Abstract
The emergence of a novel pandemic human strain of influenza A (H1N1/09) virus in April 2009 has demonstrated the need for well-validated diagnostic tests that are broadly applicable, rapid, sensitive, and specific. The analytical performance and clinical validity of results generated with the novel Roche RealTime Ready Influenza A/H1N1 Detection Set using the LightCycler 2.0 instrument were characterized. Analytical performance was assessed by processing respiratory samples spiked with H1N1/09 and seasonal influenza A virus, a set of seasonal influenza A virus subtypes, and samples containing common viral and bacterial respiratory pathogens. The clinical validity of results was assessed in comparison to other assays by analyzing 359 specimens at three clinical sites and one reference laboratory. Direct sequencing was used to resolve samples with discrepant results. The assay detected virus concentrations down to <50 RNA copies per reverse transcription (RT)-quantitative PCR (qPCR). Various influenza A virus subtypes were covered. The analytical specificity was 100%. High clinical validity was demonstrated by the 99% positive agreement between seasonal influenza A viruses, 98% positive agreement between H1N1/09 viruses, and 88% agreement between negative results. The analytical sensitivity was compared to those of three other RT-qPCR assays and was found to be equivalent. The novel Roche RealTime Ready Influenza A/H1N1 Detection Set can be utilized on the widely used LightCycler platform. We demonstrate its usefulness for the rapid detection and surveillance of pandemic H1N1/09 influenza A virus infections.
In April 2009, a new influenza A H1N1 virus strain (H1N1/09) was identified in Mexico and was reported by the CDC and WHO (2, 4). Despite preventive measures, this agent rapidly spread. WHO consequently declared pandemic alert phase 6 in June 2009, indicating that a global pandemic is under way (21). It is now clear that the eight RNA gene segments of the new virus are a mixture of components from avian, pig, and human influenza viruses, presumably recombined as a result of a series of viral coinfections and gene reassortments (15). Unlike seasonal influenza virus strains, the new H1N1/09 virus was rapidly transmitted among children and young adults, whereas people older than 65 years of age were rarely affected (11). In the majority of cases, disease severity has been mild (4), but reports also showed a higher-than-usual proportion of serious illness and death in young healthy adults (7). Higher hospitalization rates and case fatalities have also been observed for persons with underlying medical conditions or pregnancy (7, 19). Experiences gained in the past southern and northern hemisphere's winter season demonstrate that complicated cases of H1N1/09 infections can pose significant additional burdens on hospitals and intensive care units (ICUs) (7, 19). Questions have come up regarding whether the numbers of available ICU beds and respirators needed for mechanical ventilation will be up to the increased demand in all affected countries. Also, in this context, concerns still remain that the new virus will acquire virulence in a second or third wave of disease, a scenario which has its precedent in the severe 1918 influenza pandemic (17).
The H1N1/09 pandemic demonstrates the need for reliable tools that enable rapid laboratory confirmation and follow-up of infections. For public health laboratories, it is important to be able to conduct surveillance and monitor the dynamics of pandemic H1N1/09 virus infection in a population. Reliable surveillance is a prerequisite for the timely detection of potential changes in the pathogen's virulence that might arise by accumulating mutations. Shortly after the emergence of a novel influenza virus strain, the virus is usually first identified by PCR amplification with broadly reactive primers, followed by the sequencing of PCR products (10). Although this method is relatively time-consuming and insensitive, it enables the identification of specific marker sequences, which can then be compared to sequence database entries of known pathogens. On the basis of these data, more sensitive and specific diagnostic assays can be developed. Among currently available routine laboratory methods, reverse transcription (RT)-quantitative PCR (qPCR) assays have proven to be adequate for surveillance purposes and for the rapid laboratory-guided clinical diagnosis of influenza virus infection. It has largely replaced traditional virus culture- and antigen-based methods, mainly because of the shorter time to result and higher sensitivity and specificity (13). Shortly after the emergence of pandemic H1N1/09, the CDC developed an RT-qPCR protocol for the detection of the novel pathogen (22). This assay was the first to be approved by the FDA with an emergency-use authorization (EUA). Several other commercial and noncommercial RT-qPCR assays for the detection of the H1N1/09 virus have been developed in the meantime, but validation data on these assays are still limited (3, 9, 12, 14, 20). The present study characterizes the analytical performance and clinical validity of test results generated with the novel Roche RealTime Ready Influenza A/H1N1 Detection Set, which is based on an established assay design (12, 18). Study results presented here are the basis of the test's EUA issued by the FDA in November 2009.
MATERIALS AND METHODS
Samples and sample collection.
The clinical performance characteristics of the RealTime Ready Influenza A/H1N1 Detection Set on the LightCycler 2.0 instrument were established by comparing the respective test results with those obtained with the CDC real-time RT-PCR (rRT-PCR) Swine Flu Panel or other validated tests (22). This retrospective study was conducted by analyzing well-characterized clinical specimens at two U.S. clinical testing sites (Evanston and Houston), one site in Mexico, and one U.S. reference laboratory (Albuquerque).
(i) Evanston.
