Abstract
There is no reliable and simple diagnostic marker available to diagnose recent hepatitis C virus (HCV) infection. It has been shown that the avidity of specific IgG antibody is low in primary viral infection and increases with time. We report the development of an anti-HCV avidity assay derived from a commercially available test. A panel of 117 sera was first examined for IgG avidity. It was composed of samples from patients with recent (group 1, n = 14), chronic (group 2, n = 70), and resolved (group 3, n = 33) HCV infections. Avidity index (AI) values observed in recently infected patients were significantly lower (12.0% ± 9.2% [mean ± standard deviation]) than those found in chronic carriers (83.1% ± 15.2%). Using a threshold of 43.0%, this assay distinguished between groups 1 and 2 with very high sensitivity (98%) and specificity (100%). For group 3, a broader distribution of the AI values was observed (54.8% ± 27.3%), suggesting that this index would not be useful in HCV RNA-negative patients. Blind validation of the test was carried out with a panel of 36 serum samples from 17 HCV seroconverters. The assay described here is a useful tool to distinguish recent from chronic infection in HCV-viremic patients.
Hepatitis C virus (HCV) can be acquired or transmitted via any blood-borne route. Intravenous drug use has become the main transmission mechanism of HCV in Western countries (5). Patients with acute HCV infection are generally asymptomatic. Those who are symptomatic may exhibit jaundice but more often complain of fatigue, nausea, abdominal pain, or flulike symptoms. An average of 30% of patients with acute hepatitis C experience spontaneous viral clearance during the first 3 months after the clinical onset of the disease (15). Chronic disease should be considered if viremia persists for more than 6 months. Chronic hepatitis C is also generally clinically silent for a sustained period of time. This results in fortuitous diagnosis at a late stage of disease in most cases.
There is no reliable and simple diagnostic marker currently available to diagnose recent hepatitis C virus infection. Increases in serum aminotransferase may be indicative of a recent infection but are also seen in exacerbation phases of chronic hepatitis C. They may also arise due to other acute disorders, such as alcohol-induced hepatitis. Specific IgM responses to HCV do not serve as useful markers in the diagnosis of recent hepatitis C, because IgM may be present at similar levels in both recent and chronic disease (14, 20). HCV seroconversion remains the only useful marker of recent HCV infection.
An accurate estimate of HCV incidence would be useful to target interventions to high-risk populations but is a major challenge for epidemiologists. The avidity of an antibody may be an early and reliable marker of recent viral infection. Avidity increases progressively with time after exposure to an immunogen due to rapid mutations in the DNA coding for the variable part of the antibody (8, 19). Antibodies of low avidity are usually indicative of recent infection. Avidity assays have been developed for many viral infections (rubella virus, cytomegalovirus, varicella-zoster virus, and HIV-1) (2, 6, 7, 10, 16). Commercial immunoassays for anti-HCV antibody detection have been adapted to test for avidity. They have confirmed that anti-HCV avidity increases with time after primary infection (3, 11, 17). However, the limited evaluation of such assays in small populations has hampered their development for use in clinical practice. More recently, the development of an “in-house” anti-HCV IgG avidity assay, using a combination of target antigens, has been validated with seroconversion panels and samples from chronically infected individuals (12).
Here, we report the development of an anti-HCV avidity assay, derived from a commercially available third-generation enzyme-linked immunosorbent assay (ELISA). The test was evaluated using a panel of established recent and chronic HCV infections. The usefulness and the clinical significance of the anti-HCV avidity index (AI) are also discussed.
MATERIALS AND METHODS
Study population.
Tested serum samples were divided into four groups: groups 1 to 3, designated the training sample, and a fourth group, the validation sample. The validation sample allowed validation of the parameters estimated with the training sample.
