Abstract
Little is known about the physiological mechanisms subserving the experience of air hunger and the affective control of breathing in humans. Acute hunger for air after inhalation of CO2 was studied in nine healthy volunteers with positron emission tomography. Subjective breathlessness was manipulated while end-tidal CO2- was held constant. Subjects experienced a significantly greater sense of air hunger breathing through a face mask than through a mouthpiece. The statistical contrast between the two conditions delineated a distributed network of primarily limbic/paralimbic brain regions, including multiple foci in dorsal anterior and middle cingulate gyrus, insula/claustrum, amygdala/periamygdala, lingual and middle temporal gyrus, hypothalamus, pulvinar, and midbrain. This pattern of activations was confirmed by a correlational analysis with breathlessness ratings. The commonality of regions of mesencephalon, diencephalon and limbic/paralimbic areas involved in primal emotions engendered by the basic vegetative systems including hunger for air, thirst, hunger, pain, micturition, and sleep, is discussed with particular reference to the cingulate gyrus. A theory that the phylogenetic origin of consciousness came from primal emotions engendered by immediate threat to the existence of the organism is discussed along with an alternative hypothesis by Edelman that primary awareness emerged with processes of ongoing perceptual categorization giving rise to a scene [Edelman, G. M. (1992) Bright Air, Brilliant Fire (Penguin, London)].
Guz (1), in analyzing respiratory control, stated that we are only at an early stage in understanding how higher brain centers can control breathing and experience breathlessness in humans. There is an established neural organization in the medulla, midbrain, and hypothalamus that controls the chemically determined arousal of air hunger in response to changes of arterial blood CO2. It is of great interest to identify the cortical regions that subserve the invasion of the stream of consciousness by the urge to breathe. The body's mechanisms for monitoring CO2 levels involve pH changes of interstitial fluid of the neurons of the ventral medulla (2). Hypothalamic neurons function also as central chemoreceptors that are sensitive not only to hypoxia but also to hypercapnia (3).
The results of studies of Banzett and colleagues (4, 5) are important for delineating the effects of hunger for air from those of hypercapnia. Experiments involving mechanical ventilation and variation of partial pressure CO2 (PCO2) of inspired air showed that the sensation of air hunger became intense with a relatively small rise of end-tidal PCO2 if ventilation is held constant, whereas spontaneously breathing subjects increased ventilation in response to comparable CO2 increases, which relieved their sensation of air hunger.
Guz (1) has also proposed that it is not the neural output to the muscles of breathing that is particularly relevant to the genesis of air hunger. The key is an intact brainstem respiratory oscillator that responds to stimulation. Mechanically ventilated curarized subjects reported severe air hunger when end-tidal PCO2 was increased (6). Similarly, air hunger was found with increased end-tidal PCO2 in quadriplegics, where the brainstem respiratory oscillator is intact but its activity cannot be transmitted via bulbo spinal fibers to the anterior horn cells of the respiratory musculature (7). The clinical data on the “locked-in” syndrome highlight the presence of unknown elements in respiratory control. Here a lesion of the ventral pons and lower midbrain involving motor tracts to the rest of the body will remove all voluntary control of muscle movement except eye elevation (8). Sensation is intact. Breathing is normal, very regular, and maintains a normal PCO2. Whereas voluntary effort to change breathing has no effect, emotion will disrupt breathing, indicating that emotional pathways (presumably limbic) to the brainstem are anatomically separate from the corticobulbar pathways. Patients have ventilatory sensitivity to inhaled carbon dioxide with associated breathlessness.
In our experiments, rapid onset of hypercapnia and air hunger was induced by subjects breathing an 8% CO2, 92% O2 mixture. The experiment is reported in two sections. The first comparison used a face mask for breathing the CO2 (CO2FM), and the measures of this combined effect of hypercapnia and air hunger in the subjects were compared with breathing oxygen by using a face mask (O2FM), also to a rest condition, and to a paced breathing condition (9). The data reported here compare positron emission tomography (PET) scans acquired with the CO2FM stimulus to scans acquired while breathing the CO2via a mouthpiece (CO2MP). The subjective sensation of greater ease of breathing in the case of the mouthpiece relative to the face mask involved a significantly reduced sensation of air hunger even though the end-tidal PCO2 was the same for the two conditions.
Methods
The procedures for selection of subjects, the apparatus, the experimental procedures for administration of the gas mixture (8% CO2, 92% O2), the measures of respiratory function, and the baseline measurements have been described previously (9). Inspired gases were provided by an open circuit consisting of a face mask (Hudson RCI Air Cushion mask No. 1276) or a mouthpiece attached to a tee connector with one-way valves to regulate the direction of the inspired and expired gases. The nostrils were occluded while using the mouthpiece (1.7 cm × 2.2 cm aperture). Mouthpiece scans were run as a block and alternated with the block of face mask scans randomized across subjects so that only one change of the breathing apparatus was done per subject. Positioning of the head was checked after each change. After each PET scan made during the inhalation of CO2, a questionnaire was read asking the subject to rate on a scale 0–100 various physical sensations, most importantly air hunger. The methods used for PET and MRI scanning and for statistical analysis have been described previously (9). For the comparison of the CO2FM condition to the CO2MP condition, clusters of regional cerebral blood flow (rCBF) change having statistical significance (Z > 3.75; cluster size > 40 voxels; voxel size 8 mm3) are reported in Table 2. Omnibus significance was calculated on the extrema points by using an empirical estimate of the independent elements in the subtraction image (resels) rather than simply being estimated on the basis of the theoretical number of independent voxels (see ref. 9).