During early 2009, NorthShore University HealthSystem analyzed over 6,000 nasopharyngeal swab samples for the detection of influenza virus and specifically the novel H1N1/09 virus. Testing was done in the Molecular Diagnostics Laboratory using RT-qPCR methods: the EraGen (Madison, WI) ASR reagents for influenza A and B viruses and an in-house-developed PCR/melt curve assay for H1, H3, and novel influenza virus discrimination. Influenza virus subtyping was based on matrix gene sequence variations reported previously by Stone et al. (16) and validated to demonstrate the accurate detection of the novel H1N1 virus (8). Evaluation of in-house testing involved comparative testing by viral culture and by PCR performed at the Illinois Department of Public Health using the CDC PCR assay (22). Samples came from individuals of all ages and included samples from inpatients and outpatients and referral samples. After testing, the remaining viral transport medium was frozen at −80°C in 0.5-ml aliquots. For this study, a 125-sample set defined to include positive and negative samples was used. Samples were entirely stripped of identifying numbers and labeled only with a code linked to the flu result but no sample identification (ID). Institutional review board (IRB) approval was obtained for the study and later extended to include an additional 20 negative samples. The nasopharyngeal samples from NorthShore University HealthSystem were collected by using rayon swabs and placed into 3 ml of universal viral transport medium (VTM) for viruses, chlamydiae, mycoplasmas, and ureaplasmas (Becton Dickinson, Sparks, MD).
(ii) Houston.
Nasal wash samples came from individuals of all age groups and included samples from inpatients and outpatients and referral samples. After initial testing, the remaining material was frozen at −80°C in 0.5-ml aliquots. For this study, a 126-sample set defined to include positive and negative samples was used. The sample set was analyzed in a blinded and randomized manner. IRB approval was obtained for the study.
(iii) Mexico.
Fifty-five human nasal swab samples were collected from individuals in Mexico City and resuspended in 2.5 ml of VTM (Becton Dickinson). Samples were aliquoted at 0.5 ml and stored at −70°C.
All samples were tested for the presence of seasonal influenza A virus and pandemic H1N1/09 virus, and negative samples were precharacterized by the CDC real-time RT-PCR protocol (22).
Virus stock material and serial dilutions.
Culture supernatants of the seasonal influenza virus isolate A/Brisbane/59/07-like (H1N1) and the pandemic influenza virus isolate A/Hamburg/05/09 (H1N1/09), obtained from the University of Marburg, Marburg, Germany, were used to generate a series of spiked samples for an assessment of the analytical performance of the RT-qPCR assays. To facilitate sample homogenization, a “spiking solution” (Sw1-1 and InfA1-1) was prepared from undiluted culture stocks by adding 200 μl of each stock supernatant to 300 μl of a sterile 0.9% NaCl solution and thorough mixing. From this 500 μl of “spiking solution,” 50 μl was added to 450 μl of pooled nasal wash solution collected from healthy volunteers (Sw1-2 and InfA1-2). From this 500 μl, further 10-fold serial dilutions were generated by consecutively adding 50 μl to 450 μl of nasal wash solution (Sw1-3 and -4 and InfA1-3 and -4). The same procedure was repeated with pooled nasal swab solution generated by resuspending nasal swabs (collected from healthy volunteers) in 1 ml of a sterile 0.9% NaCl solution per swab, prior to spiking. Pooled nasal wash and nasal swab solutions were tested for the absence of seasonal influenza A, influenza B, or pandemic influenza (H1N1/09) virus RNA by RT-qPCR prior to spiking.
Nucleic acid isolation and determination of virus stock RNA concentrations.
Total nucleic acids from spiked samples and clinical samples were isolated by using the MagNA Pure LC Total Nucleic Acid Isolation Kit—High Performance (Roche) on a MagNA Pure LC instrument (Roche), according to the instructions in the manufacturer's external lysis protocol. To determine the concentration of the specific viral RNA contained in the spiked solutions, 200 μl of the solutions Sw1-1, -2, and -3 and InfA1-1, -2, and -3 was added to 300 μl lysis buffer and mixed well under safety cabinets before starting automated nucleic acid isolation. Elution was done with 100 μl elution buffer provided with the isolation kit. The concentration of influenza A and H1N1/09 virus-specific RNA was determined by analyzing the eluates with two different RT-qPCR assays (10, 12). Briefly, 10 μl of eluate was used for reverse transcription (RT) in a total reaction volume of 20 μl using Moloney murine leukemia virus (M-MuLV) reverse transcriptase (Roche) according to the manufacturer's instructions. Two replicates containing 10 μl of each RT product (corresponding to 5 μl eluate) were analyzed by using the TaqMan universal PCR master mix, No AmpErase UNG (Applied Biosystems), and primers and probes as previously described (10, 12). Cycling was performed with a StepOnePlus instrument (Applied Biosystems). PCR standard curves for the absolute quantification of viral RNA were obtained by reverse transcription and amplification of a series of defined amounts of precharacterized, in vitro-transcribed, and assay-specific RNA transcript standards generated as previously described (20). The specific RNA concentrations of the stock dilutions were obtained by comparing the respective quantification cycles to the standard curves. Each sample was quantitated in duplicate, and the geometric mean was reported as the final quantitative result. For the quantification of Sw1-4 and InfA1-4, the obtained quantitative results were extrapolated one step further. Sw1-4 and InfA1-4 were then used as the first-step dilution (“dilution 1:1” in Table S1 in the supplemental material) for the determination of the analytical sensitivities of the assays. The concentration of specific viral RNA (measured in genome copies [cp] per milliliter) in the undiluted virus stocks was calculated from these quantitative results by multiplying the RNA quantities of Sw1-4 and InfA1-4 by a factor of 6.25 × 105. This factor was derived by considering all dilution steps introduced during sample preparation (×20, 5 μl PCR input from 100 μl eluate; ×2.5, isolation from 200 μl of 500 μl Sw1-4; ×1,000, three 10-fold dilution steps from Sw1-1 to Sw1-4; ×2.5, 200 μl of virus culture stock added to 300 μl of a 0.9% NaCl solution to generate the “spiking solution”; ×5, conversion from 200 μl−1 to 1 ml−1 of virus stock supernatant).