For the avidity test, we investigated three panels of sera: group 1 sera were from patients with recent primary HCV infection, group 2 were from patients with chronic HCV infection, and group 3 were from patients with resolved HCV infection (Table 1). Group 1 consisted of the first HCV antibody-positive sera from 14 patients with recent primary HCV infection. For 10 cases, anti-HCV seroconversion was observed, and the delay between the last negative and the first positive anti-HCV determination never exceeded 6 months. For the other cases (cases T11 to T14), the first anti-HCV-positive sample was obtained close to the alanine aminotransferase (ALT) peak (>1,000 IU/ml) (3 weeks prior to the ALT peak and 4 weeks after). Looking at sequential samples, an increase in intensity of the immunoblot profile (RIBA HCV 3.0 [Chiron Corporation, CA]) can confirm recent infections (Table 2). Consecutive sera (n = 39) from five of these untreated and immunocompetent patients (samples T1, T2, T3, T13, and T14) were analyzed for changes in HCV IgG avidity over time. Group 2 samples were from 70 patients with chronic HCV infection who were anti-HCV IgG positive and HCV RNA positive for more than 6 months. Group 3 samples were from 33 individuals positive for anti-HCV IgG but negative for serum HCV RNA. No group 2 or group 3 patients had a history of antiviral therapy.
TABLE 1.
Initial characteristics of training and validation samples
| Sample type and groupa | No. of patients | Median age in yr (range) | No. of males/ females | No. of patients with HCV genotype no.: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | NAb | ||||
| Training samples | |||||||||
| Group 1 | 14 | 37 (21-54) | 5/9 | 9 | 0 | 4 | 1 | 0 | 0 |
| Group 2 | 70 | 43 (24-79) | 50/20 | 36 | 4 | 13 | 3 | 3 | 11 |
| Group 3 | 33 | 39 (24-80) | 18/15 | 33 | |||||
| Validation samples | 17 | 58 (16-76) | 9/8 | 8 | 6 | 1 | 0 | 0 | 2 |
Group 1, recent HCV infection; group 2, chronic HCV infection; group 3, resolved HCV infection.
NA, not available
TABLE 2.
Epidemiological and biological characteristics of patients recently infected with HCV (group 1)
| Case | Age (yr) | Sex | HCV risk factor | No. of mo since last anti-HCV-negative sample | Immunoblot resultsd at time of first anti-HCV IgG-positive sample |
HCV genotypee | ALT peak serum level (IU/liter)f | Treatment | Evolution | HIV coinfection | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Core | NS3 | NS4 | NS5 | ||||||||||
| T1 | 25 | M | Unknown | 0, 5b | ++ | ++++ | − | − | 1b | 1,692 | Yes | Recovery | No |
| T2 | 54 | F | Nosocomial | 1 | +++ | ++++ | ++ | ++ | 1b | 2,697 | Yes | Recovery | No |
| T3a | 39 | M | Drug user | 1b | − | ++++ | ++++ | − | 1b | 463 | No | Chronicity | Yes |
| T4 | 46 | F | Partner of drug user | 1, 5b | − | ++ | − | − | 1 | NA | No | Chronicity | No |
| T5 | 26 | F | Drug user | 2 | ++++ | + | − | − | 3 | 260 | No | Recovery | No |
| T6 | 36 | F | Drug user | 2b | ++++ | ++ | − | − | 3 | 840 | Yes | Recovery | No |
| T7 | 38 | F | Partner of drug user | 3 | ++++ | ++++ | ++++ | +++ | 1b | 1,283 | Unknown | No | |
| T8 | 20 | M | Drug user | 4 | ++++ | − | − | − | 1a | 185 | No | Chronicity | No |
| T9a | 51 | M | Drug user | 5 | − | ++++ | +++ | − | 4 | 232 | No | Recovery | Yes |
| T10 | 39 | F | Drug user | 6 | ++++ | ++++ | ++ | + | 3 | 262 | No | Chronicity | No |
| T11c | 27 | F | Drug user | Unknown | + | ++++ | ++++ | − | 1a | 1,030 | No | Chronicity | No |
| T12c | 38 | F | Drug user | Unknown | ++ | + | − | − | 3 | 1,699 | No | Recovery | No |
| T13c | 21 | M | Drug user | Unknown | +++ | +++ | + | − | 1b | 2,179 | Yes | Recovery | No |
| T14c | 31 | F | Drug user | Unknown | +++ | +++ | − | ++ | 1b | 2,064 | No | Recovery | No |
This patient was coinfected with HIV but was not immunocompromised.
HCV RNA was detected with Amplicor HCV detection kit 2.0 (Roche Diagnostics Corporation, NJ).
We did not observe seroconversion in this patient but did observe increased intensity of the anti-HCV IgG profile in a third-generation strip immunoblot assay (RIBA HCV 3.0 [Chiron Corporation, CA]).
