, and K. M. Kam. 2009. Development of a simple and low-cost real-time PCR method for the identification of commonly encountered mycobacteria in a high throughput laboratory. J. Appl. Microbiol. 107:1433-1439. [DOI] [PubMed] [Google Scholar]
- 23.Lim, S. Y., B. J. Kim, M. K. Lee, and K. Kim. 2008. Development of a real-time PCR-based method for rapid differential identification of Mycobacterium species. Lett. Appl. Microbiol. 46:101-106. [DOI] [PubMed] [Google Scholar]
- 24.Massenkeil, G., M. Opravil, M. Salfinger, A. von Graevenitz, and R. Lüthy. 1992. Disseminated coinfection with Mycobacterium avium complex and Mycobacterium kansasii in a patient with AIDS and liver abscess. Clin. Infect. Dis. 14:618-619. [DOI] [PubMed] [Google Scholar]
- 25.McNabb, A., K. Adie, M. Rodrigues, W. A. Black, and J. Isaac-Renton. 2006. Direct identification of mycobacteria in primary liquid detection media by partial sequencing of the 65-kilodalton heat shock protein gene. J. Clin. Microbiol. 44:60-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, N., T. Cleary, G. Kraus, A. K. Young, G. Spruill, and H. J. Hnatyszyn. 2002. Rapid and specific detection of Mycobacterium tuberculosis from acid-fast bacillus smear-positive respiratory specimens and BacT/ALERT MP culture bottles by using fluorogenic probes and real-time PCR. J. Clin. Microbiol. 40:4143-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mun, H. S., H. J. Kim, E. J. Oh, H. Kim, Y. G. Park, G. H. Bai, J. Do, C. Y. Cha, Y. H. Kook, and B. J. Kim. 2007. Direct application of AvaII PCR restriction fragment length polymorphism analysis (AvaII PRA) targeting 644 bp heat shock protein 65 (hsp65) gene to sputum samples. Microbiol. Immunol. 51:105-110. [DOI] [PubMed] [Google Scholar]
- 28.Nakanaga, K., N. Ishii, K. Suzuki, K. Tanigawa, M. Goto, T. Okabe, H. Imada, A. Kodama, T. Iwamoto, H. Takahashi, and H. Saito. 2007. “Mycobacterium ulcerans subsp. shinshuense” isolated from a skin ulcer lesion: identification based on 16S rRNA gene sequencing. J. Clin. Microbiol. 45:3840-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinsky, B. A., and N. Banaei. 2008. Multiplex real-time PCR assay for rapid identification of Mycobacterium tuberculosis complex members to the species level. J. Clin. Microbiol. 46:2241-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenzweig, D. Y. 1996. Nontuberculous mycobacterial disease in the immunocompetent adult. Semin. Respir. Infect. 11:252-261. [PubMed] [Google Scholar]
- 31.Simmon, K. E., J. I. Pounder, J. N. Greene, F. Walsh, C. M. Anderson, S. Cohen, and C. A. Petti. 2007. Identification of an emerging pathogen, Mycobacterium massiliense, by rpoB sequencing of clinical isolates collected in the United States. J. Clin. Microbiol. 45:1978-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor, Z., C. M. Nolan, and H. M. Blumberg. 2005. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recommend. Rep. 54(RR-12):1-81. [PubMed] [Google Scholar]
- 33.Wagner, D., and L. S. Young. 2004. Nontuberculous mycobacterial infections: a clinical review. Infection 32:257-270. [DOI] [PubMed] [Google Scholar]
- 34.Wolinsky, E. 1992. Mycobacterial diseases other than tuberculosis Clin. Infect. Dis. 15:1-10. [DOI] [PubMed] [Google Scholar]