Determination of virus stock PFU and TCID50.
The seasonal and pandemic influenza virus strains A/Brisbane/59/07-like and A/Hamburg/05/09 were propagated and harvested according to standard influenza virus culture techniques in Marburg. The amount of PFU was determined for the H1N1/09 strain to be 7.0 × 105 PFU/ml by means of a plaque assay. For the seasonal H1N1 isolate, the median tissue culture infective dose (TCID50) was determined to be 108.2 TCID50/ml (calculated PFU, 1.1 × 108 PFU/ml [see http://www.lgcstandards-atcc.org/ for details about the mathematical conversion]). Besides cp/ml, the sensitivity is also reported as PFU/ml. However, the ratio between the PFU and the RNA copy numbers can vary considerably between strains and harvests. Consequently, these ratios are not to be viewed as absolute but provide merely a relative comparison between the assays.
Design of primers and probes and implementations of RT-qPCR assays for detection of seasonal influenza A and pandemic H1N1/09 viruses.
Real-time RT-qPCR oligonucleotides for influenza A H1N1/09 virus targeting the hemagglutinin (HA) gene (“Inf A/H1 assay”) were designed as previously described (12). Briefly, several primer-and-probe combinations were evaluated experimentally, and the most efficient combination was chosen in terms of established RT-qPCR quality characteristics (e.g., by comparing the quantification cycles, sigmoid shape of the amplification curves, total level of the fluorescence signal, reproducibility of the results in replicates, and stability of the baseline fluorescence level [data not shown]). Table 1 shows the sequences of the primers and probes used for the Inf A/H1 assay: H1SWS, H1SWAs, and H1SWP (78 bp). Previously reported PCR primers targeting the matrix (MA) gene (“Inf A/M2 assay”) of influenza A virus were used for the general influenza A virus RT-qPCR (18): M_InfA_F, M_InfA_R, and M_InfA_TM (95 bp) (Table 1). Primers and probes were obtained as part of the RealTime Ready Influenza A/H1N1 Detection Set (Roche). The kit includes an internal control that targets the human myostatin (MSTN) gene as a common nucleic acid in patient samples and verifies the adequacy of the sample and reaction. The primers and probes for Inf A/M2 and the internal control were combined during the reaction setup, and both reactions were performed in the same capillary. The set was used in combination with the RealTime Ready RNA Virus Master kit (Roche), which is a reaction mix for one-step RT-qPCR using LightCycler instruments (Roche). The reaction setup was done according to the manufacturer's instructions. A typical 20-μl reaction mixture consisted of 5 μl nucleic acid extract, 4 μl of 5× reaction buffer, 0.4 μl of 50× enzyme blend, 3.0 μl of Inf A/M2 or Inf A/H1 primer-probe mix (final concentrations of 400 nM each primer and 200 nM probe), and 7.6 μl PCR-grade water (Inf A/H1 assay). For the Inf A/M2 assay, 3.0 μl of primer-probe mix for the human myostatin extraction control and 4.6 μl PCR-grade water were added. Thermal cycling was done with LightCycler 2.0 instruments (Roche) and comprised a 30-min RT step at 50°C, a 15-min initial enzyme activation step at 95°C, and 45 cycles of 94°C for 15 s and 60°C for 30 s.
TABLE 1.
Primer and probe sequences
| Assay and gene target | Primer or probe | Amplicon size (bp) | Primer or probe sequence (5′-3′)b | nt positions or accession no.a |
|---|---|---|---|---|
| Inf A/H1 | FJ966082 | |||
| HA | H1SWS | 78 | CATTTGAAAGGTTTGAGATATTCCC | 380-404 |
| HA | H1SWAs | ATGCTGCCGTTACACCTTTGT | 457-437 | |
| HA | H1SWP | FAM-ACAAGTTCATGGCCCAATCATGACTCG-BBQ | 409-435 | |
| Inf A/M2c | CY038773 | |||
| MA | M_InfA_F | 95 | AAGACCAATCCTGTCACCTCTGA | 175-197 |
| MA | M_InfA_R | CAAAGCGTCTACGCTGCAGTCC | 269-248 | |
| MA | M_InfA_TM | FAM-TTTGTGTTCACGCTCACCGT-BBQ | 215-234 | |
| Sequencing | ||||
| HA | HAH1-58F | 1,619 | TTATGTATAGGTTATCATGCGAA | 58-80 |
| HA | HAH1-1657R | GACCCATTAGAGCACATCCA | 1676-1657 | |
| HA | HAH1-149F | 717 | TAGAAGACAAGCATAACGGGAAA | 149-171 |
| HA | HAH1-865R | CTGGTGTATCTGAAATGATAATA | 865-843 | |
| HA | S1910F | 828 | CATTTCAGATACACCAGTCCACGA | 849-872 |
| HA | HAH1-1657R | GACCCATTAGAGCACATCCA | 1676-1657 |
nt, nucleotide. Reference sequence accession numbers are for the GenBank/EMBL/DDBJ databases.