The results shown were based on criteria provided by the manufacturer for RIBA HCV 3.0 (Chiron Corporation, CA). The presence (+) or absence (−) of antibodies against the core, NS3, NS4, and NS5 proteins is indicated. The intensity of the antigen line reaction is indicated as follows: ++++, very strongly positive; +++, strongly positive; ++, moderately positive; +, weakly positive.
HCV genotype was determined using the Versant HCV genotype assay 1.0 (LiPA [Bayer, Austria]).
A normal ALT value is <35 IU/liter. NA, not available.
Blind validation of the test was carried out with a panel of 36 serum samples from 17 HCV seroconverters (V1 to V17), provided by the French National Blood Transfusion Institute (INTS; Paris, France) (Table 1). All of these samples were from patients who had undergone regular hemodialysis; their seroconversion date could thus be accurately estimated. All samples tested positive for HCV RNA. For 29 serum samples, the time since the last anti-HCV-negative sample was less than 6 months. The seven other samples showed longer times since the last anti-HCV-negative sample: 6.1 months, 14 months, 16 months, 18 months, 27 months, 44 months, and 85 months.
Anti-HCV IgG was tested with the HCV 3.0 ELISA test system (Ortho-Clinical Diagnostics Inc., Johnson and Johnson Company, NJ) and confirmed with an RIBA HCV 3.0 immunoblot. HCV RNA was detected with either Amplicor HCV detection kit 2.0 (Roche Diagnostics Corporation, NJ) or Abbott RealTime HCV (Abbott Molecular Inc., IL).
Anti-HCV IgG avidity measurement.
We used reagents and microplates from the Ortho HCV 3.0 ELISA test system. Serum samples were prediluted (10 μl in 100 μl of the kit specimen diluent). Twenty microliters of diluted samples and 200 μl of specimen diluent were then incubated for 1 h at 37°C.
Each sample was loaded into two wells. The sample was aspirated, and the two wells were filled with 200 μl of washing buffer with or without 6 M urea and incubated for 30 min at room temperature. Two control samples, one for recent HCV primary infection (collected 45 days after the ALT peak) and one from a patient with chronic infection, were included in each run.
AI was determined as the ratio of absorbance after incubation with urea to absorbance after incubation without urea and was expressed as a percentage.
For absorbance values between 2.0 and 3.5, AI determination was calculated directly as described above. For values above saturation (absorbance, >3.5), the sample was diluted 1:4 with sterile water before determining the AI. Samples with low absorbance values (<2.0) were systematically retested without dilution for more reliable AI determination. We selected the concentration of urea (6 M) and the duration of contact (30 min) in a preliminary test to determine which parameters enabled the most accurate identification of recent and chronic infections.
To assess the variability of the assay, intra- and interassay coefficients of variation (CVs) were evaluated by testing three sera with different AI values, i.e., high (90%), intermediate (65%), and low (20%). Fifteen replicates were obtained for the determination of intraassay variation and 10 for interassay variation.
Statistical analysis.
We used the Kruskal-Wallis test, a nonparametric analogue of one-way analysis of variance, to compare the avidity indexes of samples from groups 1, 2, and 3. The Mann-Whitney U test was then used to compare the avidity indexes between two groups. All tests were two sided, and P values below 0.05 were considered to be statistically significant. For groups 1 and 2, the discriminant capacity of the avidity index was analyzed using receiver operating characteristic (ROC) curves. The area under the ROC curves was calculated. We determined optimal values in terms of both sensitivity and specificity by determining the crossover point of the two curves. A linear regression with two slopes was used to show the evolution of the avidity index over time for 5 patients of group 1.
RESULTS
Variability of the assay.
The mean intraassay coefficients of variation were 4.8% for high AI values, 5.0% for intermediate values, and 6.6% for low values. The mean interassay CVs for high, intermediate, and low AI values were 4.0%, 5.4%, and 13.1%, respectively.
Anti-HCV IgG avidity index for the training sample.