Table 2.
Cortical and sub-cortical activations and deactivations from comparison of the CO2 face mask condition to the CO2 mouthpiece condition
| Activations | ||||||
|---|---|---|---|---|---|---|
| area | BA | x | y | z | Size | Z-score |
| R cingulate gyrus | 24 | 0 | 4 | 22 | 81 | 4.56 |
| R cingulate gyrus | 24 | 2 | −10 | 26 | 68 | 4.44 |
| R cingulate gyrus | 24 | 1 | 22 | 12 | 60 | 3.93 |
| L cingulate gyrus | 24 | −16 | 22 | 26 | 51 | 4.01 |
| R ant temp pole | 20 | 42 | −4 | −20 | 106 | 4.36 |
| L ant temp pole | 21 | −40 | −2 | −20 | 86 | 4.17 |
| L ant temp pole | 21 | −34 | −2 | −27 | 43 | 3.90 |
| L ant insula | −34 | 8 | 18 | 57 | 4.40 | |
| R ant insula/claustrum | 22 | 26 | 10 | 90 | 4.05 | |
| R fusiform gyrus | 37 | 42 | −54 | −10 | 75 | 4.25 |
| L lingual gyrus | 19 | −16 | −78 | −2 | 103 | 4.79 |
| L lingual gyrus | 17 | −20 | −90 | −10 | 55 | 4.21 |
| R lingual gyrus | 19 | 20 | −76 | −4 | 75 | 4.64 |
| R amygdala | 24 | −12 | −8 | 54 | 3.86 | |
| R thalamus (pulvinar) | 20 | −28 | 4 | 50 | 4.36 | |
| R thalamus (pulvinar) | 2 | −36 | 8 | 59 | 4.01 | |
| Periaqueducal grey | 2 | −18 | −10 | 52 | 4.25 | |
| Hypothalamus | 0 | −6 | −15 | 71 | 4.21 | |
| R inf parietal lobule | 40 | 42 | −46 | 28 | 45 | 4.17 |
| R mid frontal gyrus | 9 | 32 | 10 | 22 | 80 | 3.86 |
| Deactivations | ||||||
| L inf frontal gyrus | 47 | −26 | 34 | −10 | 88 | −5.67 |
| L medial frontal gyrus | 10 | −12 | 20 | −18 | 75 | −4.85 |
| L medial frontal gyrus | 11 | −18 | 38 | −12 | 81 | −4.58 |
| R medial frontal gyrus | 11 | 21 | 34 | −11 | 58 | −4.15 |
| R ventr cingulate | 32 | 2 | 24 | −8 | 84 | −3.87 |
| L thalamus | −11 | −28 | 12 | 101 | −5.04 | |
| L thalamus | −14 | −21 | 8 | 58 | −4.54 | |
| L thalamus | −2 | −14 | 4 | 93 | −4.22 | |
| L caudate | −12 | −4 | 14 | 69 | −4.65 | |
| L lentiform nucleus | −28 | −18 | 2 | 45 | −3.87 | |
| R lentiform nucleus | 19 | 0 | −5 | 72 | −3.79 | |
| L inf parietal lobule | 40 | −52 | −26 | 24 | 92 | −4.46 |
| R somatosensory cortex | 4 | 57 | −12 | 32 | 67 | −3.91 |
| R insula | 38 | −4 | 8 | 42 | −3.76 | |
Regions shown have significance |Z| > 3.75 and a large cluster size (voxels > 40; voxel volume 8 mm3).
Correlation Analysis.
A correlation value (r value) was computed as a voxel-wise correlation of breathlessness intensity ratings with the PET images from the CO2FM and CO2MP scans for each subject. As for the conditional contrast, local extrema were identified within the image of correlation values by using a previously described three-dimensional search algorithm (10–12). Omnibus significance (γ-2 statistic) was performed on such extrema. The correlation image was converted to a Z-score image, and P values were assigned from the Z distribution. Only positive correlations (Z > 2.97; P < 0.002) forming contiguous clusters (cluster size > 25; 200 mm3) are reported herein. These methods and analysis software are the same as those used to detect rCBF correlations with plasma Na+ concentration (13).
Results
Respiratory Results.
The results of a number of the experimental conditions have been reported previously (9). Herein the CO2FM and CO2MP conditions are compared with each other and to baseline measurements (Table 1). The inspired CO2 for the CO2FM (7.8 ± 0.3%) and the CO2MP (7.3 ± 0.2%) were slightly different because of the apparatus but not significantly so (P > 0.10). The end-tidal CO2 for the CO2FM and CO2MP conditions (8.8 ± 0.1% and 8.6 ± 0.1%, respectively) were also not significantly different from each other (P = 0.13), but both were highly significantly increased relative to baseline (5.4 ± 0.1%; P < 0.001). The subjective score of breathlessness for the CO2FM condition (73 ± 5) was significantly greater than for the CO2MP condition (55 ± 4) (P < 0.05). Although end-tidal CO2 was similar in the two instances, and respiratory rate was raised equivalently relative to baseline, consistent with the sense of easier breathing and reduced air hunger with the use of the mouthpiece, the minute ventilation was less with the CO2MP (17.0 ± 2.3 liters/min) compared with CO2FM (22.7 ± 3.0 liters/min), a difference that was not statistically significant (P = 0.28). Both minute ventilation volumes were very highly significantly increased relative to baseline. In both CO2 conditions, there was an increase of heart rate relative to baseline (P < 0.01), with no statistical difference between CO2 conditions (P = 0.28).