Hydrolysis probes were labeled with 6-carboxyfluorescein (FAM) at the 5′ end and a BlackBerry (BBQ) quencher at the 3′ end.
Primers and probes reported in reference 18.
Analytical sensitivity, specificity, and inclusivity of the RT-qPCR assays.
For assessing the limit of detection (LOD) of both RT-qPCR assays, a dilution series of 1:1, 1:5, 1:10, 1:50, and 1:100 was generated, starting with the virus stock dilutions SW1-4 and InfA1-4 (“dilution 1:1” in Table S1 in the supplemental material). To mimic natural sample matrix effects of clinical respiratory samples, nasal wash and nasal swab solutions were used as diluents. Table S1 in the supplemental material shows the virus concentrations in each dilution step. The respective LODs were assessed by testing 21 replicates of each of the five dilution steps in five independent nucleic acid isolation and RT-qPCR runs: (i) pandemic H1N1/09 (A/Hamburg/05/09) virus spiked in nasal swab or nasal wash solution and analyzed by the Inf A/H1 assay, (ii) pandemic H1N1/09 (A/Hamburg/05/09) virus spiked in nasal swab or nasal wash solutions and analyzed by the Inf A/M2 assay, and (iii) seasonal H1N1 (A/Brisbane/59/07-like) virus spiked in nasal swab or nasal wash solutions and analyzed by the Inf A/M2 assay. The Inf A/H1 assay was not run with the seasonal influenza A virus dilution series because the design and preliminary experiments indicated a high specificity of this assay for the pandemic H1N1/09 virus strain. Crossing points (Cps) were recorded for each analysis, and the assay result was called positive or negative (data not shown).
Fractions of positive results for each concentration were subject to probit regression analysis (95% LOD) by using the SAS software package, version 9.1 (SAS Institute Inc.).
The analytical specificities of the assays were determined by testing a set of commonly encountered viral and bacterial pathogens (a detailed list is given in Table S2 in the supplemental material). Viral samples were initially identified and quantified by RT-qPCR or qPCR during routine diagnostic workup. For this study, the respective respiratory samples were treated as described above with respect to nucleic acid isolation and assay setup. Precharacterized cultures of 18 different bacterial species were used for the analysis of specificity at concentrations of >106 CFU/ml. To assess the analytical inclusivity of the Inf A/M2 assay with respect to broad reactivity with influenza A virus and the analytical specificity of the Inf A/H1 assay with respect to broad reactivity with the novel (H1N1) 2009 strain, a set of human and animal influenza virus isolates was tested (Table S2). These influenza virus samples were analyzed in duplicate at concentrations of 102 and 103 copies per RT-qPCR and comprise 14 circulating virus subtypes of different geographic origins collected in 3 decades.
Comparison of different assays for the detection of pandemic H1N1/09 virus.
A previously characterized influenza A/H1N1/09 virus reference sample was kindly provided by the European Network for Diagnostics of Imported Viral Diseases (ENIVD). A lyophilized sample contained 7.2 × 106 RNA copies/ml of A/Hamburg/4/2009 as measured by RT-qPCR by an ENIVD expert laboratory. It was reconstituted in 100 μl of 0.9% sodium chloride and serially 10-fold diluted in 0.9% sodium chloride. Nucleic acid extraction was accomplished by using the MagNA Pure LC Total Nucleic Acid Isolation Kit—High Performance (Roche) on a MagNA Pure LC instrument (Roche) as described above but using the fully automated procedure (200-μl input volume and 100-μl elution volume). Each dilution was extracted in duplicate, and here, total RNA was aliquoted and stored at −80°C for subsequent analysis. Three different RT-qPCR assays were compared with the RealTime Ready Influenza A/H1N1 Detection Set (Roche). One assay was the in-house version of the RealTime Ready assay, which was conducted as previously described (12). The second assay was the CDC-like assay by TIB Molbiol (Berlin, Germany). The third assay was the Artus Infl./H1 LC/RG RT-PCR kit (Qiagen, Hilden, Germany). Each assay was performed according to the recommendations of the manufacturer. All assays comprised two individual RT-qPCRs targeting the HA and MA genes, respectively. Each dilution was tested in duplicates by each of the four assays, resulting in a total of 4 (2 × 2) RT-qPCRs per dilution and assay.
Clinical sample preparation and analysis.
Total nucleic acids were isolated from 300 μl of the respective clinical sample and resuspended in 100 μl buffer using the MagNA Pure LC Total Nucleic Acid Isolation Kit—High Performance (Roche) on MagNA Pure LC instruments (Roche) according to the manufacturer's external lysis protocol.