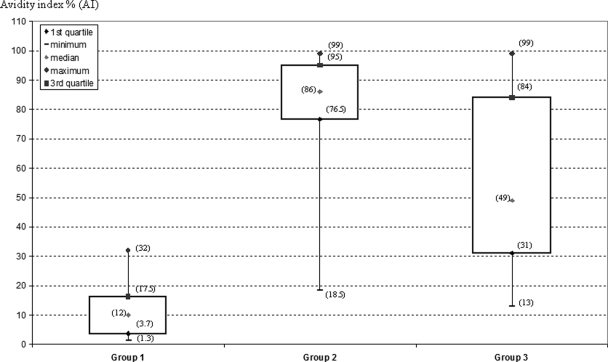
Overall, mean AI values differed significantly between the three patient groups (P < 0.0001) (Fig. 1). The mean AI value ± standard deviation (SD) was significantly lower for patients with recent HCV infection (group 1) (12.0% ± 9.2%) than for patients with chronic HCV infection (group 2) (83.1% ± 15.2%) (P < 0.0001). The AI values were intermediate in patients with resolved infection (group 3) (54.8% ± 27.3%). In this group of patients, in whom HCV infection spontaneously cleared, there was a broader distribution of the AI values, suggesting that this index is less useful in HCV RNA-negative patients.
FIG. 1.
Anti-HCV IgG AI values for the training sample. Group 1 (recent primary HCV infection; n = 14), group 2 (chronic HCV infection; n = 70), and group 3 (resolved HCV infection; n = 33). The data for the 3 groups were described using box plots (median value and interquartile range). For each group, AI values corresponding to the median, the minimum, the maximum, and the 1st and 3rd quartile are indicated in parentheses.
The performance of the assay to discriminate between patients with recent infection and those with chronic infection was analyzed for groups 1 and 2 by ROC curve analysis. Using a threshold of 43%, the sensitivity and specificity of the assay reached 98% and 100%, respectively. The area under the ROC curves is equal to 0.99. All samples from group 1 had AI values below this threshold, whereas 69 out of 70 samples from group 2 had AI values above the threshold.
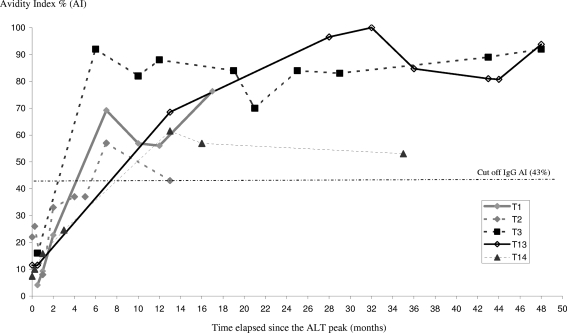
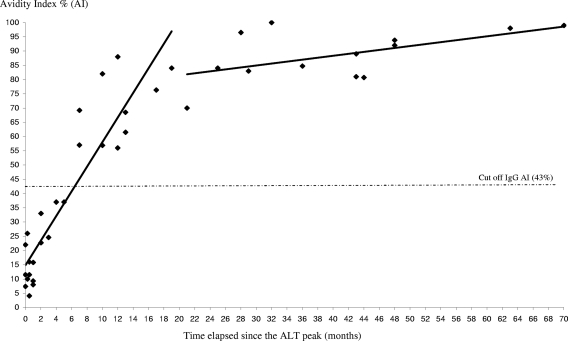
Information on the dates of ALT peak was available for five patients in group 1 (patients T1, T2, T3, T13, T14). AI increased with time over the first 20 months (r2 = 0.862 [95% confidence interval, 0.712 to 0.937; P < 0.0001]) (Fig. 2 and 3).
FIG. 2.
Changes in anti-HCV IgG AI values over time for five patients with recent primary HCV infection (group 1). The evolution of the AI for patients T1, T2, T3, T13, and T14 is shown. The observation period (50 months) is expressed as months after the ALT peak. The threshold of 43% is indicated by a dashed line.
FIG. 3.
Increase in anti-HCV IgG AI values over time since the ALT peak, shown for five patients from group 1 (recent primary infection). The results from a linear regression with two slopes are shown. The threshold of 43% is indicated by a dashed line.
Anti-HCV IgG AI for the validation sample.