Table 1.
Group mean respiratory results (±SEM) for the eight subjects
| Condition | Insp CO2, % | Exp CO2, % | Resp. rate, min−1 | Tidal volume, liter | Min vent, liter/min | Heart rate, min−1 | Breathlessness |
|---|---|---|---|---|---|---|---|
| Rest | 0.1 | 5.4 (0.1) | 11 (0.2) | 0.72 (0.05) | 6.2 (0.2) | 67 (1) | 0 |
| CO2FM | 7.8 (0.3)*** | 8.8 (0.1)*** | 15 (0.6) | 1.51 (0.19) | 22.7 (3.0)*** | 78 (3)* | 73 (5) |
| CO2MP | 7.3 (0.2)*** | 8.6 (0.1)*** | 14 (0.6) | 1.24 (0.17) | 17.0 (2.3)*** | 72 (2)* | 55 (4) |
Significance of increase of CO2FM and CO2MP conditions relative to the rest condition: *, P < 0.01; **, P < 0.001; ***, P < 0.0001. Statistical tests were not made separately for respiration rate and tidal volume, because these measures are combined to form the minute ventilation rates.
PET Results
Conditional Contrast of CO2FM vs. CO2MP.
Omnibus statistical analysis was significant (total number of independent extrema = 678, γ-2 Z score = 4.6, P < 0.0001) two-tailed (10). Relatively greater air hunger in the CO2FM trials was accompanied by significant regional activations in paralimbic/limbic areas and in subcortical areas, e.g., in anterior, middle, and posterior cingulate cortex [Brodmann's area (BA)24/32/23], bilateral activation of the anterior temporal poles (BA20), and anterior insula/claustrum, right fusiform gyrus (BA37), and bilaterally in lingual gyri (BA19) (see Fig. 1 and Table 2). Other activations included right pulvinar, right amygdala region, hypothalamus, midbrain tegmentum (14), and also areas in neocortex—right inferior parietal lobule (BA40) and right dorsal prefrontal cortex (BA9).
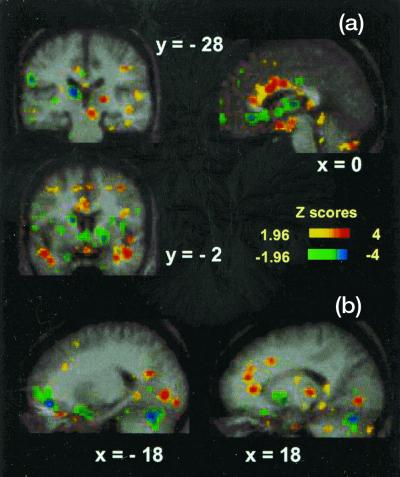
Figure 1.
PET activations and deactivations for the CO2FM vs. CO2MP conditional contrast of displayed on the average MRI brain image of the nine subjects. a shows activations in the anterior cingulate region (x = 0), bilaterally in the middle temporal gyrus (y = −2), and in the right midbrain (y = −28). Deactivations are evident in the left caudate nucleus (y = −2), the medial anterior cingulum (x = 0), and the left pulvinar and left inferior parietal lobule (y = −28). b shows activations in the left lingual gyrus (x = −18) and right anterior cingulate, posterior thalamus, and lingual gyrus (x = 18). Deactivations can be observed in the left medial frontal gyrus, bilaterally in the cerebellum, and in the right lentiform nucleus (x = 18).
Significant deactivations occurred in inferior pre- and orbitofrontal cortex (BA47/10/11), left thalamus, left caudate and lentiform nuclei, left inferior parietal lobule (BA40), right middle insula, and right precentral gyrus (BA4) (see Fig. 1 and Table 2).
Correlation with Breathlessness Ratings.
Omnibus statistical analysis was significant (number of independent extrema = 671, γ-2 Z-score = 4.97, P < 0.0001) (10). Significant correlations (Fig. 2 and Table 3) were present in the anterior and middle cingulate cortex (BA24/32), parahippocampal gyrus, insula/claustrum, left sublenticular periamygdalar region, fusiform and lingual gyrus, and right anterior temporal pole (BA20). Significant negative correlations (not tabulated) were present in superior (BA8/6) and inferior prefrontal cortex (BA11/25/47), anterior and posterior cingulate (BA32/30), and mediodorsal thalamus.
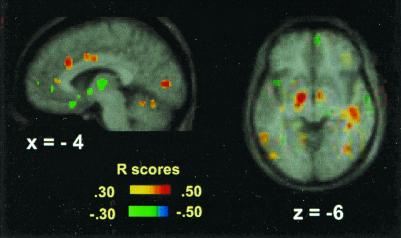
Figure 2.