The detection of pandemic influenza A/H1N1/09 and seasonal influenza viruses was performed by two methods. The first method utilized the RealTime Ready Influenza A/H1N1 Detection Set (Roche) with the RealTime Ready RNA Virus Master kit (Roche). Thermal cycling was done with LightCycler 2.0 instruments (Roche) under the conditions described above. The second method utilized the CDC protocol for RT-PCR for influenza A(H1N1) virus using primers and probes described in the protocol (22) and an Invitrogen One-Step RT-PCR SuperScript III Platinum quantitative kit. Thermal cycling was done with a LightCycler 2.0 instrument (Roche) under the following conditions: 50 min at 50°C, 2 min at 95°C, 45 cycles of 15 s at 95°C, and 30 s at 55°C. All reactions for both methods were performed according to the manufacturers' instructions by using 5 μl of total RNA in a 20-μl total reaction mixture volume. For both detection methods an internal control (the human myostatin gene for the Roche method and RNase P for the CDC protocol) was included.
(i) Evanston.
For analysis with the CDC reference method, 500 μl of VTM from the nasopharyngeal swab sample was utilized for total nucleic acid isolation using a NucliSENS EasyMAG nucleic acid extractor (bioMérieux, France) and standard protocols. Total RNA was eluted in 60 μl of NucliSENS EasyMAG Extraction Buffer 3 and stored at 4°C for RT-PCR analysis the same day and/or frozen at −80°C for longer storage.
(ii) Albuquerque.
As a reference method, the Focus Diagnostics Influenza A H1N1 (2009) Real-Time RT-PCR Assay Revision A from 4 August 2009 was used.
PCR amplification and confirmation of discordant RT-qPCR results by sequencing.
For analysis of samples with discordant RT-qPCR results, control sequencing was carried out. RT-PCR was performed by using the One-Step RT-PCR kit (Qiagen) in a total volume of 25 μl, including 2.5 mM MgCl2 and 0.4 μM each primer.
The HA gene was sequenced partially. Table 1 shows the primer and probe sequences. The initial amplification was performed by using specific primers for pandemic influenza viruses A/H1N1/09, HAH1-58F, and HAH1-1657R (1,619 bp). An aliquot (2.5 μl) from the first round of the PCR was then used as a template in a second round of PCR with primers HAH1-149F and HAH1-865R (717 bp), and S1910F and HAH1-1657R (828 bp), respectively. The PCR products were purified by using QIAquick columns (Qiagen) and sequenced in both directions with the second-round amplification primers.
Nucleotide sequences of PCR products were determined by using the BigDye Terminator cycle sequencing kit (Applied Biosystems) and separated on a model 3100 genetic analyzer (Applied Biosystems). Nucleotide sequences of PCR products were aligned and analyzed by using Clustal X and MEGA 4.0 software, respectively.
RESULTS
Analytical sensitivity, specificity, and inclusivity of the RT-qPCR assays.
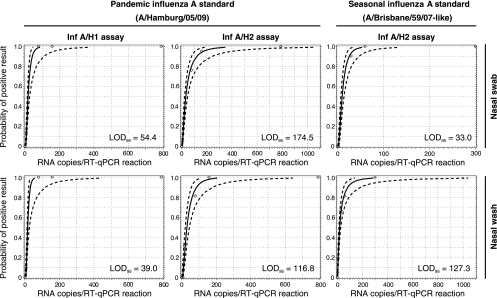
The analytical sensitivities of the Inf A/H1 and Inf A/M2 assays were assessed in terms of the 95% LOD. This common technical specification indicates the minimal target nucleic acid concentration down to which a given assay will detect the analyte with at least a 95% probability. LODs were derived by testing 21 replicates of each of the five dilution steps (see Table S1 in the supplemental material) in five independent nucleic acid isolation and RT-qPCR runs. Figure 1 shows the probit analysis plots statistically inferred from the experiment results. The 95% LODs were (i) 54.4 copies per RT-qPCR for pandemic H1N1/09 (A/Hamburg/05/09) virus spiked in nasal swab solution and 39.0 copies per RT-qPCR in nasal wash solution when analyzed with the Inf A/H1 assay, (ii) 174.5 copies per RT-qPCR for pandemic H1N1/09 virus spiked in nasal swab solution and 116.8 copies per RT-qPCR in nasal wash solution when analyzed with the Inf A/M2 assay, and (iii) 33.0 copies per RT-qPCR for seasonal H1N1 (A/Brisbane/59/07-like) virus spiked in nasal swab solution and 127.3 copies per RT-qPCR in nasal wash solution when analyzed with the Inf A/M2 assay.
FIG. 1.
Probit analysis of the pandemic H1N1/09 influenza (Inf A/H1) and influenza A (Inf A/M2) virus RT-qPCR assays. Diamond datum points represent the observed proportion of positive test results of all replicates tested at a given RNA input concentration. The statistically predicted proportion of positive results (solid line) and 95% confidence intervals (broken lines) are indicated. LOD95, 95% limit of detection.