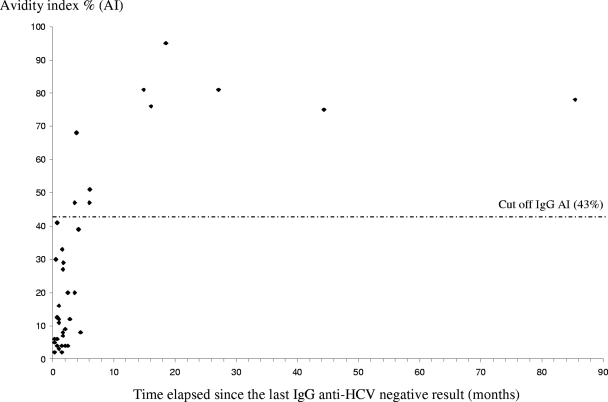
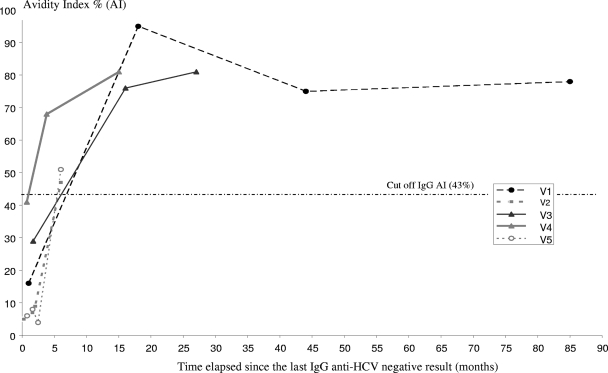
Early seroconversion was observed for 29 sera from the validation sample. These sera had been drawn from patients within 6 months after the last anti-HCV-negative serum. The mean AI ± SD was 16.4% ± 16.3%. Two samples had AI values above 43% (sensibility, 93%). For samples with more than a 6-month delay between the last anti-HCV-negative and the first anti-HCV-positive determinations (seven sera), the mean value of AI ± SD was 73% ± 16.1%; all these sera had an AI above 43% (specificity, 100%) (Fig. 4). We were able to show changes in AI over time for five patients (patients V1 to V5) with available sequential samples (Fig. 5).
FIG. 4.
Anti-HCV IgG AI values for patient from the validation sample. The observation period is expressed as months after the last anti-HCV-negative result. The threshold of 43% is indicated by a dashed line.
FIG. 5.
Changes in anti-HCV IgG AI values over time for five patients from the validation sample. The observation period is expressed as months after the last anti-HCV IgG-negative result. The threshold of 43% is indicated by a dashed line.
DISCUSSION
Distinguishing recent from chronic HCV infection may be useful in a variety of contexts. Determining whether a recently discovered viremia in an individual corresponds to an early phase or to chronic infection may allow improved management of the patient. At the population level, distinguishing recently infected from chronically infected patients may allow the estimation of HCV incidence through serosurveys.
There are no reliable one-shot assays available for the identification of recent HCV infections. Recombinant immunoblot assays on sequential samples can indicate recent infection when an increase in either intensity or the number of reactive antigens is observed, but at least two samples are needed.
Here, we describe the modification of a commercial assay for measurement of HCV antibody affinity. This assay is based on treatment with urea, which disrupts the weak antigen-antibody complex. Avidity index was determined by comparing ELISA results for sera treated with and without urea. Several adaptations of commercial immunoenzymatic assays have been reported for determination of anti-HCV IgG avidity. First, Ward et al. reported an adaptation of a second-generation anti-HCV ELISA (17). In a more recent work, Coppola et al. used the same third-generation ELISA as us (HCV 3.0 ELISA test system [Ortho-Clinical Diagnostics]) (3).
In the training sample, we observed a rise in AI over time during the first 20 months after the ALT peak, followed by a plateau phase in which AI values remained stable (Fig. 3). The maximum for AI was reached after 6 and 7 months, respectively, for patients T3 and T2. The rise was slower for patient T13: the maximum was reached after 32 months. Kanno and Kazuyama reported that the maximum value for AI was observed after an average of 25 months in untreated patients (11). A more rapid rise in AI values was observed by Coppola et al. Sixteen to 20 days after the onset of symptoms, the mean AI value was already 65% in 27 patients with recent HCV infection (4). However, these data are not comparable with our results, because a different denaturating agent was used (guanidine, 1 M).