Positive and negative correlations of breathlessness scores with rCBF during CO2 FM and CO2 MP scans displayed on the average MRI brain image. Significant correlations are evident in the lingual gyrus, anteriorly and rostrally in the cingulate gyrus (x = −4), and in the sublenticular area and right temporal gyrus (z = −6). The color coding of the correlation coefficients is shown.
Table 3.
Positive correlation of breathlessness scores with rCBF during CO2 trials
| Area | BA | x | y | z | Size | Z-score |
|---|---|---|---|---|---|---|
| L cingulate gyrus | 32 | −3 | 19 | 28 | 46 | 3.34 |
| L cingulate gyrus | 24 | −6 | −8 | 32 | 48 | 3.18 |
| L cingulate gyrus | 32 | −12 | 8 | 40 | 28 | 3.02 |
| R ant temp pole | 20 | 24 | −6 | −30 | 34 | 3.52 |
| R ant temp pole | 21 | 43 | 1 | −20 | 47 | 3.37 |
| L ant temp pole | 20 | −36 | −4 | −26 | 70 | 3.35 |
| R ant insula/claustrum | 24 | 20 | 17 | 49 | 3.45 | |
| L post insula | −29 | −36 | 21 | 35 | 3.21 | |
| R fusiform gyrus | 37 | 38 | −39 | 0 | 25 | 2.97 |
| R middle temporal gyrus | 21 | 48 | −32 | −3 | 39 | 3.00 |
| L lingual gyrus | 18 | −4 | −82 | 5 | 44 | 3.81 |
| L sublenticular area | −12 | −10 | −6 | 114 | 3.34 | |
| R inf parietal lobule | 40 | 33 | −43 | 20 | 45 | 3.31 |
| R mid frontal gyrus | 9 | 27 | 20 | 26 | 48 | 3.14 |
The threshold for inclusion is P < 0.002.
Discussion
Breathing through a face mask evoked a significantly increased sense of “air hunger” relative to using a mouthpiece, although end-tidal CO2 was equivalent in the two conditions. The results of the conditional contrast of CO2FM and CO2MP, and the parametric correlation of rCBF with breathlessness intensity concurred in delineating a distributed network of limbic/paralimbic, subcortical, and neocortical brain regions associated with increased subjective sense of air hunger.
Paralimbic Increases
Anterior Cingulate Cortex (ACC).
Multiple sites (12 with Z > 3.1) in anterior and middle cingulate cortex (BA24/32) were activated by a greater sense of air hunger (Figs. 1–3 and Tables 2 and 3). A previous study of CO2-induced breathing reported ACC activation but did not delineate the effects of hypercapnia from the sense of air hunger (15). Activation of the insula has also been reported (15, 16) and occurred here.
Figure 3.
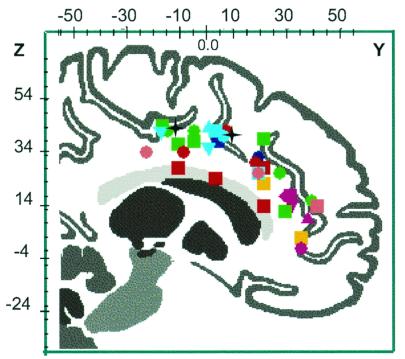
Locations in Talairach coordinates of the activations in the anterior cingulate cortex in published studies of hunger for air, thirst, hunger, micturition pain, and REM sleep (automatic rendering from brainmap search and view software, University of Texas Health Science Center, San Antonio). Air Hunger: Brannan and coworkers (9) CO2FM inhalation vs. CO2MP inhalation (red square); breathlessness correlation (red circle). Thirst: Denton et al. (25) maximum thirst vs. rest (green square); Denton et al. (13) plasma [Na+] correlation (green circle). Hunger: Tataranni et al. (30) 36-h fast vs. satiation (orange square). Micturition: Blok et al. (37, 50); a series of comparisons involving micturition, withholding urine, and empty bladder (magenta). Pain Rainville et al. (34); a series of comparisons involving painful hot vs. warm or neutral (blue); other comparisons (cyan or pink) involving painful heat vs. warm nonpainful or neutral (30, 51–55). Sleep: Maquet et al. (41) REM sleep (black star).
Anterior Temporal Poles.
Bilateral activations were present in anterior inferior temporal cortex (BA20), consistent with reports of effects of aversive emotional states (17, 18).
Fusiform and Lingual Gyri.
Involvement in the right fusiform gyrus was found in a previous study of CO2-stimulated breathing (15). The lingual gyrus has been reported as a site of early sensory processing enhancement in case of aversive visual stimuli (19, 20). In the present study, the activation of fusiform and lingual gyri in the absence of visual stimulation (subjects' eyes were kept closed) is particularly interesting. This effect may be caused by the distributed activation of linked regions in paralimbic cortex by intensely aversive stimuli, possibly serving the adaptive purpose of enhancing and preparing the visual system for the detection of a possible danger in the environment. Activation of right inferior parietal (BA40) and dorsal prefrontal cortex (BA9) is consistent with the specialized role of the two areas in the control of vigilance/arousal (21).
Deactivations.
Inferior prefrontal and orbitofrontal cortex (BA47/11/10), left caudate and lentiform nuclei and left thalamus and left inferior parietal cortex (BA40) were deactivated in association with increased air hunger. It is possible that deactivation of these regions may be associated with release of inhibitory control or behavioral suppression, functions associated with the orbitofrontal cortex (22). Finally, the deactivation of right sensorimotor cortex (BA4, M1-mouth region) and right insular cortex may be explained by unmatched oral sensations associated with holding the mouthpiece in the oral cavity during the CO2MP trials only.