The analytical specificity of the Inf A/H1 assay and the analytical inclusivity of the Inf A/M2 assay were evaluated by testing a set of precharacterized influenza A virus culture samples (see Table S2 in the supplemental material). No cross-reaction was observed with any influenza A virus strains when using the Inf A/H1 assay. In particular, the assay did not amplify samples of recently circulating seasonal influenza A virus subtypes, including H1N1 and H3N2. These data indicate a high specificity of the Inf A/H1 assay for the pandemic H1N1/09 strain. The Inf A/M2 assay yielded positive results for all influenza A virus strains analyzed, including strains that were first isolated >30 years ago and avian influenza virus (H7N7). This indicates a broad reactivity of the Inf A/M2 assay against influenza A virus. No cross-reaction was observed with a set of other commonly encountered viral and bacterial pathogens (Table S2).
Comparison of different assays for the detection of pandemic H1N1/09 virus.
Using a previously characterized A/H1N1/09 reference sample, three different RT-qPCR assays were compared with the RealTime Ready Influenza A/H1N1 Detection Set. Each assay contained individual RT-PCRs for the HA gene as well as for the MA gene. All assays showed comparable performances and reliably detected a 1 × 10−5 dilution of the reference sample (see Table 3). Random hits were observed at even lower concentrations. No significant differences (i.e., >100-fold) in sensitivity were observed with respect to the HA and MA genes between assays.
TABLE 3.
Comparison of the analytical sensitivities of four RT-qPCR assays for the detection of influenza A/H1N1/09 virus in quadruple test series
| Assaya | Sensitivity (test 1/test 2/test 3/test 4) to A/Hamburg/4/2009 at a log10 dilution of: |
|||||
|---|---|---|---|---|---|---|
| 1 × 10−2 | 1 × 10−3 | 1 × 10−4 | 1 × 10−5 | 1 × 10−6 | 1 × 10−7 | |
| HA gene target | ||||||
| ROC | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/−/+/− | −/−/−/− |
| PAN | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/− | −/−/+/− |
| CDC-L | +/+/+/+ | +/+/+/+ | +/+/+/− | +/+/+/− | −/−/−/− | −/−/−/− |
| ART | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/− | −/−/−/− |
| MA gene target | ||||||
| ROC | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/−/−/− | −/−/−/− |
| PAN | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/−/−/− | −/−/−/− |
| CDC-L | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/−/+/− |
| ART | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/+/+/+ | +/−/+/− | +/−/+/− |
ROC, Roche RealTime Ready Influenza A/H1N1 Detection Set; PAN, assay described previously by Panning et al. 12; CDC-L, CDC-like rRT-PCR Swine Flu Panel (TIB Molbiol, Berlin, Germany); ART, Artus Infl./H1 LC/RG RT-PCR kit (Qiagen, Hilden, Germany).
Performance of the RealTime Ready Influenza A/H1N1 Detection Set with clinical samples.
The clinical performance of the RealTime Ready Influenza A/H1N1 Detection Set was assessed by analyzing a total of 359 clinical specimens at four study sites, including 114 pandemic influenza H1N1/09 virus samples, 76 seasonal influenza A virus samples, and 169 negative samples. Test specimens covered three main specimen types: nasal swab, nasal wash, and nasopharyngeal swab samples (see Table S3 in the supplemental material). Table 2 shows a summary of the results. The positive agreement between study and alternative assays for pandemic H1N1/09 was 98%. The positive agreement for seasonal influenza A virus was 99%. The agreement for the negative samples was 88%. From 169 samples typed negative by the alternative assays, 13 were determined to be positive for seasonal influenza A virus and 7 were determined to be positive for pandemic H1N1/09 by the Roche RealTime Ready Influenza A/H1N1 Detection Set. Two out of 114 samples were determined to be positive for pandemic H1N1/09 by the alternative assays but positive for seasonal influenza A virus by the Roche assay. One out of 76 samples was determined to be positive for seasonal influenza A virus by the alternative assays but negative by the Roche test. Detailed results for each clinical study site are given in Table S3.
TABLE 2.
Clinical performance characteristics of the RealTime Ready Influenza A/H1N1 Detection Seta
| RealTime Ready Influenza A/H1N1 Detection Set | No. of results by alternative RT-qPCR assays |
Total no. of results | % agreement (95% CI) | ||
|---|---|---|---|---|---|
| Pandemic (H1N1) 2009 virus positive | Seasonal influenza A virus positive | Negative | |||
| Pandemic (H1N1) 2009 virus positive | 112 | 0 | 7 | 119 | 88, negatives (76-90) |
| Seasonal influenza A virus positive | 2 | 75 | 13 | 90 | 99, seasonal influenza A virus (93-100) |
| Negative | 0 | 1 | 149 | 150 | 98, pandemic (H1N1) 2009 virus (94-100) |
| Total | 114 | 76 | 169 | 359 | |
Alternative assays used were the CDC rRT-PCR assay for 2009 H1N1 influenza virus or the Focus Diagnostics Influenza A H1N1 (2009) real-time RT-PCR assay. CI, confidence interval.
PCR amplification and confirmation of discordant RT-qPCR results by sequencing.