In our study, subjects were defined as “recently infected” when anti-HCV seroconversion had occurred in the last 6 months. They were defined as “chronic carriers” when anti-HCV antibodies and HCV RNA were detectable for more than 6 months. AI values observed in recently infected patients (group 1) in the training sample were significantly lower than those found in chronic carriers (group 2). Using a threshold of 43%, this assay distinguished between these two disease states with very high sensitivity (98%) and specificity (100%). As the small training sample of recent infections (group 1) was a limitation of this study, the preliminary results were confirmed in a second phase of the study using a validation sample that was tested blindly. This validation sample consisted of 36 sera from 17 viremic patients with an estimated date of seroconversion. Only two of 29 samples collected within 6 months of the last anti-HCV-negative sample had an AI value above 43.0%.
For the first case, we observed a very rapid rise of the avidity index, from only 4% at 2 months to 47.0% at 3.6 months. For the second case, the AI was under the threshold at 0.7 months (41%) but reached 68.0% at 3.9 months. The AI values of eight samples collected after the sixth month were above the threshold.
It is well established that 20 to 30% of HCV-exposed patients spontaneously clear the virus. Their anti-HCV reactivity then declines progressively and may lead to seroreversion (13). We looked at 33 samples from patients with resolved infection who tested negative for HCV RNA and positive for HCV antibody. The AI values varied between 13% and 98% in these patients (mean value, 54.8%). Of the 33 samples studied, 13 had AI values below 43.0%, demonstrating the overlap between values corresponding to those observed early after infection and those observed in patients with resolved infection. The date of the last anti-HCV-negative sample was not available for this group of patients, so we could not determine whether they had resolved their infection recently or a long time ago. This large scattering of AI values is consistent with previous results obtained by Klimashevskaya et al. (12). Studying HCV incidence at a country level or among high-risk groups would be facilitated by the availability of a method able to date the infection. The assay described herein can be applied in viremic patients but cannot be recommended in nonviremic patients until a better characterization is established.
Drug treatment has been proven to be highly effective during early infection, highlighting the particular importance of detecting recent primary HCV infection in individual patients. Additionally, several trials have demonstrated the efficacy of a 12- or 24-week course of pegylated interferon (PEG-IFN) monotherapy among patients with acute hepatitis C when the therapy is initiated within 3 months (1, 9, 18).
Determination of the AI can also help the physician to identify the period of exposure to HCV, providing insight into the suspected route of transmission.
Footnotes
Published ahead of print on 7 July 2010.
REFERENCES
- 1.Calleri, G., G. Cariti, F. Gaiottino, F. G. De Rosa, O. Bargiacchi, S. Audagnotto, S. Quaglia, T. De Blasi, P. Romano, A. Traverso, G. Leo, R. Carbone, B. Del Mastro, M. Tinelle, P. Caramello, and G. Di Perri. 2007. A short course of pegylated interferon-alpha in acute HCV hepatitis. J. Viral Hepat. 14:116-121. [DOI] [PubMed] [Google Scholar]
- 2.Chargelegue, D., B. T. Colvin, and C. M. O'Toole. 1993. A 7-year analysis of anti-Gag (p17 and p24) antibodies in HIV-1-seropositive patients with haemophilia: immunoglobulin G titre and avidity are early predictors of clinical course. AIDS 7:S87-S90. [DOI] [PubMed] [Google Scholar]
- 3.Coppola, N., R. Pisapia, C. Marrocco, S. Martini, L. M. Vaterio, V. Messina, G. Tonziello, C. Sagnelli, P. Filippini, F. Piccinino, and E. Sagnelli. 2007. Anti-HCV IgG avidity index in acute hepatitis C. J. Clin. Virol. 40:110-115. [DOI] [PubMed] [Google Scholar]