Primal Emotion.
Several vegetative systems are generative of primal emotion, which has important bearing on the general question of the role of such states as reflecting the basis of the evolutionary emergence of primary consciousness. Among the definitions of emotion, the Oxford Dictionary (23) includes: “affection of the mind arising from bodily states.” Within a wide range of bodily states implicit in the above, some arbitrary gradation of intensity may be recognized. That is, a primal emotion might be proposed as one where there is sustained complete occupancy of the stream of consciousness with a singular compelling sensation and intention. Such states arise from vegetative systems and may be largely interoceptor-driven as, for example, with extreme thirst or hunger for air, and reflect that the existence of the creature is immediately threatened. Control of basic vegetative systems is centered in the phylogenetically ancient areas of the brain, as is also the case with arousal and sleep. Some distinction may be drawn between physiological evocation of emotion dictated by chemical events in the midbrain and those emotional states, such as love, anger, pity, and fear, where the inflow entraining the events may be situational perception determined by distance receptors (24, 25). On this relativistic and somewhat arbitrary scale, the study of hunger for air is an extreme of a primal emotion.
The views that have been advanced on the question of phylogenetic emergence of primary consciousness center on the neurophysiological organization that may have subserved first emergence of such dim awareness. Edelman (26) has argued that evolutionary development of the ability to create a “scene” led to the emergence of primary consciousness. Explicitly, a central element of the process is perceptual categorization, whereby the plethora of contemporaneous signals from the external world that are without necessary physical or causal connections are carved up into signals useful for a given species. Perceptual categorization is seen, along with control of movement, as the most fundamental process of the vertebrate nervous system (26, 27). Edelman suggests we experience primary consciousness as a “picture” or “mental image” of ongoing categorized events. The scene may be given salience by reentrant connections from the limbic brainstem system, which give “value-category” memory.
An alternative theory to this primarily distance-receptor concept is that primary consciousness may have arisen with basic vegetative systems like air hunger and, accordingly, from interoceptor-initiated brain events (24, 25). That is, the evolutionary origin may be from primal emotions and sensations arising from chemical sensors and receptors, both internal and surface, signaling that the existence of the organism was immediately threatened—specifically primal emotions such as hunger for air, thirst, pain, hunger, and extreme temperature change. Control of basic vegetative systems is centered in the ancient areas of the brain (rhomboencephalon, mesencephalon, and diencephalon), as is the case with the elemental process of arousal itself and of sleep.
Hunger for air is a compelling primal emotion like severe thirst. In fish and amphibians, the respiratory response is to O2, but ascent of the phylogenetic scale involved evolution of a rapid response mechanism to CO2. In mammals, its genesis is in the medulla and midbrain consequent to chemical changes in the respiratory neurones, the ensuing state of arousal involving the reticular activating system of the periaqueductal gray. Edelman and Tononi (27) draw attention to the fact that there is evidence the reticular activating system may make a more specific contribution to conscious experience over and above that implied by suggesting it has an essential but permissive role. Interactions between the thalamocortical system, the reticular thalamic nucleus, and the ascending reticular activating system appear to implement a gate that facilitates thalamocortical interactions related topographically to a particular portion of the organism's spatial envelope and selectively block other portions (28).
Components of the ascending reticular activating system involving the mesencephalic and posterior hypothalamic neuronal groups and specific elements of the phylogenetically ancient cortical areas, allocortex and transitional cortex, including cingulate and insula, together with thalamus, claustrum, and amygdala, appear to act as a distributed system of activations and deactivations to contrive the compelling emotion of “hunger for air.”
Commonality of Brain Elements Subserving Primal Emotions.
There is congruence between the loci of activations and deactivations associated with hunger for air and those identified with other primal emotions. With thirst evoked by rapid i.v. infusion of hypertonic sodium chloride, the areas activated were the midcingulate (BA24) and anterior cingulate (BA32) (Fig. 3), the posterior cingulate, parahippocampus, insula, claustrum, posterolateral nucleus of the thalamus, and sites in the cerebellum (25, 29). Strong deactivations occurred in the amygdala, the dorsomedial thalamus, and also in brainstem areas in the region of the parabrachial and pigmented paranigral nuclei. The disappearance of the cingulate activations (particularly BA32) within 3 min of drinking water to give a sense of satiation was suggestive of their having a role in the consciousness of thirst.
Studies of hunger for food and its satiation have indicated a generally similar pattern of brain activity subserving this sensation. Thus, cerebellum, hypothalamus, thalamus, insular cortex and claustrum, and the anterior cingulate (Fig. 3) were activated, together with hippocampus, parahippocampus, precuneus, caudate, and putamen, and also the posterior orbitofrontal and anterior temporal cortex (30).
Pain has been defined by behaviorists as a phenomenon characterized by withdrawal or flight as response to stimulation in any animal, vertebrate or invertebrate. Pitts (31) states that pain is understood by the layperson and clinician as conscious suffering and is absent in the surgical patient during anesthesia. Its character as a conscious experience governing behavior apt to survival parallels other compelling primal emotions and the issue of when they emerged in phylogenesis. Pitts suggests neural structures believed to subserve nociception, such as the spinoreticular system, carrying pain fibers reported to be present in mammals, some birds, reptiles, amphibians, and fish (32). He proposes pain is perceived only during consciousness.