At one study site (Mexico [see Table S3 in the supplemental material]), the proportion of discordant RT-qPCR results was significantly higher than for the other sites (24% versus ∼4%). Discrepancies for 13 samples were caused by negative results obtained with the CDC assay, while the RealTime Ready Influenza A/H1N1 Detection Set results were positive (n = 7 for pandemic H1N1; n = 6 for seasonal influenza A virus). In order to clarify this difference, seven samples with discordant results were sequenced and analyzed for potential sequence deviations in the primer and probe binding regions. PCR products of the expected sizes were successfully amplified from all seven samples by nested PCR. Sequence analysis showed no significant differences between the samples and other precharacterized influenza A H1N1/09 virus isolates with respect to the primer and probe binding sites (data not shown).
DISCUSSION
A sudden emergence and unpredictable progress are common features of influenza pandemics (5, 6). The H1N1/09 pandemic is no exception but nevertheless has provided us with a wealth of information on various aspects of this global health threat: genetic origin, epidemiology, and impact on our health care systems, to name only a few. It has also become clear that the availability of rapid and reliable diagnostic tools for the identification of infected individuals remains crucial for surveillance and early disease containment measures. Rapid availability of test results has enabled laboratories worldwide to better support clinicians in decision making regarding whether to initiate or to continue antiviral therapy for high-risk patients (1).
RT-qPCR has become the method of choice for the laboratory diagnosis of influenza virus infections due to its increased diagnostic sensitivity, specificity, time to result, and sample throughput in comparison to traditional methods like virus culture- and antigen-based tests. Several RT-qPCR protocols for the detection of the H1N1/09 virus have been reported, but standardization of and validation data on these assays are still limited (3, 9, 14).
We report on a study of the analytical performance and clinical validity of test results generated with the novel Roche RealTime Ready Influenza A/H1N1 PCR Detection Set. The assays are based on an established design (12, 18) and have been converted to a prefabricated kit that can be run as a one-step RT-qPCR on the LightCycler 2.0 instrument (Roche) in combination with the RealTime Ready RNA Virus Master kit (Roche).
The analytical sensitivity of the Inf A/M2 and Inf A/H1 assays was determined to be in the range of 30 to 180 copies per RT-qPCR with seasonal and pandemic H1N1/09 virus spiked into two appropriate sample matrices. These results are comparable (Table 3) to those of other widely used detection assays (12, 22). We demonstrate the broad reactivity of the influenza A virus-specific assay with a panel of different influenza A virus isolates collected over >30 years, likely indicating the ability to detect strains that will circulate in the future. As expected, no cross-reactivity was detectable with other viral and bacterial pathogens. The assay performance was determined under clinical conditions: at all study sites except one, a high concordance between results was observed. Sequencing data indicate that these discordances are likely to be caused by the suboptimal sensitivity of the CDC assay to H1N1/09 detection. Several nucleotide mismatches are known within the primer and probe annealing sites of the CDC assay compared to the predominant H1N1/09 sequences in Mexico (data not shown).
Taken together, validation of the analytical and clinical performance of the novel Roche RealTime Ready Influenza A/H1N1 Detection Set demonstrates its usefulness for the rapid detection and surveillance of pandemic H1N1/09 influenza A virus infections.
Supplementary Material
Acknowledgments
We thank S. Förster, M. Bollwein, Araceli Posadas, Rocio Bazan, and Octavio Garcia for expert technical assistance. We also thank A. Baillot, M. Monazahian (Niedersächsisches Landesgesundheitsamt, Hannover, Germany), B. Schweiger, and B. Biere (Robert Koch Institut, Berlin, Germany) for kindly providing influenza virus samples for specificity testing.
Footnotes
Published ahead of print on 7 July 2010.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Anonymous. 2009. Update: novel influenza A (H1N1) virus infection—Mexico, March-May, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:585-589. [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2009. Update: swine influenza A (H1N1) infections—California and Texas, April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:435-437. [PubMed] [Google Scholar]
- 3.Carr, M. J., R. Gunson, A. Maclean, S. Coughlan, M. Fitzgerald, M. Scully, B. O'Herlihy, J. Ryan, D. O'Flanagan, J. Connell, W. F. Carman, and W. W. Hall. 2009. Development of a real-time RT-PCR for the detection of swine-lineage influenza A (H1N1) virus infections. J. Clin. Virol. 45:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawood, F. S., S. Jain, L. Finelli, M. W. Shaw, S. Lindstrom, R. J. Garten, L. V. Gubareva, X. Xu, C. B. Bridges, and T. M. Uyeki. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. [DOI] [PubMed] [Google Scholar]