- 4.Coppola, N., R. Pisapia, G. Tonziello, A. Masiello, S. Martini, M. Pisaturo, V. Messina, C. Sagnelli, M. Macera, G. Signoriello, and E. Sagnelli. 2009. Improvement in the aetiological diagnosis of acute hepatitis C: a diagnostic protocol based on the anti-HCV-IgM titre and IgG avidity index. J. Clin. Virol. 46:222-229. [DOI] [PubMed] [Google Scholar]
- 5.Esteban, J. I., S. Sauleda, and J. Quer. 2008. The changing epidemiology of hepatitis C virus infection in Europe. J. Hepatol. 48:148-162. [DOI] [PubMed] [Google Scholar]
- 6.Grangeot-Keros, L., M. J. Mayaux, P. Lebon, F. Freymuth, G. Eugene, R. Stricker, and E. Dussaix. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175:944-946. [DOI] [PubMed] [Google Scholar]
- 7.Hedman, K., and S. A. Rousseau. 1989. Measurement of avidity of specific IgG for verification of recent primary rubella. J. Med. Virol. 27:288-292. [DOI] [PubMed] [Google Scholar]
- 8.Inouye, S., A. Hasegawa, S. Matsuno, and S. Katow. 1984. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J. Clin. Microbiol. 20:525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaeckel, E., M. Cornberg, H. Wedemeyer, T. Santantonio, J. Mayer, M. Zankel, G. Pastore, M. Dietrich, C. Trautwein, M. P. Manns, and the German Acute Hepatitis C Therapy Group. 2001. Treatment of acute hepatitis C with interferon alfa-2b. N. Engl. J. Med. 345:1452-1457. [DOI] [PubMed] [Google Scholar]
- 10.Junker, A. K., and P. Tilley. 1994. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J. Med. Virol. 43:119-124. [DOI] [PubMed] [Google Scholar]
- 11.Kanno, A., and Y. Kazuyama. 2002. Immunoglobulin G antibody avidity assay for serodiagnosis of hepatitis C virus infection. J. Med. Virol. 68:229-233. [DOI] [PubMed] [Google Scholar]
- 12.Klimashevskaya, S., A. Obriadina, T. Ulanova, G. Bochkova, A. Burkov, A. Araujo, S. L. Stramer, L. H. Tobler, M. P. Busch, and H. A. Fields. 2007. Distinguishing acute from chronic and resolved hepatitis C virus (HCV) infections by measurement of anti-HCV immunoglobulin G avidity index. J. Clin. Microbiol. 45:3400-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanotte, P., F. Dubois, S. Le Pogam, C. Guerois, B. Fimbel, Y. Bacq, Y. Gruel, A. Goudeau, and F. Barin. 1998. The kinetics of antibodies against hepatitis C virus may predict viral clearance in exposed hemophiliacs. J. Infect. Dis. 178:556-559. [DOI] [PubMed] [Google Scholar]
- 14.Papatheodoridis, G. V., J. K. Delladetsima, A. Katsoulidou, V. Sypsa, M. Albrecht, G. Michel, A. Hatzakis, and N. C. Tassopoulos. 1997. Significance of IgM anti-HCV core level in chronic hepatitis C. J. Hepatol. 27:36-41. [DOI] [PubMed] [Google Scholar]
- 15.Santantonio, T., J. Wiegand, and J. T. Gerlach. 2008. Acute hepatitis C: current status and remaining challenges. J. Hepatol. 49:625-633. [DOI] [PubMed] [Google Scholar]
- 16.Thomas, H. I., S. Wilson, C. M. O'Toole, C. M. Lister, A. M. Saeed, R. P. Watkins, and P. Morgan-Capner. 1996. Differential maturation of avidity of IgG antibodies to gp41, p24 and p17 following infection with HIV-1. Clin. Exp. Immunol. 103:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward, K. N., W. Dhaliwal, K. L. Ashworth, E. J. Clutterbuck, and C. G. Teo. 1994. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J. Med. Virol. 43:367-372. [DOI] [PubMed] [Google Scholar]
- 18.Wiegand, J., P. Buggisch, W. Boecher, S. Zeuzem, C. M. Gelbmann, T. Berg, W. Kauffmann, B. Kallinowski, M. Cornberg, E. Jaeckel, H. Wedemeyer, M. P. Manns, and German HEP-NET Acute HCV Study Group. 2006. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology 43:250-256. [DOI] [PubMed] [Google Scholar]
- 19.Wolter, T., C. Gassmann, V. Vetter, and G. Bauer. 1997. Avidity determination: utilization of a basic immunological mechanism allows improvement of the serodiagnosis of infections. Clin. Lab. 43:125-135. [Google Scholar]
- 20.Zaaijer, H. L., L. T. Mimms, H. T. Cuypers, H. W. Reesink, C. L. van der Poel, S. Taskar, and P. N. Lelie. 1993. Variability of IgM response in hepatitis C virus infection. J. Med. Virol. 40:184-187. [DOI] [PubMed] [Google Scholar]