In humans, pain induced by heat (33) caused increased activation in sensory areas S1 and S2 but also in the anterior cingulate (Fig. 3) and anterior insula, the supplementary motor area cortex, claustrum, basal thalamus, and putamen. There was also reduced rCBF in the posterior cingulate. When perceived intensity of pain was reduced or amplified by hypnosis (34), it was established that increase of perceived intensity involved increased activation in the cingulate cortex. Coghill et al. (33) drew attention to the multiple distributed activations involved in the pain system, which may explain the difficulty of eliciting painful sensations by cortical stimulation, and also why discrete cortical lesions seldom lead to complete reduction of pain, but may alter certain aspects of pain experience or behavioral reactions to pain.
Micturition is a vegetative function associated with a progressively increasing desire that verges on pain when the bladder fills to maximum capacity. In this circumstance, onset of micturition involves subjective elements that parallel gratification in other instincts. Disorders, such as urge incontinence in the elderly, are powerful emotions.
A PET study of humans (35) showed strong activations in periaqueductal gray, rostral hypothalamus and the right dorsal medial pontine tegmentum (Barrington's nucleus), structures delineated in studies of micturition in cats (36, 37). Animal experiments showed stimulation of anterior cingulate, amygdala, bed nucleus of the stria terminals, and septal nuclei would initiate micturition. The PET study also found that during micturition, the right anterior cingulate (BA32/24) (Fig. 3) and right inferior frontal gyrus (BA46/47/44) were more significantly activated than in the stage of full bladder before micturition. Deactivation of the right anterior cingulate occurred when subjects on the table had a full bladder and were unable to void.
With the individual vegetative functions, as noted above, imaging shows specific areas in the medulla, mesencephalon, and diencephalon that are consistent with other neurophysiological and anatomical knowledge of the specific vegetative function concerned. There is evidence of commonality in structures involved in these primal emotions, although valence of effect (positive or negative) may differ in different emotions. That is, the mesencephalic nuclei particularly periaqueductal gray, cerebellum, hypothalamus, thalamic nuclei, amygdala, claustrum, and the ancient cortical areas of the anterior and posterior cingulate, parahippocampus, and insula, are predominantly represented. Furthermore, it might be hypothesized that this reflects an organization of distributed activations and deactivations representing the “dynamic core” (38) subserving arousal of consciousness with the primal vegetative systems. Fig. 3 shows loci of activation in the anterior cingulate with several primal emotions discussed. It is noteworthy that, whereas activations are present throughout the cingulate cortex, a particularly dense overlap of activations is present in dorsal anterior cingulate cortex around y = +20 and z = +28 (BA32) and in middle cingulate cortex around y = 0 and z = +34 (BA24).
In contrast, during slow wave sleep (SWS), PET analysis has shown negative correlation of rCBF in dorsal pons and mesencephalon, both thalami, and basal ganglia (39). The negative correlation within dorsal pons and mesencephalon during SWS may reflect the persistent decrease in the firing rate of neurones of diffuse ascending brainstem systems, which leads to hyperpolarization of thalamic nuclei (40). There is also deactivation of the orbital prefrontal cortex, anterior cingulate (BA24/BA32), and precuneus. On the other hand, with rapid eye movement (REM) sleep with dreaming, PET showed activation in the pontine tegmentum, left thalamus, both amygdaloid complexes, anterior cingulate cortex (Fig. 3), and right parietal operculum (41). Deactivations were observed bilaterally in a large area of dorsolateral prefrontal cortex, in supramarginal gyrus as well as posterior cingulate and precuneus. REM sleep is generated by nuclei of the mesopontine reticular formation (42, 43), which activate the thalamic nuclei, which in turn activate the cortical mantle (44). The amygdaloid complexes receive important connections from the dorsal pons (45) and the intralaminar thalamic nuclei (46), and themselves project to several brainstem nuclei that could induce the autonomic phenomena of REM (45). They also send their most important cortical connections to the cingulate. Also, Roland and coworkers (47) have shown that transition from relaxed wakefulness to high general attention involves activation of midbrain tegmentum and intralaminar thalamic nuclei.
However, it is evident that even if these structures did represent such a “dynamic core” subserving primal emotion, it remains wholly unexplained how the characteristic quality of the primal emotion is experienced with its inherent specificity of intention to either fight for breath, drink, eat, micturate, withdraw in the case of pain, or accede to the desire to sleep. Presumptively the cortical elements are essential to the quality of conscious experience within the elements of a “dynamic core.” Possibly the specific character of both the sensation and intentionality is determined in some way presently inexplicable by the issue of whether the ascending input to primitive cortex comes primarily from the anterior wall of the third ventricle (thirst), the hypothalamic nuclei (hunger), Barrington's nucleus (urge to pass urine), the ventral rostral medullary and dorsal tegmental area (hunger for air), the spinoreticular pathway (pain), or the dorsolateral hypothalamus area (hunger for salt). Parallel specificity exists also within the cortical areas, as for example stimulation of regions of the anterior cingulate in the BA32 and BA24 region identified by PET as activated in thirst in humans will initiate drinking in the monkey (48).