- 5.Fraser, C., C. A. Donnelly, S. Cauchemez, W. P. Hanage, M. D. Van Kerkhove, T. D. Hollingsworth, J. Griffin, R. F. Baggaley, H. E. Jenkins, E. J. Lyons, T. Jombart, W. R. Hinsley, N. C. Grassly, F. Balloux, A. C. Ghani, N. M. Ferguson, A. Rambaut, O. G. Pybus, H. Lopez-Gatell, C. M. Alpuche-Aranda, I. B. Chapela, E. P. Zavala, D. M. Guevara, F. Checchi, E. Garcia, S. Hugonnet, and C. Roth. 2009. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324:1557-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallaher, W. R. 2009. Towards a sane and rational approach to management of influenza H1N1 2009. Virol. J. 6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain, S., L. Kamimoto, A. M. Bramley, A. M. Schmitz, S. R. Benoit, J. Louie, D. E. Sugerman, J. K. Druckenmiller, K. A. Ritger, R. Chugh, S. Jasuja, M. Deutscher, S. Chen, J. D. Walker, J. S. Duchin, S. Lett, S. Soliva, E. V. Wells, D. Swerdlow, T. M. Uyeki, A. E. Fiore, S. J. Olsen, A. M. Fry, C. B. Bridges, and L. Finelli. 2009. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N. Engl. J. Med. 361:1935-1944. [DOI] [PubMed] [Google Scholar]
- 8.Kaul, K. L., K. A. Mangold, H. Du, K. M. Pesavento, J. Nawrocki, and J. A. Nowak. 1 July 2010, posting date. Influenza A subtyping. Seasonal H1N1, H3N2, and the appearance of novel H1N1. J. Mol. Diagn. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 9.Lau, S. K., K. H. Chan, C. C. Yip, T. K. Ng, O. T. Tsang, P. C. Woo, and K. Y. Yuen. 2009. Confirmation of the first Hong Kong case of human infection by novel swine origin influenza A (H1N1) virus diagnosed using ultrarapid, real-time reverse transcriptase PCR. J. Clin. Microbiol. 47:2344-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melzl, H., J. J. Wenzel, B. Kochanowski, K. Feierabend, B. Kreuzpaintner, E. Kreuzpaintner, A. Rohrhofer, S. Schreder-Meindl, H. Wollner, B. Salzberger, U. Reischl, W. Jilg, H. Wolf, and H. H. Niller. 2009. First sequence-confirmed case of infection with the new influenza A(H1N1) strain in Germany. Euro Surveill. 14:pii=19203. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19203. [DOI] [PubMed] [Google Scholar]
- 11.Neumann, G., T. Noda, and Y. Kawaoka. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panning, M., M. Eickmann, O. Landt, M. Monazahian, S. Olschlager, S. Baumgarte, U. Reischl, J. J. Wenzel, H. H. Niller, S. Gunther, B. Hollmann, D. Huzly, J. F. Drexler, A. Helmer, S. Becker, B. Matz, A. Eis-Hubinger, and C. Drosten. 2009. Detection of influenza A(H1N1)v virus by real-time RT-PCR. Euro Surveill. 14:pii=19329. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19329. [PubMed] [Google Scholar]
- 13.Petric, M., L. Comanor, and C. A. Petti. 2006. Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics. J. Infect. Dis. 194(Suppl. 2):S98-S110. [DOI] [PubMed] [Google Scholar]
- 14.Poon, L. L., K. H. Chan, G. J. Smith, C. S. Leung, Y. Guan, K. Y. Yuen, and J. S. Peiris. 2009. Molecular detection of a novel human influenza (H1N1) of pandemic potential by conventional and real-time quantitative RT-PCR assays. Clin. Chem. 55:1555-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith, G. J., D. Vijaykrishna, J. Bahl, S. J. Lycett, M. Worobey, O. G. Pybus, S. K. Ma, C. L. Cheung, J. Raghwani, S. Bhatt, J. S. Peiris, Y. Guan, and A. Rambaut. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122-1125. [DOI] [PubMed] [Google Scholar]
- 16.Stone, B., J. Burrows, S. Schepetiuk, G. Higgins, A. Hampson, R. Shaw, and T. Kok. 2004. Rapid detection and simultaneous subtype differentiation of influenza A viruses by real time PCR. J. Virol. Methods 117:103-112. [DOI] [PubMed] [Google Scholar]
- 17.Taubenberger, J. K., and D. M. Morens. 2006. 1918 influenza: the mother of all pandemics. Emerg. Infect. Dis. 12:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward, C. L., M. H. Dempsey, C. J. Ring, R. E. Kempson, L. Zhang, D. Gor, B. W. Snowden, and M. Tisdale. 2004. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J. Clin. Virol. 29:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webb, S. A., V. Pettila, I. Seppelt, R. Bellomo, M. Bailey, D. J. Cooper, M. Cretikos, A. R. Davies, S. Finfer, P. W. Harrigan, G. K. Hart, B. Howe, J. R. Iredell, C. McArthur, I. Mitchell, S. Morrison, A. D. Nichol, D. L. Paterson, S. Peake, B. Richards, D. Stephens, A. Turner, and M. Yung. 2009. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N. Engl. J. Med. 361:1925-1934. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel, J. J., H. Walch, M. Bollwein, H. H. Niller, W. Ankenbauer, R. Mauritz, H. J. Holtke, H. M. Zepeda, H. Wolf, W. Jilg, and U. Reischl. 2009. Library of prefabricated locked nucleic acid hydrolysis probes facilitates rapid development of reverse-transcription quantitative real-time PCR Assays for detection of novel influenza A/H1N1/09 virus. Clin. Chem. 55:2218-2222. [DOI] [PubMed] [Google Scholar]
- 21.WHO. 2009. World now at the start of 2009 influenza pandemic. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
- 22.WHO. 2009. CDC protocol of realtime RTPCR for influenza A (H1N1). World Health Organization, Geneva, Switzerland. http://www.who.int/csr/resources/publications/swineflu/realtimeptpcr/en/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.