Pitts (31), in considering the issue of how far down the phylogenetic tree perception of pain occurs, emphasizes that consciousness may not be an all-or-none phenomenon but varies in particular with the size of the brain, which will also limit other capacities. He cites Grossman (49), “Although the anterior thalamic and possibly mesencephalic and pontine brainstem are necessary for consciousness, they are probably not sufficient— interaction of these rather small masses of neurones with at least a certain volume of limbic cortex or neocortex must occur. The thalamic and brainstem areas may function as organizers of cortical activity and probably must themselves be included in cortical–subcortical–cortical circuits for consciousness to occur.”
Thus the view we are suggesting here is that the Edelman (26, 27) hypothesis of primary consciousness emerging with the ability to create a “scene” from categorization of divergent sensory input and the “scene” being rendered salient by convergence of massive reentrant circuits is a cogent view of early stages in evolution of perception and awareness determined by distance receptor inflow and is a major functional influence on development of the brain. However, in terms of the first emergence of dim awareness in animal evolution, we suggest it came from the interoceptor-driven primal vegetative emotions signaling that the existence of the organism was threatened. Thus, primary consciousness is ignited by the neural organization postulated above and involves essential elements in the rhomboencephalon, mesencephalon, hypothalamus, thalamus, amygdala, and areas of the ancient allocortex and transitional cortex—anterior and posterior cingulate, insula and claustrum, and parahippocampus. The process of rostral anatomical elaboration and specialization of the brain with the evolution of cytoarchitecture of the cortex with its corticothalamic connections as well as direct connections to the hypothalamus, midbrain, medulla, and cerebellum was the phylogenetic circumstance that was conditional for the complex genetic reflex mechanisms of the hypothalamus, mesencephalon, and medulla to be modified by emergent conscious awareness embodying intentionality with subjectivity inherent. Options could be exercised by the organism.
Acknowledgments
We gratefully acknowledge helpful comments from Profs. Per Roland, Simon Gandevia, John Pappenheimer, and George Paxinos. This work was supported by the National Health and Medical Research Council of Australia, the Howard Florey Biomedical Foundation of the United States, the Robert J., Jr. and Helen C. Kleberg Foundation, and the Harold G. and Leila Y. Mathers Charitable Foundation.
Abbreviations
- PET
positron emission tomography
- rCBF
regional cerebral blood flow
- BA
Brodmann's area
- PCO2
partial pressure CO2
- FM
face mask
- MP
mouthpiece
- REM
rapid eye movement
References
- 1.Guz A. Respir Physiol. 1997;109:197–203. doi: 10.1016/s0034-5687(97)00050-9. [DOI] [PubMed] [Google Scholar]
- 2.Pappenheimer J R. Harvey Lect. 1965. 71–94. [Google Scholar]
- 3.Horn E M, Waldrop T G. Respir Physiol. 1998;114:201–211. doi: 10.1016/s0034-5687(98)00087-5. [DOI] [PubMed] [Google Scholar]
- 4.Block-Salisbury E, Spengler C M, Brown R, Banzett R B. Am J Respir Crit Care Med. 1998;157:415–420. doi: 10.1164/ajrccm.157.2.9701024. [DOI] [PubMed] [Google Scholar]
- 5.Banzett R B, Lansing R W, Evans C E, Shea S A. Respir Physiol. 1996;103:19–31. doi: 10.1016/0034-5687(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 6.Gandevia S C, Killean K, McKenzie D R, Crawford M, Allen G M, Gorman R B, Hales J P. J Physiol. 1993;470:85–107. doi: 10.1113/jphysiol.1993.sp019849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banzett R B, Lansing R W, Reid M B, Adams L, Brown R. Respir Physiol. 1989;76:53–67. doi: 10.1016/0034-5687(89)90017-0. [DOI] [PubMed] [Google Scholar]
- 8.Heywood P, Murphy K, Corfield D R, Morell M J, Howard R S, Guz A. Respir Physiol. 1996;106:13–20. doi: 10.1016/0034-5687(96)00060-6. [DOI] [PubMed] [Google Scholar]
- 9.Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, Abplanalp B, Stofer K, Denton D, Fox P T. Proc Natl Acad Sci USA. 2001;98:2029–2034. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox P T, Mintun M A, Reiman E M, Raichle M E. J Cereb Blood Flow Metab. 1988;8:642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- 11.Fox P T, Perlmutter J S, Raichle M E. J Comput Assist Tomogr. 1985;9:141–153. doi: 10.1097/00004728-198501000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Fox P T, Mintun M A. J Nucl Med. 1988;30:141–149. [PubMed] [Google Scholar]
- 13.Denton D A, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Fox P. Proc Nat Acad Sci USA. 1999;96:2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paxinos G, Huang X. Atlas of the Human Brain Stem. San Diego: Academic; 1995. [Google Scholar]
- 15.Corfield D R, Fink G R, Ramsay S C, Murphy K, Harty H R, Watson J D G, Adams L, Frackowiak R S J, Guz A. J Physiol. 1995;488:77–84. doi: 10.1113/jphysiol.1995.sp020947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banzett R B, Mulniar H E, Murphy K, Rosen S D, Wise R J S, Adams L. NeuroReport. 2000;11:2117–2120. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- 17.Reiman E M, Lane R D, Ahern G L, Schwartz G E, Davidson R J, Friston K J, Yun L S, Chen K. Am J Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- 18.Liotti M, Mayberg H S, Brannan S K, McGinnis S, Jerabek P, Fox P. Biol Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- 19.Taylor S F, Liberzon I, Fig L M, Decker L R, Minoshima S, Koeppe R A. NeuroImage. 1998;8:188–197. doi: 10.1006/nimg.1998.0356. [DOI] [PubMed] [Google Scholar]
- 20.Knight D C, Smith C N, Stein E A, Helmstetter F J. NeuroReport. 1999;10:3665–3670. doi: 10.1097/00001756-199911260-00037. [DOI] [PubMed] [Google Scholar]
- 21.Pardo J V, Fox P F, Raichle M E. Nature (London) 1991;349:61–65. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- 22.Stuss D T, Benson D F. The Frontal Lobes. New York: Raven; 1986. [Google Scholar]
- 23.The New Shorter Oxford Dictionary (1993) (Clarendon, Oxford).
- 24.Denton D A, McKinley M J, Weisinger R S. Proc Natl Acad Sci USA. 1996;93:7397–7404. doi: 10.1073/pnas.93.14.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denton D A, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Lancaster J, Fox P. Proc Natl Acad Sci USA. 1999;96:5304–5309. doi: 10.1073/pnas.96.9.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelman G M. Bright Air, Brilliant Fire. London: Penguin; 1992. [Google Scholar]
- 27.Edelman G M, Tononi G. A Universe of Consciousness. New York: Basic; 2000. [Google Scholar]
- 28.Scheibel A N. The Reticular Formation Revisited. New York: Raven; 1980. [Google Scholar]
- 29.Parsons L M, Denton D, Egan G, McKinley M, Shade R, Lancaster J, Fox P T. Proc Natl Acad Sci USA. 2000;97:2332–2336. doi: 10.1073/pnas.040555497. . (First Published February 25, 2000; 10.1073/pnas.040555497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tataranni P A, Gautier J-F, Chen K, Uecker A, Bandy D, Salbe A D, Pratley R E, Lawson M, Reiman E M, Ravussin E. Proc Natl Acad Sci USA. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitts G C. Perspect Biol Med. 1994;37:275–284. doi: 10.1353/pbm.1994.0085. [DOI] [PubMed] [Google Scholar]
- 32.Dennis S G, Melzach R. Animal Pain: Perception and Alleviation. Bethesda, MD: Am. Physiol. Soc.; 1983. [Google Scholar]
- 33.Coghill R C, Talbot J D, Evans A C, Meyer E, Gjedde A, Bushnell M C, Duncan G H. J Neurosci. 1994;14:4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rainville P, Duncan G H, Price D D, Carrier B, Bushnell M C. Nature (London) 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 35.Blok B F M, Willemsen A T M, Hotstege G. Brain. 1997;120:111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- 36.Noto H, Ropplo J R, Steers W D, de Groat W C. Brain Res. 1991;549:95–105. doi: 10.1016/0006-8993(91)90604-t. [DOI] [PubMed] [Google Scholar]
- 37.Blok B F M, de Weerd H, Hostege G. J Comp Neurol. 1995;359:300–309. doi: 10.1002/cne.903590208. [DOI] [PubMed] [Google Scholar]
- 38.Tononi G, Edelman G M. Science. 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- 39.Maquet P, Degueldre C, Delfiore G, Aerts J, Peters J-M, Luxen A, Franck G. J Neurosci. 1997;17:2807–2812. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steriade M, McCarley R W. Brainstem Control of Wakefulness and Sleep. New York: Plenum; 1990. [Google Scholar]
- 41.Maquet P, Peters J-M, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Nature (London) 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 42.Jouvet M. Arch Ital Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- 43.Jones B E. Neuroscience. 1991;40:637–656. doi: 10.1016/0306-4522(91)90002-6. [DOI] [PubMed] [Google Scholar]
- 44.Steriade M, Jones E G, Llinas R. Thalamic Oscillations and Signalling. New York: Wiley; 1990. [Google Scholar]
- 45.Amaral D G, Price J L, Pittzanen A, Carmichael S T. The Amygdala, Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York: Wiley–Liss; 1992. [Google Scholar]
- 46.Aggleton J P, Burton M J, Passingham R E. Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- 47.Kinomura S, Larsson J, Gulyas B, Roland P E. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 48.Robinson B W, Mishkin M. Exp. Brain Res. 1968. 330–366. [DOI] [PubMed] [Google Scholar]
- 49.Grossman R G. Information Processing in the Nervous System. New York: Raven; 1980. [Google Scholar]
- 50.Blok B F, Sturms L M, Hostege G. Brain. 1998;121:111–121. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- 51.Vogt B A, Derbyshire S P, Jones A K. Eur J Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 52.Craig A D, Reiman E M, Evans A O, Bushnell M C. Nature (London) 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 53.Talbot J D, Marrett S, Evans A, Meyer E, Bushnell M C, Duncan G H. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- 54.Derbyshire S W, Jones A K, Gyulai F, Clark S, Townsend D, Firestone L L. Pain. 1997;73:431–435. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 55.Derbyshire S W, Jones A K. Pain. 1998;76:127–135. doi: 10.1016/s0304-3959(98)00034-7. [DOI] [PubMed] [Google Scholar]